Introduction
Intraocular hemorrhage means bleeding inside the eye. Bleeding can occur from any of the structures of the eye where there is a presence of vasculature. It can bleed inside the anterior chamber, vitreous cavity, retina, choroid, suprachoroidal space, or optic disc. It could occur either because of trauma or in association with systemic illness, or very rarely, it could occur spontaneously. Intraocular hemorrhage can be subdivided depending on the location of the bleed:
Hyphema
Bleeding from the iris, ciliary body, trabecular meshwork, and associated vasculature into the anterior chamber (bordered by cornea anteriorly, iridocorneal angles laterally, and lens posteriorly) is known as hyphema.[1] Microhyphema- a very minimal amount of blood in the anterior chamber, which is detectable only on microscopic examination.
Vitreous Hemorrhage
Bleeding in and around the anterior chamber of the eye is known as vitreous hemorrhage. It can be further subclassified as:
- Intragel hemorrhage
- Extravasation of blood into space lined anteriorly by an anterior hyaloid membrane, laterally by non-pigmented ciliary epithelium, and posteriorly by the posterior hyaloid membrane is known as intragel hemorrhage.
- Properties of intragel hemorrhage:
- Settle inferiorly,
- Clots easily,
- As the RBC degenerates, the color of the vitreous hemorrhage changes from bright red to yellow.
- Preretinal hemorrhage can be subdivided into 2 categories:
- Subhyaloid hemorrhage is located between the internal limiting membrane and posterior subhyaloid membrane.
- Boat-shaped configuration: If the posterior hyaloid is intact, subhyaloid hemorrhage is immobile. If the posterior hyaloid is detached, subhyaloid hemorrhage shifts with the eye movement. It is most commonly seen in patients with proliferative diabetic retinopathy.
- Sub-ILM hemorrhage is bleeding between the internal limiting membrane and the nerve fiber layer of the retina is known as sub-ILM hemorrhage. It is immobile. It also has a boat-shaped configuration, with the upper border being horizontal. Sub ILM hemorrhage is most commonly seen with Valsalva retinopathy, Terson syndrome, and Retinal microaneurysm.
- Subhyaloid hemorrhage is located between the internal limiting membrane and posterior subhyaloid membrane.
- If there is hemorrhage inside the Berger space, Cloquet canal, or canal of petit, it is also known as vitreous hemorrhage.[2]
Suprachoroidal hemorrhage occurs due to the rupture of long or short ciliary arteries into the suprachoroidal space between the choroid and sclera. It usually occurs intraoperatively and postoperatively, after trauma, and very rarely spontaneously. It can be subclassified as:
- Intraoperative - also known as expulsive suprachoroidal hemorrhage.
- Postoperative - also known as delayed suprachoroidal hemorrhage.
Retinal hemorrhages are important markers signifying local or systemic vascular abnormality, which needs to be thoroughly investigated. Retina hemorrhages can occur at the following locations:
- Flame-shaped hemorrhages are located in the nerve fiber layer.
- Dot and blot hemorrhages are located in the Outer plexiform layer - Inner nuclear layer (OPL-INL)complex.
- Subhyaloid hemorrhages are located between the internal limiting membrane and the posterior hyaloid membrane. It is boat-shaped in the configuration.
- Sub-RPE hemorrhage is located between the retinal pigment epithelium and Bruch membrane.
- Subretinal hemorrhage- It is located between the RPE(retinal pigment epithelium) and the photoreceptor layer.
Disc hemorrhage (also known as Drance hemorrhage)
- Linear hemorrhages which are perpendicular to the optic disc
- The most common location is at the superotemporal or inferotemporal margin.
Submacular hemorrhage needs a special mention as it is directly responsible for the quality of vision.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Etiology of Intraocular Hemorrhage
- Systemic Causes
- Diabetes Mellitus
- Hypertension
- Trauma
- Blood dyscrasias
- Bleeding and coagulation disorders
- Shaken baby syndrome
- Purtscher retinopathy
- Terson syndrome
- Head injury
- Valsalva maneuver
- Drug-induced (e.g., intravenous streptokinase injection and other anti-coagulants)
- Anemia
- Hemoglobinopathy (like sickle cell retinopathy and thalassemia)
- Ocular Causes
- Ocular trauma
- Proliferative diabetic retinopathy
- Arterial microaneurysm
- Retinal tear with rupture of bridging vessels
- Venous occlusive disease
- Severe ocular hypotony
- Any intraocular surgical intervention
- Hyphema - It can occur in traumatic injuries(blunt or penetrating),post-surgical, bleeding from iris neovascularization(most common cause is proliferative diabetic retinopathy, bleeding and coagulation disorders, herpetic keratouveitis (Candy-cane hypopyon) It can occur in the following conditions-
- Post-traumatic hyphema can occur after blunt or penetration trauma due to direct or indirect injury to the anterior segment vasculature.
- Post-surgical hyphema can occur after any intraocular surgical procedures.
- Spontaneous hyphema:
- Local causes: rubeosis iridis(as in proliferative diabetic retinopathy, ocular ischemia), iris tumors like iris melanoma, retinoblastoma, uveitis, juvenile xanthogranuloma, herpetic keratouveitis (candy-cane hypopyon).
- Systemic causes: leukemia, VWD(von Willebrand disease), hemophilia.
- Patient on antiplatelet and/or thrombolytic medications like aspirin, warfarin, etc.
- Vitreous hemorrhage can occur:
- Due to systemic or local predisposing conditions leading to retinal vascular wall changes and changes in the blood viscosity and composition.
- Due to local traction over the vascular sheath leading to breakthrough bleed.
- Due to retinal ischemia leading to the formation of neovessels, which are devoid of pericytes, they can easily rupture, leading to vitreous hemorrhage.
- In rare circumstances, vitreous hemorrhage can occur spontaneously.
- Causes of vitreous hemorrhage include the following:
- Trauma
- Proliferative diabetic retinopathy
- Retinal venous occlusion
- Vasculitis (like Eales disease)
- Retinopathy of prematurity
- Wet ARMD
- Idiopathic polypoidal choroidal vasculopathy
- Valsalva retinopathy
- Sickle cell retinopathy
- Ruptured retinal arterial microaneurysms
- As a complication of any intraocular surgery
- Secondary to systemic hematological disorders like Anemia, leukemia, thrombocytopenia, hemophilia, thalassemia, and other bleeding and coagulation disorders
- Shaken baby syndrome
- Secondary to intraocular tumor
- Terson's syndrome
- Suprachoroidal hemorrhage: Ocular predispositions for the development of suprachoroidal hemorrhage are the history of glaucoma, aphakia, preoperative raised intraocular pressure, history of previous ocular intervention or trauma. History of trauma, high myopia, prolonged, complicated ocular surgery, posterior capsule rent with vitreous loss,s sudden marked hypotony, excessive vomiting, and Valsalva-like maneuvers like coughing or sneezing intraoperatively or early postoperatively.
- Retinal hemorrhage and macular hemorrhage:[3] Hemorrhages in the retina occurs in pathological conditions like diabetic retinopathy, hypertensive retinopathy retinal vein occlusion, wet ARMD, IPCV(idiopathic polypoidal choroidal vasculopathy), macroaneurysm, Valsalva retinopathy, sickle cell retinopathy, Terson syndrome, Purtscher retinopathy, shaken baby syndrome, leukemic retinopathy, bacterial endocarditis
- Optic disc hemorrhage: The exact etiology for the development of disc hemorrhage is a matter of debate for decades. Disc hemorrhage is thought to occur secondary to mechanical stretching near the lamina cribrosa or due to ischemic microinfarction at the optic disc head due to glaucomatous or non-glaucomatouscauses.[4]
Epidemiology
Traumatic eye injury can cause shearing force over the normal blood vessels and can rupture them causing an ocular bleed. It can occur at any age and without any gender predisposition. However, traumatic injuries are more common in young males owing to more outdoor activities and heavy work. Traumatic injury is more common in children during the summer season owing to increased outdoor activities during summer vacations.
The incidence of traumatic hyphema is approximately 12 injuries per 100,000 population. Males are three to five times more affected than females.[5] The incidence of traumatic hyphema in children is around 70%, with 10-20 years of children being more affected.[5]
In a study by Butner et al., it was noted that in patients of vitreous hemorrhage, 34.1% of the cases were of diabetic retinopathy, 22.4% of patients were of a retinal break without retinal detachment, 14.9% cases were of rhegmatogenous retinal detachment, and 13% cases were of retinal vein occlusion[6]. Other causes accounting for the rest of 16% included posterior vitreous detachment, retinal vasculitis, sickle cell retinopathy, ARMD, tumor, retinopathy of prematurity, leukemias, acute retinal necrosis, HIV related retinopathy, and rarely patients of uveitis.[6]
The incidence and prevalence of intraocular hemorrhage are directly correlated with the incidence and prevalence of the predisposing factor, which leads to the bleeding.[7]
In a study by Obuchowska et al., it was discussed that the incidence of suprachoroidal hemorrhage after all ocular surgeries was 0.29%.[8]
A study by Al-hitq et al. showed that the incidence of submacular hemorrhage was 5.4 per million per annum. 52% of their patients had past history of Age-Related Macular Degeneration.
Shaken baby syndrome incidence is between 15 and 30 per 100 000 children under the age of 1 year.[9][10]
Pathophysiology
Three main pathophysiologic mechanisms of intraocular hemorrhage include
- Bleeding from normal vessels(secondary to trauma)
- Bleeding from abnormal vessels(associated with systemic hypertension and uveitis)
- Bleeding from new formed immature vessels (fibrovascular membranes and neoplasia)
Hyphema
- In blunt trauma, the coup-countercoup forces exert shearing stress on the blood vessels of the iris, ciliary body, and trabecular meshwork, resulting in rupture of the vessels and bleeding inside the anterior chamber.
- In penetrating trauma, direct injury to the ocular structures and their vasculature may lead to bleeding inside the eye.
- Post-operative /post-procedural hyphema- Performing any ocular surgery in the presence of inflammation and infection increases the probability of developing hyphema. Iatrogenic injury to the iris, trabecular meshwork, or ciliary body can lead to intraoperative or early post-operative bleed. An anterior chamber intraocular lens is known to cause chronic irritation of the iris and can increase the chance of bleeding inside the eye. Scleral fixated intraocular lens implantation can lead to injury of the ciliary body and the posterior surface of the iris and can lead to intraocular bleed. Iris claw lens can also acutely rupture iris vessels leading to intra-operative or early post-operative bleed. Procedures like Nd-YAG laser iridotomy or posterior capsulotomy can lead to hyphema.
- UGH, Syndrome- It was first described by a scientist named Ellingson in 1978. It is a triad of uveitis, glaucoma, and hyphema, seen in patients having intraocular lens implantation (most commonly anterior chamber IOL), in which there is continuous iris chafing because of the IOL which leads to chronic inflammation with iris transillumination defects, pigment dispersion, and hyphema obstructing the trabecular meshwork leading to glaucoma.
- In spontaneous hyphema cases, either the normal vessels become fragile secondary to some local or systemic causes, or there is bleeding from the neovessels.
Vitreous hemorrhage:[2] Bleeding inside the vitreous cavity behaves differently from the bleeding elsewhere in the body.
- Vitreous bleeding settles inferiorly.
- Vitreous bleeding clots rapidly.
- There is very poor initial polymorphonuclear lymphocyte response leading to a mild inflammatory reaction in the initial phase allowing the eye to limit inflammatory damage to the adnexal structures.
- According to a study by Sanders et al., blood from the vitreous cavity clears at the rate of 1% per day.[11]
- As the RBC degenerates, the color of the hemorrhage changes from red to yellow.
Suprachoroidal hemorrhage: Two hypotheses have been proposed for explaining the pathophysiology of suprachoroidal hemorrhage.
- One histopathologically proven hypothesis is that sudden hypotony leads to choroidal effusion, which is significant enough to stretch the walls of the long or short ciliary arteries leading to their rupture and bleeding into the suprachoroidal space.[12][13]
- Another hypothesis states that when sudden hypotony occurs, patients who already have damaged or weak posterior ciliary arteries have chances of rupture and bleeding into the suprachoroidal space.[14][15]
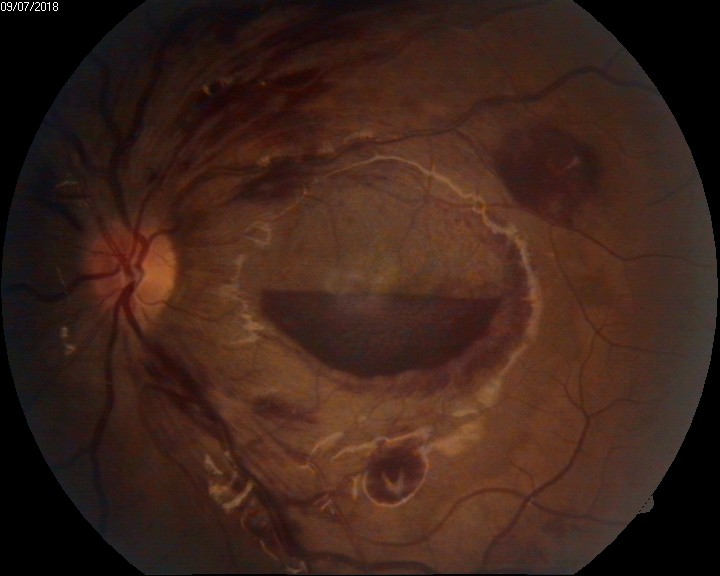
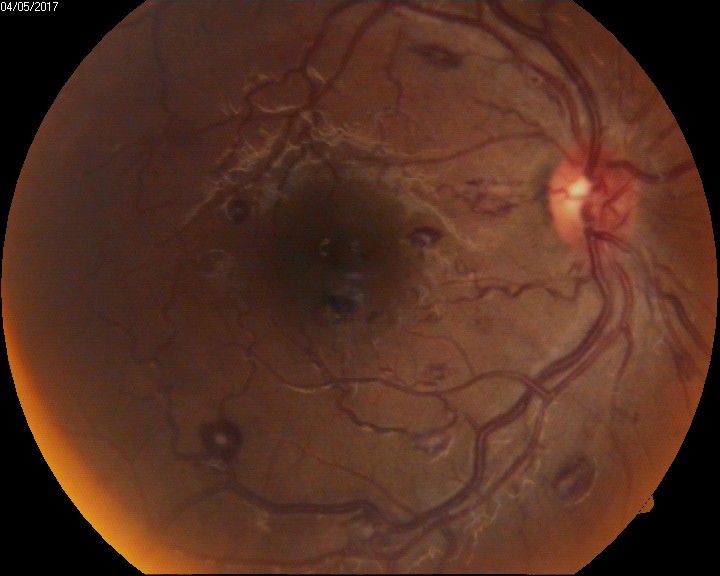
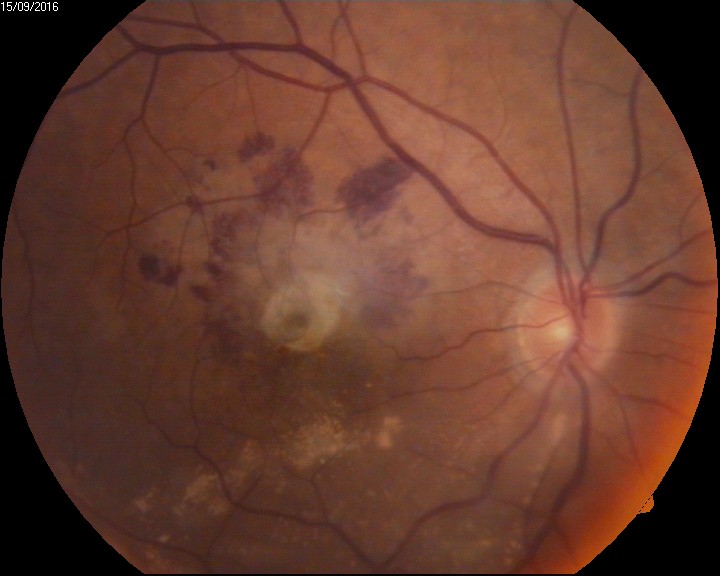
Retinal hemorrhages: Retinal hemorrhages are classified depending on the site of bleeding- Sub RPE hemorrhage(between the RPE and Bruch's membrane), Sub-retinal hemorrhage(Between the RPE and neurosensory retina), Intraretinal hemorrhage (Dot and Blot hemorrhages), and RNFL hemorrhage (Flame- shaped hemorrhage, Roth spots, splinter hemorrhages). See Image. Intraocular Hemorrhage, Roth Spots). Sub ILM hemorrhage is considered a part of the pre-retinal type of vitreous hemorrhage. Hemorrhages in the various layers of the retina are either due to some systemic predisposing factors or due to sudden shearing stress over the vessels and very rarely spontaneously.
Optic Disc hemorrhage:[16][17] The exact mechanism for the development of disc hemorrhage is debatable. Two hypotheses have been proposed-mechanical theory and vascular theory. Mechanical theory suggests that neurodegenerative changes secondary to stretching and remodeling of the connective tissue and/or stretching due to glial tissue formation lead to a shearing effect over the vessels over the disc leading to hemorrhage. The vascular theory proposes that ischemia secondary to infarction at the optic disc or disruption of the blood-retinal barrier leads to hemorrhage at the optic disc.[18] In the presence of Disc hemorrhage, glaucoma should always be ruled out. The presence of disc hemorrhage in a known case of glaucoma is considered a marker for the progression of the disease.
Shaken baby syndrome: It is a result of child abuse where the child is subjected to severe trauma leading to subdural and retinal hemorrhages.[19]
History and Physical
A detailed history of the origin, duration, and progress of the condition should be thoroughly investigated. Any associated history of traumatic injury, road traffic accident, physical assault, chest compression should be inquired.
Thorough systemic history regarding diabetes, hypertension, increased intracranial tension, metabolic disorders, bleeding and coagulation disorders, kidney and cardiac disorders should be inquired.
History of any medication intake should be inquired.
In patients of macular hemorrhage, additional findings like central scotoma, metamorphosis, micropsia, loss of color, and contrast sensitivity merit inquiry.
A thorough slit-lamp examination should be done to know the size, extent, location, and severity of the bleed, associated complications with it.
Hyphema can be classified into four grades depending on its height from the inferior limbus on slit-lamp examination.
- Grade 0-microhyphema (RBCs scattered in the anterior chamber)
- Grade I- hyphema involving less than 1/3rd of the anterior chamber
- Grade II-hyphema involving 1/3 to 1/2 of the anterior chamber
- Grade III-hyphema involving more than 1/2 but less than the total of the anterior chamber
- Grade IV- a total anterior chamber filled with fresh blood is considered as total hyphema.
Intraocular pressure should be repeatedly monitored.
Gonioscopy should be performed in hyphema, iris, and angle neovascularization patients and in patients of traumatic ocular injury to rule out angle recession.
Pupillary reflexes should be assessed by dilating the pupil.
Dilated fundus examination should be done to rule out the presence of vitreous hemorrhage and for detailed retinal examination to find out the cause of bleeding.
In conditions where visibility is hampered, B scan ultrasonography should be performed to rule out additional findings like a dislocated lens or intraocular lens, presence of the foreign body, vitreous hemorrhage, retinal detachment, optic nerve avulsion, choroidal detachment, and suprachoroidal hemorrhage.
In cases of traumatic injuries, a CT scan of the orbit should be advised to rule out any orbital fractures, presence of the intraocular foreign body, and rule out any associated brain injuries.
Ocular investigations include OCT(optical coherence tomography), OCT-A (optical coherence tomography angiography), FFA (fundus fluorescein angiography), ICG (indocyanine green angiography). Fundus photography should be done to locate the cause of vitreous hemorrhage, retinal hemorrhages, and macular hemorrhage.
In patients of macular hemorrhage, additional findings like scotoma, metamorphosis, micropsia should be inquired.
Gonioscopy should be performed in hyphema, iris, and angle neovascularization patients and in patients of trauma to rule out angle recession.
Suprachoroidal hemorrhage: It occurs mostly intraoperatively or postoperatively.
- In cataract surgery, it mostly occurs intraoperatively.[12]
- In glaucoma surgery, it mostly occurs postoperatively.[12]
- Symptoms of suprachoroidal hemorrhage include the following:
- Severe pain
- Nausea and vomiting
- Diminution of vision postoperatively.
- Signs suggestive of suprachoroidal hemorrhage include the following:
- Loss of the red reflex
- A sudden increase in intraocular pressure
- Anterior chamber swallowing
- Bulging posterior capsule
- Expulsion of intraocular content
- Iris prolapse from the wound along with other intraocular content
- Anterior expulsion of iris, lens, retina, choroid
- Choroidal elevation
Evaluation
Hyphema
- The diagnosis is made by slit-lamp examination.
- Open globe injury should be ruled out in traumatic hyphema cases. CT scan should be done in traumatic cases to rule out Orbital fracture and IOFB. USG should be done to rule out vitreous hemorrhage, IOFB, lens dislocation, and retinal detachment.
- Complete blood count, bleeding and coagulation disorders, hypertension, history of any anticoagulant medications should be ruled out in patients with spontaneous hyphema.
Vitreous Hemorrhage
- In patients with vitreous hemorrhage, systemic metabolic stability should be evaluated first.
- Most common causes of Vitreous hemorrhage like diabetes mellitus, hypertension, trauma, chest compression, raised intracranial tension, systemic autoimmune vasculitis, history of tuberculosis, bleeding or coagulation disorders, history of anticoagulant drugs intake should be investigated first.
- All routine blood tests like CBC, ESR, Hb, BT, CT, serum homocysteine, PT with INR, peripheral blood smear, Mantoux test, chest X-ray should be investigated.
- If Retina is hazily visible through the bleed, the cause of bleeding should be thoroughly investigated with the help of indirect ophthalmoscopy, 90D or 78D slit lamp guided biomicroscopic examination to look for precise posterior segment involvement.
- If any macular pathology like wet ARMD, IPCV, choroidal rupture at the macula, ruptured microaneurysm, retinal venous occlusion, diabetic macular edema is suspected on indirect ophthalmoscopic examination, optical coherence tomography (OCT) and optical coherence tomography angiography (OCT-A) should be done to find out the respective causes. It is especially useful to differentiate sub-ILM from the sub-hyaloid type of bleed.
- If the fundus is visible, fundus fluorescein angiography(FFA) should be done to determine the cause, extent, location of the retinal ischemic stimulus, and indocyanine green angiography (ICG) is necessary if any choroidal involvement is suspected.
- In cases where visibility of the retina is an issue, B-scan ultrasonography should be done for the diagnosis of Vitreous hemorrhage and to look for associated findings of retinal detachment, posterior vitreous detachment, lens drop, intraocular foreign body, traction at the posterior pole to differentiate sub-hyaloid hemorrhage (SHH) from intragel vitreous hemorrhage.
Suprachoroidal Hemorrhage
- It is a spot diagnosis clinically.
- In cases where distorted intraocular architecture does not allow posterior segment evaluation, B- scan ultrasonography helps in the diagnosis of SCH.
- It can also help localize the site and extent of bleed so that we could plan for surgical drainage based on USG guidance.
- It also helps to rule out associated retinal detachment or vitreous hemorrhage.
- It is also useful for post-surgical follow-up of the patients to assess the condition of the eye.
- A-scan shows a typical high reflective spike at the wall of the SCH followed by an area of lower reflective spike indicating clotted blood in the suprachoroidal space.
- CT scan and MRI also helps to demarcate the site and extent of bleed.
- Fundus fluorescein angiography(FFA), Indocyanine green angiography(ICG), Optical coherence angiography (OCT) are helpful to rule out the causes of loss of vision but can only be useful if proper examination of the posterior segment is visible.
Retinal hemorrhages, Disc hemorrhages, and macular hemorrhages are spot clinical diagnosis on indirect ophthalmoscopic examination and 78D or 90D slit-lamp biomicroscopic examination. To know the site, extent, and location of the hemorrhages, an OCT 5 line raster scan along the hemorrhage is very helpful. OCT and OCT-A might provide additional information regarding the location of the hemorrhage, associated signs of edema, subretinal fluid, any tractional component, and the integrity of the outer and inner retinal layers. FFA is useful to look for any ischemic areas, differentiate hemorrhages from neovascularization, and plan for FFA guided photocoagulation if required. ICG is useful to get precise information regarding choroidal pathology as choroidal circulation is very nicely differentiated on ICG angiography.
Treatment / Management
In cases of traumatic injury, systemic condition, and cardiorespiratory status should be stabilized first. Once the systemic stability is ensured, then a proper ocular examination should be carried out.
Patients of hyphema and vitreous hemorrhage are advised sitting or semireclined position.
Patients are advised to elevate the head end of the bed to a minimum of 30 degrees while sleeping so that blood settles inferiorly, allowing clearing of the visual axis.
Avoid coughing, sneezing, vomiting, and heavy weight lifting.
The patient should be advised to rest the eye, avoid excessive activities like reading, playing, watching TV, and computer works. Preferably eye patch should be applied to rest the eye.
The strict control of systemic predisposing factors like diabetes mellitus, hypertension, cardiovascular instability should be done.
Topical anesthetic agents like proparacaine can be instilled if the patient is not allowing proper eye examination.[20](A1)
Intraocular pressure should be regularly monitored, and if required, topical agents like beta-blockers (timolol maleate), alpha agonist (brimonidine tartrate), carbonic anhydrase inhibitors (dorzolamide, brinzolamide), prostaglandin analogs (latanoprost, travoprost, bimatoprost). Systemic agents like intravenous mannitol or oral glycerol or acetazolamide could be used in conditions of acute uncontrolled glaucoma.
Once the glaucoma is ruled out, cycloplegic agents like cyclopentolate or homatropine eye drops could be added for pain control, to allow reabsorption of blood, and to avoid pupillary play, which could stretch the injured blood vessels, thereby increasing the probability of rebleeding.[21][22](B3)
Topical steroid therapy with prednisolone acetate 1%or dexamethasone sodium phosphate 0.1% helps to reduce the inflammation and allows faster clearing of the blood and stabilizing the inflamed vessels to avoid the risk of rebleeding.[23](A1)
If the hyphema is responding to the medical line of management, surgical intervention could be avoided, and the hyphema could resolve completely with the medical line of management in most of the cases.
In conditions like corneal staining, penetrating corneal injury, and in cases of raised intraocular pressure not responding to medical line of management, surgical intervention with anterior chamber lavage and management of other associated complications should be carried out.[24] Sickle cell anemia patients require extra precaution as there might be a drastic increase in intraocular pressure due to the sickling of RBCs, obstructing the trabecular meshwork. If for more than 24 hours, the intraocular pressure is more than 25 mmHg, or the IOP is fluctuating to more than 30 mmHg for 2 to 4 days, surgical interventions should be considered.[25]
In patients with vitreous hemorrhage where the fundus is not visible, B- scan ultrasonography should be carried out to look for associated findings of retinal detachment. If the retina is attached, vitreous hemorrhage could be treated with the medical line of management and reviewed every 1 to 2 weeks.
If the patient shows signs of improving vitreous hemorrhage, the patient could be managed conservatively with the medical line of management. In patients of proliferative retinopathies with ischemic retina, if the retina is even sparsely visible, laser photocoagulation should be initiated to limit the ischemic stimulus. Laser photocoagulation converts the ischemic retina to an anoxic retina, reducing the anti-VEGF load, reducing the chances of rebleeding. If the patient does not show signs of improvement, the patient should be considered for intravitreal Anti-VEGF medication.
Doses of commonly used intravitreal injections:[26]
- Bevacizumab- 1.2 5mg/0.05 ml
- Ranibizumab - 0.5 mg or 0.3 mg /0.05 ml
- Aflibercept -2 mg/0.05 ml
- Pegaptanib- 0.3 mg/0.9 ml [only FDA approved for neovascular age related macular degeneration]
If the hemorrhage does not resolve with the above management, pars plana vitrectomy should be considered. Indication of Vitrectomy in diabetic patients include:[26]
- Non-clearing vitreous hemorrhage (vitreous/subhyaloid/pre-macular)
- Tractional retinal detachment with macula off
- Combined tractional with rhegmatogenous retinal detachment
- Anterior segment neovascularization with the invisibility of the posterior segment
- Ghost cell glaucoma
- Thick epiretinal membrane
- Vitreomacular traction
Many surgeons nowadays inject intravitreal anti-VEGF medication in non-resolving vitreous hemorrhage 7 days prior for pars plana vitrectomy surgery.[27] (B2)
In vitrectomy surgery, three pars plana ports are made, one for the infusion line, one for the light pipe, and the third for the microinstruments. A fourth port can be made if a chandelier light source is used in cases of bimanual vitrectomy.[28]Core vitrectomy with guarded PVD (posterior vitreous detachment) induction is done. The peripheral vitreous is shaved. All bleeders are cauterized. All tractional components are removed. Fluid- Air exchange is done. If required, ERM peeling with or without ILM (internal limiting membrane) peeling is done with due precautions. Endophotocoagulation of the nonperfused areas is done, sparing the macula. At the end of the surgery, tamponade in the form of air, fluid, gases (C3F8, SF6), or silicone oil, can be injected.
Cryotherapy can be done to ablate the peripheral ischemic retina. Cryotherapy is thought to disrupt the blood-retinal barrier, induce inflammation, and thereby help remove RBCs.Because of the intense inflammation generation because of cryotherapy, it is not considered the first-line treatment for ablation of ischemic areas. It is indicated only in conditions of nonvisibility of fundus and to prevent port site neovascularisation.[29]
Diathermy procedure is obsolete nowadays because of its side effects and difficult maneuvering.
In cases of rubeosis iridis, pan-retinal photocoagulation with or without anti-VEGF medication might be helpful in the early phases. In the advanced stages, the new vessels lead to fibrosis and contracture, leading to permanent distortion of the angles leading to refractory glaucoma, which might eventually require a glaucoma drainage implant). The prognosis is, however, poor in such cases. Painful blind eyes may need diode laser cyclophotocoagulation or cyclocryotherapy.[26]
Isolated retinal hemorrhage not involving the macula can be observed, and systemic predisposing factors must be stabilized. Disc hemorrhages per se don't cause vision loss. Glaucoma should be ruled out, and if present appropriate antiglaucoma treatment should be started. Systemic metabolic dysfunction should also be corrected if disc hemorrhages are due to nonglaucomatous causes.
Roth spots are usually asymptomatic and resolve spontaneously with the management of the underlying condition.
Subhyaloid hemorrhage could be observed if not involving the macular area. Subhyaloid hemorrhage involving the macular area may lead to a drastic loss of vision. It could be treated with Q switched 1064 nm Nd: YAG laser (energy 2.1 to 11.5 mJ) focused at the anterior border of subhyaloid hemorrhage at the lower margin inferior and away from the fovea.[30] Care should be taken not to injure the retinal vessels and cause iatrogenic injury to the retina. It allows the blood to flow into the vitreous cavity-causing improvement in the visual acuity but causing an increase in the number of floaters.[31](B3)
Suprachoroidal hemorrhage is an ophthalmic emergency, and prompt diagnosis and urgent management on the operating table are key to saving acute expulsion of the ocular contents. If SCH is detected on the table(intraoperative SCH), immediate closure of all the wound sites should be done, and tight sutures should be taken at all entry wounds. If the expulsion continues despite the attempt to close the wound site, posterior sclerotomies or 25 gauge trocar assisted transconjunctival suprachoroidal hemorrhage drainage should be attempted to soften the eye.[12] Once the expulsion is under control, the anterior chamber should be formed by air or viscoelastic substances. Postoperatively IOP should be controlled by topical or systemic antiglaucoma drugs. Steroid drops could be given to decrease the inflammation. Cycloplegic agents could be given to decrease the pain and spasm. Oral or systemic analgesic medication should be given for pain control(avoid antiplatelet agents like NSAIDS and Aspirin to prevent further bleeding).[32](B3)
The Conservative line of management of postoperative SCH is the same as described above. If the IOP is continuously higher, causing severe ocular pain, surgical intervention might be required. Point of highest SCH is assessed, conjunctival peritomy is done at that quadrant, and scleral cut of around 3 to 4 mm is done radially until efflux of SCh occurs. If needed, slight pressure could be applied at the edge of the cut to expel out the blood from the suprachoroidal space.[33] Intraoperative anterior chamber collapse should be avoided with the help of air, gas, liquid, or viscoelastic substances. If there's an associated finding of retinal detachment, vitreous hemorrhage, or any other visually threatening posterior segment pathology requiring intervention, after SCH is drained, pars plana vitrectomy should be done. Adequate precaution should be taken to keep the infusion cannula inside the vitreous cavity and not in the suprachoroidal space to avoid choroidal effusion.[12] (B3)
Macular hemorrhage is a sight-threatening situation requiring timely intervention as long-standing heme accumulation at the macula could lead to photoreceptor degeneration leading to permanent visual loss.[34] If the cause of the macular bleed is due to the involvement of retinal circulation, anti-VEGF medications like bevacizumab (non-FDA approved) and ranibizumab (FDA approved) could be used. In pathologies involving the choroidal circulation, Intravitreal Aflibercept has shown to have a favorable response. A steroid implant could be considered in cases of refractory macular edema and hemorrhage not responding adequately to anti-VEGF medications. Even photodynamic therapy (PDT) could be used in patients not responding to anti-VEGF medication.[32] Pneumatic displacement alone or combined with intravitreal tissue plasminogen activator injection could be attempted to clear the macula of the bleed with a face-down position for some days to clear out the blood.[35] Macular translocation surgery could be attempted in patients with macular hemorrhage but does not show promising results.[36](B2)
Differential Diagnosis
Differential Diagnosis of Hyphema[37]
- Traumatic hyphema
- Hyphema secondary to intraocular procedures[38]
- Herpetic keratouveitis (blood-stained hypopyon) - secondary to HSV and VZV virus.
- UGH (uveitis glaucoma hyphema) syndrome[39]
- Uveitis
- Fuch's heterochromic iridocyclitis (Amsler sign - hemorrhage in the aspirated fluid from vitreous tapping or anterior chamber paracentesis due to iris atrophy. It is supposed to be due to rupture of fragile iris vasculature because of iris atrophy)
- Juvenile xanthogranuloma[40]
- Hyphema due to neovascularization of iris and angle secondary to severe retinal ischemia secondary to proliferative diabetic retinopathy, CRVO, Ocular ischemic syndrome.[41]
Differential Diagnosis of Vitreous Hemorrhage
- Vitritis (associated findings should be ruled out like keratic precipitates, anterior chamber reaction, posterior synechiae, festooned pupil, complicated cataract. The fundus should be screened to rule out chorioretinitis, retinitis, choroiditis, vasculitis, exudative retinal detachment)
- Primary intraocular lymphoma (Masquerade Syndrome)
- Endophytic (retinoblastoma in children)
- Asteroid Hyalosis(not visually depriving)
- Retinal detachment(It has to be differentiated from posterior hyaloid detachment associated with vitreous hemorrhage on B scan ultrasonography).[42]
Differential Diagnosis of Retinal Hemorrhages
- Retinal hemorrhages seen in children could be due to vacuum or forceps delivery, birth trauma, Secondary to Shaken baby syndrome, Coats' disease, PHPV(Persistent hyperplastic primary vitreous), Retinopathy of Prematurity, Hematological disorders like severe anemia, sickle cell retinopathy.[43]
- Retinal hemorrhages in adults could be due to trauma, Diabetic retinopathy, Hypertensive retinopathy, Retinal venous occlusion, Ocular ischemic syndrome, sickle cell retinopathy, juxtafoveal telangiectasia, retinal microaneurysm, raised intracranial tension, bleeding or coagulation disorders, Valsalva retinopathy.[43]
- If Roth spots are seen, thorough systemic evaluation to rule out the cause for the same should be done.[44]
Differential Diagnosis of Disc Hemorrhages[45]
Linear hemorrhages along the disc margin should be differentiated from other causes of hemorrhages apart from glaucoma, which includes the following:
- Posterior vitreous detachment from disc area[46]
- ischemic optic neuropathies
- Grade IV hypertensive retinopathy (see Image. Intraocular Hemorrhage, Disc Hemorrhage in Grade 4 Papilloedema)
- Diabetic papillopathy
- Systemic hypertension
- Leukemia
- SLE
- Vascular anomaly at the disc
Differential Diagnosis of Suprachoroidal Hemorrhage[47][48][49][48]
- Retrobulbar hemorrhage
- Choroidal effusion (Serous type)
- Hemorrhagic choroidal detachment
- Acute angle-closure glaucoma
- Choroidal hemangioma
- Posterior scleritis
Differential Diagnosis of Macular Hemorrhage[50]
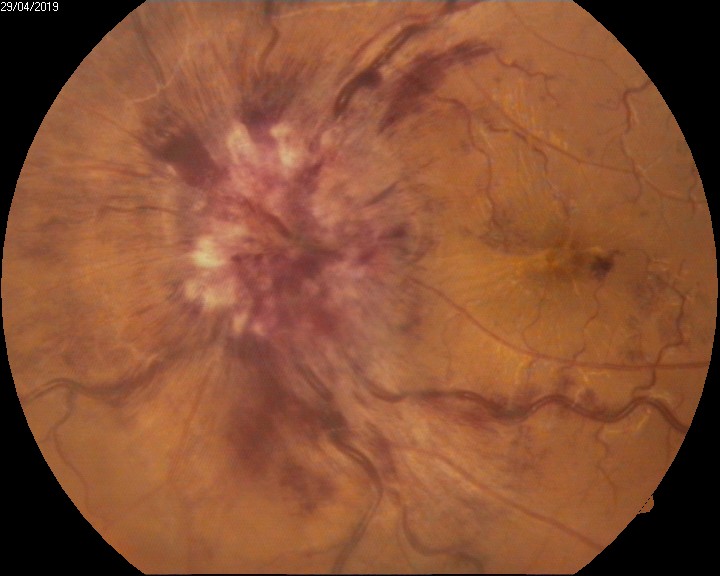
- Choroidal neovascularisation secondary to age-related Macular Degeneration (see Image. Intraocular Hemorrhage, Hemorrhagic Choroidal Neovascular Membrane)
- Type I(under the RPE),
- type II (under the neurosensory retina),
- type III (RAP lesion)
- High myopia
- Idiopathic polypoidal choroidal vasculopathy[34]
- Choroidal rupture secondary to trauma[32]
- Angiod streaks
- Presumed ocular histoplasmosis (POHS)
- Chorioretinitis or retinochoroiditis
- Secondary to laser photocoagulation
- Secondary to intraocular foreign body
- Ruptured retinal artery microaneurysm
- Valsalva retinopathy
- Anemic retinopathy
- Leukemic retinopathy
- Secondary to an intraocular tumor (primary or metastatic)
- Secondary to diabetic retinopathy and retinal venous occlusion
- Secondary to bleeding and coagulation disorders.
Prognosis
The prognosis of various types of an intraocular hemorrhage depends on the location of bleed, amount of bleed, the severity of the condition, rate of clearing of blood, whether the blood is obscuring the visual acuity or not, associated complications along with the hemorrhage (corneal staining, tractional retinal detachment, pre retinal fibrosis, ischemic optic atrophy, neovascular glaucoma)and severity of involvement of the macular region.[51]
Hyphema, if not associated with other vision-threatening complications, is having a relatively good prognosis. More the grade of Hyphema, the higher the chances of glaucoma and the less chance of recovery to normal vision.[37]
According to a study by Sanders et al., blood from the vitreous cavity clears at the rate of 1% per day.[11]
Prognosis of vitreous hemorrhage due to posterior vitreous detachment is good.[2]
Prognosis of vitreous hemorrhage or hyphema due to trauma depends on the associated complications associated with the traumatic insult. Choroidal rupture at the macular region is associated with a bad prognosis.
Terson syndrome, anemic retinopathy, Valsalva retinopathy have a relatively good prognosis (see Image. Intraocular Hemorrhage, Anemic Retinopathy).[52]
Hemorrhage due to wet ARMD, PCV has a relatively bad prognosis as chronic heme collection at the macula leads to damage of the photoreceptor layer and scarring. [53]
Prognosis of retinal vein occlusion depends on the severity of the ischemic stimulus. Ischemic CRVO has the worst prognosis of all types of RVO. The prognosis of Nonischemic BRVO is the best. If retinal vein occlusion is associated with macular ischemia, the prognosis is bad.[54]
Prognosis of retinal hemorrhage secondary to systemic metabolic dysfunction or retinal venous occlusions depends on the degree of resolution of the underlying pathologic condition.[55]
Traumatic retinal hemorrhages usually have a good prognosis if not associated with choroidal rupture involving the macular area.[55]
Subhyaloid hemorrhage if treated early with Nd: YAG hyaloidotomy is associated with a better prognosis.[56]
Prognosis of suprachoroidal hemorrhage, either intraoperative or postoperative, is guarded and depends on preoperative visual acuity and occurrence of retinal detachment along with the suprachoroidal hemorrhage.[49]
Disc hemorrhage is associated with the possibility of glaucoma, and the prognosis of the condition depends on the progression and severity of glaucoma.[57]
Complications
Complications of Hyphema
- Increase in intraocular pressure[58]
- Chances of rebleeding[59]
- Corneal staining[37]
- In patients of sickle cell anemia, the hypoxic environment inside the anterior chamber increases the chances of sickling, leading to blockage of trabecular meshwork by the sickled RBCs leading to further aggravation of the condition.[60]
Complications of Vitreous Hemorrhage
- Epiretinal membrane formation
- Pigmentary retinopathy
- Pre-retinal fibrosis
- Strabismus (long-standing hemorrhage involving the visual axis)
- Occlusion amblyopia (if the hemorrhage is obstructing the visual axis at an early age)
- Tractional retinal detachment
- Secondary rhegmatogenous retinal detachment.
- Neovascular glaucoma
Complications of Suprachoroidal Hemorrhage
Suprachoroidal hemorrhage is one of the most dreadful complications of any intraocular surgery. Once it occurs, visual recovery is very difficult despite all measures.
Complications of Macular Hemorrhage [43]
- Photoreceptor cell damage
- Submacular fibrosis
- Retinal neovascularization(secondary to ischemia)
- Vitreoretinal fibrovascular proliferation.
- Chronic heme collection can lead to macular scarring leading to a drastic loss of vision.
Deterrence and Patient Education
Any patient presenting with an intraocular hemorrhage for the first time should always undergo detailed ocular screening to find out the root cause of the bleed. The patient should be counseled properly regarding the prognosis of intraocular hemorrhage depending on the location of the bleed. Early diagnosis, management, and follow-up visits at a timely interval depending on the location of bleed and severity of presentation are mandatory. Systemic control of diabetes, hypertension, bleeding disorders, coagulation disorders is necessary to avoid irreversible worsening of intraocular hemorrhage. Subconjunctival hemorrhage and periorbital ecchymosis have a good prognosis with almost full recovery on their own.
Cold compression might help to hasten the recovery. In the presence of hyphema or vitreous hemorrhage, sitting or semi recline position might help keep the blood inferiorly, thereby not obstructing the visual axis causing relatively less discomfort in visual acuity. Lifestyle modification should be done along with proper systemic and topical medications to halt the progression of any bleeding or coagulation disorders. Heavy weight lifting, excessive coughing, sneezing, Valsalva maneuvering, excessive stress, lack of sleep should be avoided in the presence of hemorrhage at any location in the eye. If the intraocular hemorrhage, which is hampering visual acuity, does not clear with medical management, surgical intervention becomes mandatory.
Enhancing Healthcare Team Outcomes
Any patient presenting with intraocular hemorrhage should not only receive treatment solely by an ophthalmologist in isolation, but it also requires a multispecialty evaluation by an endocrinologist, nephrologist, cardiologist, and neurologist. Interprofessional communication can lead to better patient management. The patient will most often present to the primary health care provider or nurse practitioner, and these professionals should be aware of the condition as it is treatable. Prompt referral to an ophthalmologist is necessary.
These patients can then be followed by their primary clinicians and should ensure correct dosing on the medication management aspect of the condition. Nursing will be the first department to come in contact with the patients on follow-up and can assess the treatment progress as well as evaluate the compliance with both medication and lifestyle measures, and report any issue to the primary care physician. This collaborative, interprofessional team approach to care can ensure optimal patient outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Sankar PS, Chen TC, Grosskreutz CL, Pasquale LR. Traumatic hyphema. International ophthalmology clinics. 2002 Summer:42(3):57-68 [PubMed PMID: 12131583]
Spraul CW, Grossniklaus HE. Vitreous Hemorrhage. Survey of ophthalmology. 1997 Jul-Aug:42(1):3-39 [PubMed PMID: 9265701]
Level 3 (low-level) evidenceKaur B, Taylor D. Retinal haemorrhages. Archives of disease in childhood. 1990 Dec:65(12):1369-72 [PubMed PMID: 2103739]
Quigley HA,Addicks EM,Green WR,Maumenee AE, Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Archives of ophthalmology (Chicago, Ill. : 1960). 1981 Apr; [PubMed PMID: 6164357]
Level 3 (low-level) evidenceKennedy RH, Brubaker RF. Traumatic hyphema in a defined population. American journal of ophthalmology. 1988 Aug 15:106(2):123-30 [PubMed PMID: 3400754]
Butner RW, McPherson AR. Spontaneous vitreous hemorrhage. Annals of ophthalmology. 1982 Mar:14(3):268-70 [PubMed PMID: 7092037]
Winslow RL, Taylor BC. Spontaneous vitreous hemorrhage: etiology and management. Southern medical journal. 1980 Nov:73(11):1450-2 [PubMed PMID: 7444506]
Level 2 (mid-level) evidenceObuchowska I, Mariak Z. Risk factors of massive suprachoroidal hemorrhage during extracapsular cataract extraction surgery. European journal of ophthalmology. 2005 Nov-Dec:15(6):712-7 [PubMed PMID: 16329055]
Level 2 (mid-level) evidenceBarlow KM, Minns RA. Annual incidence of shaken impact syndrome in young children. Lancet (London, England). 2000 Nov 4:356(9241):1571-2 [PubMed PMID: 11075773]
Level 3 (low-level) evidenceKelly P, Farrant B. Shaken baby syndrome in New Zealand, 2000-2002. Journal of paediatrics and child health. 2008 Mar:44(3):99-107 [PubMed PMID: 18086144]
Level 2 (mid-level) evidenceSanders D, Peyman GA, Fishman G, Vlchek J, Korey M. The toxicity of intravitreal whole blood and hemoglobin. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. Albrecht von Graefe's archive for clinical and experimental ophthalmology. 1975 Dec 4:197(3):255-67 [PubMed PMID: 813541]
Level 3 (low-level) evidenceChu TG, Green RL. Suprachoroidal hemorrhage. Survey of ophthalmology. 1999 May-Jun:43(6):471-86 [PubMed PMID: 10416790]
Level 3 (low-level) evidenceObuchowska I, Mariak Z, Stankiewicz A. [The evaluation of incidence of massive suprachoroidal hemorrhage n the material of the Department of Ophthalmology, Medical Academy in Bialystok from 1990 to 2000]. Klinika oczna. 2002:104(2):93-5 [PubMed PMID: 12174463]
Level 2 (mid-level) evidenceObuchowska I, Mariak Z. [A new approach towards pathogenesis and treatment of massive suprachoroidal hemorrhage]. Klinika oczna. 2002:104(2):138-42 [PubMed PMID: 12174457]
Scott IU, Flynn HW Jr, Schiffman J, Smiddy WE, Murray TG, Ehlies F. Visual acuity outcomes among patients with appositional suprachoroidal hemorrhage. Ophthalmology. 1997 Dec:104(12):2039-46 [PubMed PMID: 9400763]
Level 2 (mid-level) evidenceUhler TA, Piltz-Seymour J. Optic disc hemorrhages in glaucoma and ocular hypertension: implications and recommendations. Current opinion in ophthalmology. 2008 Mar:19(2):89-94. doi: 10.1097/ICU.0b013e3282f3e6bc. Epub [PubMed PMID: 18301280]
Level 3 (low-level) evidenceSeol BR, Jeoung JW, Park KH. Ocular and systemic risk factors associated with recurrent disc hemorrhage in primary open-angle glaucoma. PloS one. 2019:14(9):e0222166. doi: 10.1371/journal.pone.0222166. Epub 2019 Sep 16 [PubMed PMID: 31525246]
Park HL, Kim JW, Park CK. Choroidal Microvasculature Dropout Is Associated with Progressive Retinal Nerve Fiber Layer Thinning in Glaucoma with Disc Hemorrhage. Ophthalmology. 2018 Jul:125(7):1003-1013. doi: 10.1016/j.ophtha.2018.01.016. Epub 2018 Mar 2 [PubMed PMID: 29486903]
Blumenthal I. Shaken baby syndrome. Postgraduate medical journal. 2002 Dec:78(926):732-5 [PubMed PMID: 12509690]
Gharaibeh A, Savage HI, Scherer RW, Goldberg MF, Lindsley K. Medical interventions for traumatic hyphema. The Cochrane database of systematic reviews. 2013 Dec 3:12(12):CD005431. doi: 10.1002/14651858.CD005431.pub3. Epub 2013 Dec 3 [PubMed PMID: 24302299]
Level 1 (high-level) evidenceWalton W, Von Hagen S, Grigorian R, Zarbin M. Management of traumatic hyphema. Survey of ophthalmology. 2002 Jul-Aug:47(4):297-334 [PubMed PMID: 12161209]
Level 3 (low-level) evidenceGragg J, Blair K, Baker MB. Hyphema. StatPearls. 2024 Jan:(): [PubMed PMID: 29939579]
Farber MD, Fiscella R, Goldberg MF. Aminocaproic acid versus prednisone for the treatment of traumatic hyphema. A randomized clinical trial. Ophthalmology. 1991 Mar:98(3):279-86 [PubMed PMID: 2023746]
Level 1 (high-level) evidenceWeiss JS, Parrish RK, Anderson DR. Surgical therapy of traumatic hyphema. Ophthalmic surgery. 1983 Apr:14(4):343-5 [PubMed PMID: 6866433]
Deutsch TA, Weinreb RN, Goldberg MF. Indications for surgical management of hyphema in patients with sickle cell trait. Archives of ophthalmology (Chicago, Ill. : 1960). 1984 Apr:102(4):566-9 [PubMed PMID: 6704013]
Shukla UV, Tripathy K. Diabetic Retinopathy. StatPearls. 2024 Jan:(): [PubMed PMID: 32809640]
Faisal SM, Tahir MA, Cheema AM, Anjum MI. Pars plana vitrectomy in vitreous hemorrhage with or without Intravitreal Bevacizumab a comparative overview. Pakistan journal of medical sciences. 2018 Jan-Feb:34(1):221-225. doi: 10.12669/pjms.341.12683. Epub [PubMed PMID: 29643911]
Level 2 (mid-level) evidenceWang ZY, Zhao KK, Li JK, Rossmiller B, Zhao PQ. Four-port bimanual 23-gauge vitrectomy for diabetic tractional retinal detachment. Acta ophthalmologica. 2016 Jun:94(4):365-72. doi: 10.1111/aos.12951. Epub 2016 Feb 8 [PubMed PMID: 26855122]
Ross WH, Gottner MJ. Peripheral retinal cryopexy for subtotal vitreous hemorrhage. American journal of ophthalmology. 1988 Apr 15:105(4):377-82 [PubMed PMID: 3358430]
Raymond LA. Neodymium:YAG laser treatment for hemorrhages under the internal limiting membrane and posterior hyaloid face in the macula. Ophthalmology. 1995 Mar:102(3):406-11 [PubMed PMID: 7891977]
Level 3 (low-level) evidenceKhadka D, Bhandari S, Bajimaya S, Thapa R, Paudyal G, Pradhan E. Nd:YAG laser hyaloidotomy in the management of Premacular Subhyaloid Hemorrhage. BMC ophthalmology. 2016 Apr 18:16():41. doi: 10.1186/s12886-016-0218-0. Epub 2016 Apr 18 [PubMed PMID: 27090882]
Casini G, Loiudice P, Menchini M, Sartini F, De Cillà S, Figus M, Nardi M. Traumatic submacular hemorrhage: available treatment options and synthesis of the literature. International journal of retina and vitreous. 2019:5():48. doi: 10.1186/s40942-019-0200-0. Epub 2019 Dec 11 [PubMed PMID: 31890278]
Rezende FA, Kickinger MC, Li G, Prado RF, Regis LG. Transconjunctival drainage of serous and hemorrhagic choroidal detachment. Retina (Philadelphia, Pa.). 2012 Feb:32(2):242-9. doi: 10.1097/IAE.0b013e31821c4087. Epub [PubMed PMID: 22127221]
Anantharaman G, Sheth J, Bhende M, Narayanan R, Natarajan S, Rajendran A, Manayath G, Sen P, Biswas R, Banker A, Gupta C. Polypoidal choroidal vasculopathy: Pearls in diagnosis and management. Indian journal of ophthalmology. 2018 Jul:66(7):896-908. doi: 10.4103/ijo.IJO_1136_17. Epub [PubMed PMID: 29941728]
Ohji M, Saito Y, Hayashi A, Lewis JM, Tano Y. Pneumatic displacement of subretinal hemorrhage without tissue plasminogen activator. Archives of ophthalmology (Chicago, Ill. : 1960). 1998 Oct:116(10):1326-32 [PubMed PMID: 9790631]
Level 3 (low-level) evidenceChen FK, Patel PJ, Uppal GS, Tufail A, Coffey PJ, Da Cruz L. Long-term outcomes following full macular translocation surgery in neovascular age-related macular degeneration. The British journal of ophthalmology. 2010 Oct:94(10):1337-43. doi: 10.1136/bjo.2009.172593. Epub 2010 May 21 [PubMed PMID: 20494910]
Level 2 (mid-level) evidenceShiuey Y, Lucarelli MJ. Traumatic hyphema: outcomes of outpatient management. Ophthalmology. 1998 May:105(5):851-5 [PubMed PMID: 9593386]
Level 2 (mid-level) evidenceKaplowitz K, Nobe M, Abazari A, Honkanen R. Trabeculectomy for traumatic hyphema in sickle cell trait. Seminars in ophthalmology. 2015 Jul:30(4):297-304. doi: 10.3109/08820538.2013.847108. Epub 2013 Nov 19 [PubMed PMID: 24251431]
Level 3 (low-level) evidenceZemba M, Camburu G. Uveitis-Glaucoma-Hyphaema Syndrome. General review. Romanian journal of ophthalmology. 2017 Jan-Mar:61(1):11-17 [PubMed PMID: 29450365]
Pantalon A,Ștefănache T,Danciu M,Zurac S,Chiseliță D, Iris juvenile xanthogranuloma in an infant - spontaneous hyphema and secondary glaucoma. Romanian journal of ophthalmology. 2017 Jul-Sep; [PubMed PMID: 29450403]
Adeoti C, Isawumi M, Ashaye A, Olomola B. The anterior segment of the eye in diabetes. Clinical ophthalmology (Auckland, N.Z.). 2012:6():667-71. doi: 10.2147/OPTH.S27313. Epub 2012 May 7 [PubMed PMID: 22654491]
Witmer MT, Cohen SM. Oral anticoagulation and the risk of vitreous hemorrhage and retinal tears in eyes with acute posterior vitreous detachment. Retina (Philadelphia, Pa.). 2013 Mar:33(3):621-6. doi: 10.1097/IAE.0b013e3182671006. Epub [PubMed PMID: 23108264]
Level 2 (mid-level) evidenceKanukollu VM, Ahmad SS. Retinal Hemorrhage. StatPearls. 2024 Jan:(): [PubMed PMID: 32809612]
Ruddy SM,Bergstrom R,Tivakaran VS, Roth Spots 2020 Jan; [PubMed PMID: 29494053]
Koh YY, Lai CC, Chen HSL, Yeung L, Ku WC, Chuang LH. Optic disc hemorrhage in nonglaucomatous eyes: A cross-sectional study with average 8-year follow-up. PloS one. 2020:15(8):e0237796. doi: 10.1371/journal.pone.0237796. Epub 2020 Aug 17 [PubMed PMID: 32804983]
Level 2 (mid-level) evidenceRoberts TV, Gregory-Roberts JC. Optic disc haemorrhages in posterior vitreous detachment. Australian and New Zealand journal of ophthalmology. 1991 Feb:19(1):61-3 [PubMed PMID: 2039627]
Level 3 (low-level) evidenceWirostko WJ, Han DP, Mieler WF, Pulido JS, Connor TB Jr, Kuhn E. Suprachoroidal hemorrhage: outcome of surgical management according to hemorrhage severity. Ophthalmology. 1998 Dec:105(12):2271-5 [PubMed PMID: 9855159]
Level 2 (mid-level) evidenceLaube T, Brockmann C, Bornfeld N. Massive suprachoroidal hemorrhage: Surgical management and outcome. GMS ophthalmology cases. 2015:5():Doc10. doi: 10.3205/oc000032. Epub 2015 Oct 23 [PubMed PMID: 27625954]
Level 3 (low-level) evidenceWang LC, Yang CM, Yang CH, Huang JS, Ho TC, Lin CP, Chen MS. Clinical characteristics and visual outcome of non-traumatic suprachoroidal haemorrhage in Taiwan. Acta ophthalmologica. 2008 Dec:86(8):908-12. doi: 10.1111/j.1755-3768.2008.01266.x. Epub 2008 Jul 8 [PubMed PMID: 18631331]
Level 2 (mid-level) evidencevan Zeeburg EJ, van Meurs JC. Literature review of recombinant tissue plasminogen activator used for recent-onset submacular hemorrhage displacement in age-related macular degeneration. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 2013:229(1):1-14. doi: 10.1159/000343066. Epub 2012 Oct 12 [PubMed PMID: 23075629]
Ziemianski MC, McMeel JW, Franks EP. Natural history of vitreous hemorrhage in diabetic retinopathy. Ophthalmology. 1980 Apr:87(4):306-12 [PubMed PMID: 7393537]
Schultz PN, Sobol WM, Weingeist TA. Long-term visual outcome in Terson syndrome. Ophthalmology. 1991 Dec:98(12):1814-9 [PubMed PMID: 1775315]
Level 2 (mid-level) evidenceel Baba F, Jarrett WH 2nd, Harbin TS Jr, Fine SL, Michels RG, Schachat AP, Green WR. Massive hemorrhage complicating age-related macular degeneration. Clinicopathologic correlation and role of anticoagulants. Ophthalmology. 1986 Dec:93(12):1581-92 [PubMed PMID: 2433658]
Level 3 (low-level) evidenceIp M, Hendrick A. Retinal Vein Occlusion Review. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2018 Jan-Feb:7(1):40-45. doi: 10.22608/APO.2017442. Epub 2017 Dec 27 [PubMed PMID: 29280368]
Hansen JB, Killough EF, Moffatt ME, Knapp JF. Retinal Hemorrhages: Abusive Head Trauma or Not? Pediatric emergency care. 2018 Sep:34(9):665-670. doi: 10.1097/PEC.0000000000001605. Epub [PubMed PMID: 30180101]
Jena S, Tripathy K. Vitreous Hemorrhage. StatPearls. 2024 Jan:(): [PubMed PMID: 32644557]
Budenz DL, Huecker JB, Gedde SJ, Gordon M, Kass M, Ocular Hypertension Treatment Study Group. Thirteen-Year Follow-up of Optic Disc Hemorrhages in the Ocular Hypertension Treatment Study. American journal of ophthalmology. 2017 Feb:174():126-133. doi: 10.1016/j.ajo.2016.10.023. Epub 2016 Nov 7 [PubMed PMID: 27832941]
Ashaye AO. Traumatic hyphaema: a report of 472 consecutive cases. BMC ophthalmology. 2008 Nov 26:8():24. doi: 10.1186/1471-2415-8-24. Epub 2008 Nov 26 [PubMed PMID: 19036128]
Level 2 (mid-level) evidenceLai JC, Fekrat S, Barrón Y, Goldberg MF. Traumatic hyphema in children: risk factors for complications. Archives of ophthalmology (Chicago, Ill. : 1960). 2001 Jan:119(1):64-70 [PubMed PMID: 11146728]
Level 2 (mid-level) evidenceNasrullah A, Kerr NC. Sickle cell trait as a risk factor for secondary hemorrhage in children with traumatic hyphema. American journal of ophthalmology. 1997 Jun:123(6):783-90 [PubMed PMID: 9535622]