HIV-1–Associated Toxoplasmosis
HIV-1–Associated Toxoplasmosis
Introduction
Toxoplasmosis is the commonest central nervous system (CNS) infection in patients with HIV/AIDS who are not on appropriate prophylaxis. The usual manifestation of cerebral toxoplasmosis is typically 1 or more CNS mass lesions. Other opportunistic conditions seen in AIDS that may present with mass like lesions include primary CNS lymphoma (typically associated with the Epstein-Barr virus) and progressive multifocal leukoencephalopathy (PML) which is caused by JC polyomavirus.[1][2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The causative agent is Toxoplasma gondii, an exclusively intracellular, coccidian protozoan parasite with worldwide distribution. Transmission occurs following ingestion of infectious oocysts from contaminated meat, other food, or water. The oocysts are abundant in the soil, cat litter contaminated with feline feces or undercooked meat from an infected animal (especially felines). Felines are the only animals in which T. gondii can complete its reproductive cycle. After ingestion, the organisms encyst in nucleated cells and can lie dormant within the tissues for the life of the host (latent infection). In most scenarios, disease occurs following the reactivation of latent infection because of progressive loss of cellular immunity. This can occur in the setting of advanced HIV, following solid or stem cell transplant, prolonged steroids, use of monoclonal antibodies, or chemotherapy.[4][5]
Epidemiology
The incidence of toxoplasmosis infection depends on the seropositivity of Toxoplasma gondii in the population. In the United States, the seropositivity of T. gondii is approximately 10% of the adult population. Seropositivity is higher in Africa and Europe, where it can reach up to 80%, and toxoplasma encephalitis could be as high as 25% to 50% of susceptible hosts. The seropositivity in the HIV population mirrors that of the general population.[6][7]
Even though cats and related felines have traditionally been associated with toxoplasmosis, the prevalence of infection is not related to owning a cat. In HIV toxoplasma-seropositive patients with CD4 less than 100 who are not on appropriate prophylaxis, the probability of developing reactivated toxoplasmosis is as high as 30%. Apart from oral transmission, toxoplasmosis can also be transmitted via the placenta from mother to fetus or through transplantation of an infected organ.
Pathophysiology
After ingestion of the infective forms, the organism invades the intestinal epithelium and subsequently disseminates throughout the body. At the tissues, which often is the brain but can affect any tissue, they then encyst and lie dormant until immunity wanes. The dormant forms are called bradyzoites, while the actively replicating forms are called tachyzoites. Primary toxoplasmosis is often subclinical but could rarely present symptomatically in an immunocompromised seronegative person who became recently exposed to the infective forms. In this scenario, IgM to a toxoplasma is often positive, but IgG is not, unlike most reactivation cases, when IgG is the only positive serology test. As previously outlined, in most situations, clinical toxoplasmosis in the HIV patient is due to the reactivation of latent infection as immunity wanes.[8]
Histopathology
The histology usually reveals diffuse encephalitis, cyst-containing lesions, microglial nodules, and lymphocytic vasculitis.
History and Physical
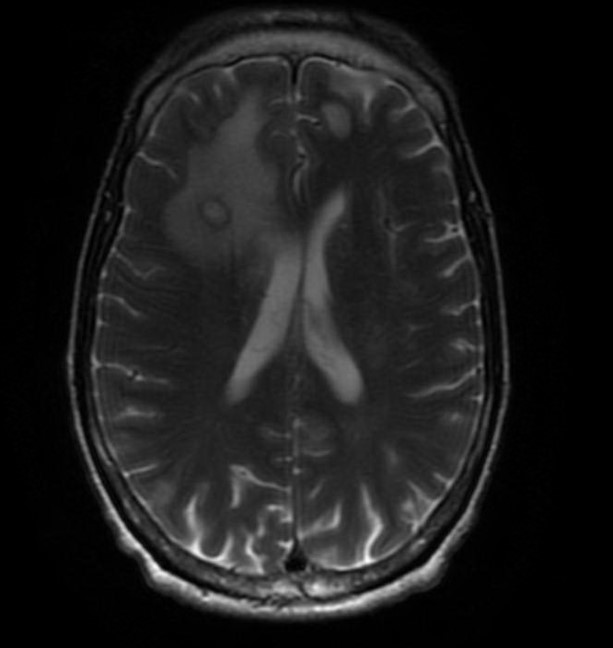
Cerebral toxoplasmosis (toxoplasmic encephalitis), usually with 1 or more ring-enhancing brain lesions, is the typical presentation in patients with HIV (see Image. Cerebral Toxoplasmosis). Symptoms are often subacute, ranging from a few days to a month. Common symptoms include a headache, confusion, and lethargy. Fever may be present but often absent. There is some suggestion that a chronic toxoplasma infection state may not be completely asymptomatic, and in some patients, behavioral changes and neuropsychiatric disorders are possible. Seizures and focal neurologic deficits are also common, occurring in up to 30% and 70% of patients, respectively. Mental status changes could range from dull affect to stupor and coma, often secondary to global encephalitis or increased intracranial pressure. On the other hand, the latent or dormant phase is often asymptomatic.
Extracerebral involvement is typically less common than CNS infection. These include pneumonitis and chorioretinitis, and less commonly, the gut, liver, heart, bladder, spinal cord, bone marrow, and testes. Rarely, toxoplasmosis may present as a disseminated disease.
Evaluation
There are no routine laboratory findings that are specific to toxoplasmosis. Lactate dehydrogenase (LDH) can be increased markedly in patients with disseminated toxoplasmosis and pulmonary disease.[2][9][10]
A presumptive diagnosis of cerebral toxoplasmosis in the HIV patient can be made as follows:
- CD4 count less than 100 cells/microliter without any effective prophylaxis
- A compatible clinical syndrome
- A positive T. gondii immunoglobulin (Ig) G antibody (Antitoxoplasma IgM antibodies are usually absent except rarely in cases of primary infection. Quantitative IgG antibody titers are not helpful in diagnosis.)
- Brain imaging (preferably MRI) that demonstrates a typical radiographic appearance
If the above criteria are present, there is at least a 90% probability the diagnosis is cerebral toxoplasmosis. However, it is important to note that a positive serology does not confirm the diagnosis, and a negative serology implies the diagnosis is not likely (though not impossible) to be cerebral toxoplasmosis.
Brain imaging often shows multiple (67%) or single (33%) ring-enhancing brain lesions often associated with edema. There is a predilection for involvement of the basal ganglia, corticomedullary junction, or brain white matter.
A more definitive diagnosis can be made by obtaining a lumbar puncture. Cerebrospinal fluid (CSF) analysis often shows mononuclear pleocytosis, elevated protein, and, sometimes, reduced CSF glucose. Polymerase chain reaction (PCR) testing for T. gondii in CSF is 100% specific but only 44% to 65% sensitive. This suggests a positive CSF PCR result establishes the diagnosis of cerebral toxoplasmosis, but a negative one does not rule it out.
When the diagnosis is in doubt, and alternative diagnoses are considered, a brain biopsy can be obtained. Findings in cerebral toxoplasmosis may show necrotic abscesses with blood vessel thrombosis and necrosis. Cysts containing bradyzoites may often be found coexisting with numerous active tachyzoites. In most cases, brain biopsy is not required for diagnosis.
Treatment / Management
The treatment of toxoplasmosis in HIV-infected patients includes:
- Antimicrobial therapy directed against T. gondii
- Antiretroviral therapy for immune recovery
Pyrimethamine and sulfadiazine are most commonly employed in treatment. Both agents synergistically and sequentially block folic acid metabolism, which is necessary to develop the parasite. Folinic acid (leucovorin) is often added to replace folate stores, which are nonselectively depleted. Initial treatment is typically for 6 weeks.[11][12][13][12](A1)
Initial therapy is followed by secondary prophylaxis or maintenance therapy, which continues until CD4 plus T-lymphocyte counts are over 200 cells/microliters for over 3 months. In addition to awaiting immune recovery, secondary prophylaxis provides continuous therapy against dormant cystic forms, which may rupture and reinitiate the infectious process at any time. Maintenance therapy, therefore, is necessary to prevent relapse.
In patients who are allergic to sulfonamides, treatment options include clindamycin, trimethoprim/sulfamethoxazole, pyrimethamine plus atovaquone plus folinic acid, pyrimethamine plus azithromycin plus folinic acid, and atovaquone alone (if unable to tolerate sulfur drugs or pyrimethamine). Spiramycin is the drug of choice in the first trimester of pregnancy, as pyrimethamine may be teratogenic.
Most clinicians often employ the use of steroids (when edema is present), but studies have shown its use to be neither beneficial nor harmful. The use of steroids in cerebral toxoplasmosis should probably be limited to impending brain herniation as the diagnosis may be clouded once the steroid is started before a definitive diagnosis is made.
The treatment course is often dramatic, with half the cases showing neurological improvement by day 3 and, in most cases, by day 7 of treatment commencement. If there is no significant improvement or worsening symptoms by days ten to 14 of therapy, repeat imaging and possibly brain biopsy should be considered. Persistent neurologic sequelae may remain in 37% of survivors, and the death rate at 1 year could vary from 10% to 60%.
An integral part of therapy is starting antiretroviral (ART) agents as soon as feasible, usually within 2 weeks of starting antitoxoplasma therapy. Immune reconstitution inflammatory syndrome (IRIS), which can present as a paradoxical worsening of symptoms as immunity recovers, is rarer with toxoplasmosis than mycobacterial and cryptococcal infections. Even though there are no studies about the optimal timing of ART in toxoplasmosis, early ART has clear benefits and should not necessarily be delayed beyond 2 weeks. [14][15](B3)
Differential Diagnosis
The differential diagnoses for HIV-1–associated toxoplasmosis include the following:
- CNS lymphoma
- CNS tuberculosis
- Cryptococcus
- Neurosyphilis
- Cardioembolic stroke
- Cytomegalovirus infection
Complications
Complications of HIV-1–associated toxoplasmosis include the following:
- Changes in personality
- Seizures
- Cranial nerve palsy
- Hemiparesis
- Hemianopia
- Ataxia
- Aphasia
Postoperative and Rehabilitation Care
Once antibiotic treatment has started, improvement is gradual and may take several weeks. Imaging studies need to be repeated in 4 to 6 weeks to determine if the lesion is decreasing in size. Long-term therapy is continued at low doses. If the CD4 count improves and the lesion resolves, one may discontinue the therapy.
Deterrence and Patient Education
Patients with HIV-1–associated toxoplasmosis should be advised to avoid eating raw or undercooked meat and wash their hands thoroughly after handling cat litter or soil
Pearls and Other Issues
Prophylaxis against reactivation of T. gondii in seropositive patients with CD4 lymphocyte counts less than 100 cells/microliter is recommended. Trimethoprim/sulfamethoxazole is mostly used. Other options include dapsone plus pyrimethamine, folinic acid, or atovaquone with or without pyrimethamine/leucovorin.
In conclusion, toxoplasmosis should always be included in the differential diagnosis of CNS lesions in advanced HIV/AIDS. Diagnosis combines clinical symptoms and signs, serology, and imaging. CSF may further support the diagnosis. Brain biopsy is often reserved for difficult cases. Treatment is often dramatic, and most patients have a favorable clinical outcome.
Enhancing Healthcare Team Outcomes
The role of the interprofessional healthcare team cannot be overemphasized in the management of HIV patients with CNS toxoplasmosis. Clinicians should emphasize safe sex practices and provide counseling on sex education. Washing hands after contact with cat litter is highly recommended. In addition, walking barefeet in contaminated soil is not recommended. Patients should be cautioned against the use of intravenous drugs. One should wash hands after coming into contact with raw meat and wear gloves when gardening. Because these patients are on many medications, including HAART, pharmacists are in the ideal position to encourage compliance and oversee the regimen. Patients with HIV and CNS toxoplasmosis require lifelong prophylaxis with trimethoprim-sulfamethoxazole; pharmacists should educate patients on potential complications if therapy is not complied with. Finally, these individuals should be told to avoid travel to areas where toxoplasmosis is endemic. Only through an established, integrative, interprofessional team approach can the morbidity and mortality of CNS toxoplasmosis be lowered in HIV patients.[16][17]
The prognosis of CNS toxoplasmosis in HIV is guarded. Relapses are very common if the treatment is discontinued. Ongoing treatment can lower the risk of recurrent infection. If the disorder is not treated adequately, complications like deafness, seizures, and blindness can occur. Infants with congenitally acquired toxoplasmosis generally have a better outcome than HIV adults.[18][19]
Media
(Click Image to Enlarge)
References
Giovane RA, Lavender PD. Central Nervous System Infections. Primary care. 2018 Sep:45(3):505-518. doi: 10.1016/j.pop.2018.05.007. Epub 2018 Jul 9 [PubMed PMID: 30115337]
Paquet C, Yudin MH. No. 285-Toxoplasmosis in Pregnancy: Prevention, Screening, and Treatment. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2018 Aug:40(8):e687-e693. doi: 10.1016/j.jogc.2018.05.036. Epub [PubMed PMID: 30103893]
Mendez OA, Koshy AA. Toxoplasma gondii: Entry, association, and physiological influence on the central nervous system. PLoS pathogens. 2017 Jul:13(7):e1006351. doi: 10.1371/journal.ppat.1006351. Epub 2017 Jul 20 [PubMed PMID: 28727854]
Rapalino O,Mullins ME, Intracranial Infectious and Inflammatory Diseases Presenting as Neurosurgical Pathologies. Neurosurgery. 2017 Jul 1 [PubMed PMID: 28575459]
Sonneville R, Magalhaes E, Meyfroidt G. Central nervous system infections in immunocompromised patients. Current opinion in critical care. 2017 Apr:23(2):128-133. doi: 10.1097/MCC.0000000000000397. Epub [PubMed PMID: 28169858]
Level 3 (low-level) evidenceCrabtree-Ramírez B, Caro-Vega Y, Shepherd BE, Grinsztejn B, Wolff M, Cortes CP, Padgett D, Carriquiry G, Fink V, Jayathilake K, Person AK, McGowan C, Sierra-Madero J, Caribbean, Central and South America Network for HIV Epidemiology (CCASAnet), of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) Program. Time to HAART Initiation after Diagnosis and Treatment of Opportunistic Infections in Patients with AIDS in Latin America. PloS one. 2016:11(6):e0153921. doi: 10.1371/journal.pone.0153921. Epub 2016 Jun 7 [PubMed PMID: 27271083]
Miura Y, Kishida S. [Neurological complications with HIV infection]. Brain and nerve = Shinkei kenkyu no shinpo. 2013 Mar:65(3):275-81 [PubMed PMID: 23475519]
Ho YC, Sun HY, Chen MY, Hsieh SM, Sheng WH, Chang SC. Clinical presentation and outcome of toxoplasmic encephalitis in patients with human immunodeficiency virus type 1 infection. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2008 Oct:41(5):386-92 [PubMed PMID: 19122919]
Brandsma D, Bromberg JEC. Primary CNS lymphoma in HIV infection. Handbook of clinical neurology. 2018:152():177-186. doi: 10.1016/B978-0-444-63849-6.00014-1. Epub [PubMed PMID: 29604975]
Christo PP, Vilela Mde C, Bretas TL, Domingues RB, Greco DB, Livramento JA, Teixeira AL. Cerebrospinal fluid levels of chemokines in HIV infected patients with and without opportunistic infection of the central nervous system. Journal of the neurological sciences. 2009 Dec 15:287(1-2):79-83. doi: 10.1016/j.jns.2009.09.002. Epub 2009 Sep 25 [PubMed PMID: 19782379]
Okome-Nkoumou M, Guiyedi V, Ondounda M, Efire N, Clevenbergh P, Dibo M, Dzeing-Ella A. Opportunistic diseases in HIV-infected patients in Gabon following the administration of highly active antiretroviral therapy: a retrospective study. The American journal of tropical medicine and hygiene. 2014 Feb:90(2):211-5. doi: 10.4269/ajtmh.12-0780. Epub 2013 Dec 9 [PubMed PMID: 24323514]
Level 2 (mid-level) evidenceThoden J, Potthoff A, Bogner JR, Brockmeyer NH, Esser S, Grabmeier-Pfistershammer K, Haas B, Hahn K, Härter G, Hartmann M, Herzmann C, Hutterer J, Jordan AR, Lange C, Mauss S, Meyer-Olson D, Mosthaf F, Oette M, Reuter S, Rieger A, Rosenkranz T, Ruhnke M, Schaaf B, Schwarze S, Stellbrink HJ, Stocker H, Stoehr A, Stoll M, Träder C, Vogel M, Wagner D, Wyen C, Hoffmann C, Deutsche AIDS Gesellschaft, Österreichische AIDS-Gesellschaft. Therapy and prophylaxis of opportunistic infections in HIV-infected patients: a guideline by the German and Austrian AIDS societies (DAIG/ÖAG) (AWMF 055/066). Infection. 2013 Sep:41 Suppl 2(Suppl 2):S91-115. doi: 10.1007/s15010-013-0504-1. Epub 2013 Sep 14 [PubMed PMID: 24037688]
Low A, Gavriilidis G, Larke N, B-Lajoie MR, Drouin O, Stover J, Muhe L, Easterbrook P. Incidence of Opportunistic Infections and the Impact of Antiretroviral Therapy Among HIV-Infected Adults in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Jun 15:62(12):1595-1603. doi: 10.1093/cid/ciw125. Epub 2016 Mar 6 [PubMed PMID: 26951573]
Level 1 (high-level) evidenceTan IL, McArthur JC. HIV-associated neurological disorders: a guide to pharmacotherapy. CNS drugs. 2012 Feb 1:26(2):123-34. doi: 10.2165/11597770-000000000-00000. Epub [PubMed PMID: 22201342]
Level 3 (low-level) evidenceMasur H, Kaplan JE, Holmes KK, U.S. Public Health Service, Infectious Diseases Society of America. Guidelines for preventing opportunistic infections among HIV-infected persons--2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Annals of internal medicine. 2002 Sep 3:137(5 Pt 2):435-78 [PubMed PMID: 12617574]
Level 3 (low-level) evidenceKaplan JE, Masur H, Holmes KK, USPHS, Infectious Disease Society of America. Guidelines for preventing opportunistic infections among HIV-infected persons--2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2002 Jun 14:51(RR-8):1-52 [PubMed PMID: 12081007]
Level 3 (low-level) evidenceKirk O, Lundgren JD, Pedersen C, Nielsen H, Gerstoft J. Can chemoprophylaxis against opportunistic infections be discontinued after an increase in CD4 cells induced by highly active antiretroviral therapy? AIDS (London, England). 1999 Sep 10:13(13):1647-51 [PubMed PMID: 10509565]
Robert-Gangneux F, Meroni V, Dupont D, Botterel F, Garcia JMA, Brenier-Pinchart MP, Accoceberry I, Akan H, Abbate I, Boggian K, Bruschi F, Carratalà J, David M, Drgona L, Djurković-Djaković O, Farinas MC, Genco F, Gkrania-Klotsas E, Groll AH, Guy E, Hirzel C, Khanna N, Kurt Ö, Junie LM, Lazzarotto T, Len O, Mueller NJ, Munoz P, Pana ZD, Roilides E, Stajner T, van Delden C, Villena I, Pelloux H, Manuel O. Toxoplasmosis in Transplant Recipients, Europe, 2010-2014. Emerging infectious diseases. 2018 Aug:24(8):1497-1504. doi: 10.3201/eid2408.180045. Epub [PubMed PMID: 30014843]
Halonen SK, Weiss LM. Toxoplasmosis. Handbook of clinical neurology. 2013:114():125-45. doi: 10.1016/B978-0-444-53490-3.00008-X. Epub [PubMed PMID: 23829904]
Level 3 (low-level) evidence