Indications
Immunoglobulins, aka antibodies, are glycoprotein molecules produced by plasma cells in response to a variety of antigenic stimuli involved in diverse physiological and pathological processes. Immunoglobulins primarily function in the adaptive arm (although “natural immunoglobulins” work in the innate arm) of the immune system and are subdivided, based on heavy chains they contain, into various classes, i.e., IgM, IgG, IgD, IgA, and IgE.[1]
IgG is subdivided into various subclasses, i.e., IgG1, IgG2, IgG3, and IgG4 (in order of decreasing abundance), and IgA into IgA1 and IgA2. IgG is the most abundant immunoglobulin with a plasma concentration range of 700-1600 mg/dL, and this constitutes about 75% to 80% of the immunoglobulins. IgA constitutes about 15% of the immunoglobulins at a plasma concentration of 70-400 mg/dL, whereas IgM has a range of 40-230 mg/dL in the plasma.[2][3][4][5]
Intravenous immunoglobulin (IVIG) is a concentrate of the pooled immunoglobulins derived from 1000 to 100000 healthy donors depending upon the manufacturer. Immunoglobulins play a pivotal role in humoral adaptive immunity; ergo, IVIG reflects a collective exposure of the donor population to their environment and can be expected to contain an antibody repertoire of multiple specificities against a broad spectrum of infectious agents (bacterial, viral, and others), self-antigens and anti-idiotype antibodies.
The composition of IVIG products closely corresponds to that of immunoglobulins in the normal human plasma, especially IgG (along with its subclasses), IgA, traces of other Igs, cytokines, and soluble receptors. IVIG products are prepared using the Cohn-Oncley procedure, the first step of which is cold ethanol precipitation used to enrich the IgG from the plasma of donors. Any two IVIG product varies with respect to the presence of excipients such as substances used to stabilize proteins and prevent aggregation of IgG (sugars such as glucose, maltose, D-sorbitol or more recently amino acids such as glycine or proline), sodium levels, pH levels, osmolality and other immunoglobulins (for example, IgA can vary from 0.06 mg to 40 mg in different preparations).[6]
IgG comprises more than 90% of the proteins in an IVIG preparation, and it is the principal component required for the therapeutic effect of IVIG. Some authors even consider IVIG to stand for intravenous IgG.[7] Thus, IVIG therapy aims to replenish sufficient amounts of IgG antibodies that passively neutralize or opsonize a broad spectrum of infectious pathogens but could also elicit an active immune response via activation of various immune cells, thus conferring protection against diverse diseases.
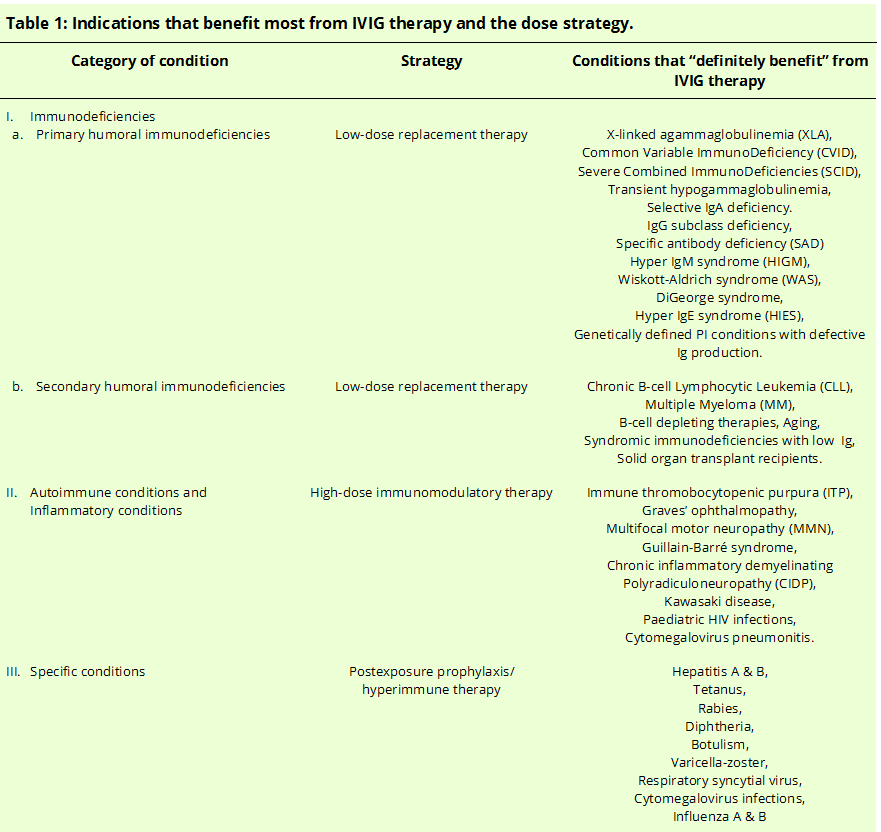
The indications for IVIG can be classified informally into a few broad categories based on the mechanism of action and the type of conditions they treat. They are as follows:
- As a replacement therapy in immunodeficiencies.
- For immunomodulatory and anti-inflammatory therapy, (a) Immunomodulation in hematological and organ-specific autoimmune disorders (b) Anti-inflammatory in rheumatic inflammatory conditions, infectious and neurological disorders.
- As a hyperimmune therapy against specific infectious agents.
Different IVIG doses (low vs. high) are administered based on the indicated medical condition because the mechanisms of action differ with different doses. Low-dose immunoglobulins serve merely as a passive replacement in immunodeficiencies (category I only). High-dose immunoglobulins take an active part and modulate the immune functions with additional anti-inflammatory activity (category II only). Of note, there is considerable overlap between autoimmunity and inflammatory conditions as they almost always co-exist. Category III, hyperimmune therapy does not fall under the purview of dose-differentiation as they are specific antibodies and are given at doses “pro re nata” so that they neutralize the small proportion of specific pathogenic antigens. The actual difference in doses and their plausible differences in mechanisms of action are discussed below in their respective sections.
According to the latest report of the American Academy of Allergy, Asthma and Immunology (AAAAI), the indications for IVIG therapy are divided into four ordinal groups depending on how the therapy benefits the patients: “Definitely beneficial,” “Probably beneficial,” “May provide benefit” and “Unlikely to provide benefit.” Here we will discuss only the first group as the number of diseases included in all the groups is too large and outside the scope of this paper.[8]
IVIG replacement therapy is the apparent treatment of choice for humoral primary immunodeficiencies, as these patients cannot mount an effective immune response towards pathogens. Humoral primary immunodeficiencies are the most common and comprise the largest patient population of primary immunodeficiency (PI) diseases.[9] Humoral PI has the most number of FDA-approved IVIG products than any other condition. These diseases are characterized by absent or low serum concentrations of Igs, recurrent infections and the need for repeated intravenous antibiotics, a lack of normal antibody response to a vaccine challenge, a normal antibody concentration with defective function, and an identifiable genetic defect.[10]
Agammaglobulinemia due to lack of B cells is the archetype condition in the group of PIs and was first described by Bruton in an 8-year old boy with recurrent pneumococcal infections. He discovered the lack of serum immunoglobulins and treated it successfully with subcutaneous immunoglobulins.[11] It was discovered later to be a genetic disorder with an X-linked inheritance pattern, and the neonates are usually protected by the maternal IgGs to protect the fetus. The disease does not usually manifest until after six months of age when the maternal IgG wanes and signs of recurrent bacterial infections become apparent. However, the average age at diagnosis with a positive family history is 2.6 years with an average lifespan of 15 to 20 years, underscoring the need for IVIG therapy for survival.[12][13] A list of other conditions that “Definitely benefit” from IVIG therapy appears in Table 1.[8]
Secondary humoral immunodeficiencies can be caused by a number of conditions, and the most common of those is malnutrition. While malnutrition is manageable and leads to the recuperation of immune defense, there are several other conditions for which treatment is unavailable or where secondary immunodeficiency is inevitable.[14] These conditions, like PIs, require low-dose IVIG therapy to avoid the risk of frequent and deadly infections. Cancers such as B-cell chronic lymphocytic leukemia (B-cell CLL) and multiple myeloma (MM) lead to humoral immunosuppression, and these conditions benefit from IVIG therapy. Other conditions that also benefit are those with B-cell depleting therapies such as lymphoma and geriatric patients with recurrent infections. In addition to the clinical picture, IgG levels in plasma can be used to guide IVIG therapy for secondary conditions, and values of IgG less than or equal to 150 mg/dL are considered severe hypogammaglobulinemia. For levels that are in the range of 150 to 600 mg/dL, additional testing of antibody levels in response to vaccines like tetanus and diphtheria should be considered before starting IVIG therapy.[15] A list of other secondary immunodeficiency conditions that benefit from IVIG therapy appears in Table 1.
In autoimmune and inflammatory conditions, two to four-fold increases in doses of IVIG, when compared to replacement doses, can bring a variety of protective changes.[16] These changes, as explained below, are complex, and various studies have shown the efficacy of a high-dose strategy in these conditions. For example, immune thrombocytopenic purpura (ITP) is an autoimmune condition characterized by isolated thrombocytopenia causing life-threatening bleeding. IVIG therapy has been shown to raise the platelet count within four days of administration, reducing the need for frequent and repeated platelet transfusions. Glucocorticoids, along with IVIG, are now considered first-line therapy in this condition and have greatly improved the lives of these patients.[17] Other such conditions are given in Table 1.
For specific therapy against infectious pathologies, hyperimmune IVIG that contains high concentrations of specific IVIG targeted against a specific organism or antigen is administered. Hyperimmune IVIG is purified from human donors who were recently vaccinated or are in the convalescent period recovering from an infection. Such hyperimmune IVIG is currently indicated for postexposure prophylaxis of hepatitis A and B, tetanus, rabies, diphtheria, botulism, varicella-zoster, respiratory syncytial virus, and cytomegalovirus infections.[18]
However, the US-Food and Drug Administration approved conditions that are indicated for IVIG therapy are dispersed among all the categories described above and include humoral PIs, ITP, B-cell CLL, common variable immunodeficiency (CVID), Kawasaki disease (KD), multifocal motor neuropathy (MMN), bone marrow transplantation and HIV infection. Along with FDA approved use of IVIG, the off-label uses of this product are growing rapidly. Other conditions where IVIG therapy demonstrates benefit include toxic epidermal necrolysis and Stevens-Johnson syndrome, neonatal sepsis, birdshot retinochoroidopathy, Henoch-Schönlein purpura, and toxic shock syndrome. IVIG is used in a multitude of other conditions where it is regarded as beneficial. The list is extensive, and the readers are advised to refer to the updates from the most recent guidelines when necessary.[8]
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
The basic structure of the IgG molecule is made up of polypeptide chains and consists of two identical heavy chains and two identical light chains forming a Y-shaped structure. The protease papain can digest the immunoglobulin into two Fab fragments and one Fc fragment. IgG-Fab is the antigen-binding fragment and mediates highly specific interactions with the antigenic epitope. IgG-Fc is the crystallizable region, which mediates the binding to Fc-gamma-receptors (FcgRs) on immune cells and/or complement proteins.[1]
Major functions of the Fab portion of IgG are neutralization of infective pathogens (by interfering with pathogen attachment to host cell receptor or by targeting various steps in their lifecycle) and non-specific opsonization by binding to the surface of microorganisms leading to phagocytosis.[19]
IgG-Fc binds to various FcgRs, which are expressed on almost all the immune cell types and can be either activatory or inhibitory in function. IgG-FcgR interaction results in pleiotropic functional consequences, including the activation or inhibition of effector immune response, expansion of regulatory T cell population (inhibits overt immune response), modulation of FcgR expression on B cells and immune cells, phagocytosis, antibody-dependent cellular cytotoxicity (ADCC), immune cell differentiation and maturation, apoptosis regulation, expression of proinflammatory mediators, modulation of the antigen-presenting cell and dendritic cell functions.[20][21][22]
In humoral immunodeficiencies, IVIG primarily acts by substituting for the lack of IgG and confers passive immunity by neutralizing bacterial toxins and viruses through the Fab portion. They also help activate the complement cascade at low doses by specific interaction with pathogens, a function that reverses in high doses to complement inactivation by non-specific interactions.[23] Polyvalent IVIG products with a higher number of donors would contain a much larger spectrum of specificities and would be more efficient in immune replacement therapies.[24] The effects of replacement dose IVIG in PIs are observable well beyond the half-life of IgG administered, suggesting the induction of active immunity.[25] This is exemplified by the activation of cellular immunity; for example, IVIG modulates T cell immunity in PIs and increases CD4 counts in CVID, induces B cell immunoglobulin production in CVID patients, and induces dendritic cell (DC) maturation.[26][27][28]
Autoimmunity is essentially an overt immune response against the body’s own tissues, and IgG autoantibodies are considered the main players in most conditions. Self-antigen is recognized by Fab fragment of IgG autoantibody, and Fc fragment relays this signal to Fc-gamma-receptors (FcgRs) on various immune cells.[21] IgG autoantibodies cause inflammation by interacting with FcgRs, neonatal FcR (FcRn), and complement proteins.[29][30][31] Autoantibodies disrupt a myriad of functions, including cellular lysis (as in ITP), triggering micro thrombosis, ADCC, complement-dependent cytotoxicity, uncontrolled neutrophil activation, stimulation of hormonal receptor (Graves disease), receptor blockade of neural transmission (MG), induction of inflammation (rheumatoid arthritis) and altered cell signaling.[32]
The immunomodulatory actions of high-dose IVIG in autoimmune and inflammatory conditions are highly complex and differ in different diseases. In general, high-dose IVIG paradoxically leads to a reversal of their effects, as opposed to their actions at replacement doses, resulting in a more immunosuppressive and anti-inflammatory phenotype.[33]
- Anti-idiotypic antibodies in IVIG, for instance, binds and neutralizes circulating autoantibodies to factor VIII.[34]
- F(ab’) neutralizes C3a and C5a anaphylatoxins that mediate inflammation in autoimmune rheumatic conditions; IVIG binds to complement proteins and sequesters them away from auto-antibodies, which helps prevent the formation of membrane attack complex in dermatomyositis.[35][36][37]
- Fc mediated effects [38]: High-dose IVIG primarily leads to an inhibitory effect.
- Monomeric IgG in high dose IVIG causes blockade of activating FcgRs by saturating them on immune cells attenuating immune complex-mediated inflammation and autoimmunity.[39] They also cause an immediate increase in platelet counts in ITP patients.[40]
- Neonatal FcRs (FcRn) on endothelial cells bind to auto-IgG and prolong its half-life by preventing its catabolism. IVIG can saturate FcRn, thereby promoting the rapid elimination of the pathogenic endogenous IgGs.[41]
- Fc-gamma-RIIb is an inhibitory IgG receptor on innate immune cells, and IVIG causes upregulation of these receptors leading to an increase of the activation threshold by immune complexes, thus suppressing inflammation.[42]
- IVIG modulation of cellular immunity:
- Inhibits activation of monocytes and macrophages.[43]
- Induction of anti-inflammatory cytokines from innate cells and leads to decreased macrophage responsiveness to interferon.[44]
- Inhibition of DC maturation and differentiation.[45][46]
- IVIG is cytotoxic to neutrophils and eosinophils, leading to a switch from a pro- to an anti-inflammatory environment.[47][48]
- IVIG can induce apoptosis by inducing the Fas apoptotic pathway in DCs and effector Th1 and Th17 cells.[49][50]
- Enhances immunosuppressive effects through the expansion of the regulatory T cell population.[51][52]
- Exerts immunosuppressive action by suppressing the proliferation of B-lymphocytes and, thus, modulation of antibody production.[53] IVIG can also induce suppression of autoreactive B lymphocytes and neutralization of their cytokines. [54]
- IVIG also modulates the activity of endothelial cell proliferation and expression of adhesion molecules in the vascular lumen leading to an anti-inflammatory action in vasculitic disorders.[55]
- Inhibition of Fas-ligand mediated apoptosis by Fas antibodies present in IVIG halts the progression of disease in toxic epidermal necrolysis.[56]
In addition to the above mechanisms, natural autoantibodies present in the IVIG also suppress some autoimmune pathophysiological mechanisms. Sialylated IgG antibodies present in IVIG preparations are shown to have an anti-inflammatory property, although this claim is controversial.[57]
Hyperimmune IgG also provides passive immunity to an infectious agent, but they are active against only one specific pathogen, unlike replacement therapy. High-dose IVIG therapy, in contrast, exerts its action through its non-specific interactions with various immune molecules and cells, as seen above. Hyperimmune IgG action is instant and leads to efficient clearance of specific pathogenic microorganisms or toxins mediating the disease.[58]
Different IVIG preparations are used at different doses in diverse diseases in a variety of patients with dissimilar immune statuses, and although research so far has shed some light on the various mechanisms of actions, it is very hard to generalize the actions, which must be considered separately for each disease in question.
Administration
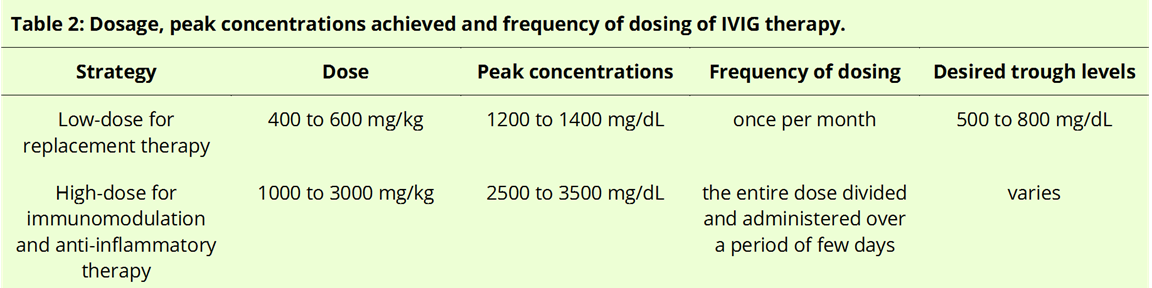
IG is available either as 5% (50 mg/dL) or 10% (100 mg/dL) liquid or as lyophilized preparations. As the name suggests, IVIG is administered intravenously, and the half-life of a typical intravenous immunoglobulin infusion is about 3 to 4 weeks. The dosage, peak concentrations achieved, and frequency of dosing, as elaborated in the text, appear in summary form in Table 2.
Low-dose replacement therapy: In PIs, IVIG is usually administered at replacement doses of 400 to 600 mg/kg per month, achieving plasma levels of 1200 to 1400 mg/dL.[8][25] IgG trough levels (defined as the lowest concentration achieved before the next dose) of 500 to 800 mg/dL are achievable with this dosing and are considered to be protective from infectious consequences in immunodeficient patients.[59][60] IVIGs administered at a frequency of once every month are usually sufficient for PIs through a wear-off effect (increased susceptibility to infection) that can occur near the end of their dosing cycle for at-risk patients and should be borne in mind.[61] In cases of acute infections in immunodeficient patients, a short-term course of high-dose IVIG merit consideration for treatment as in enteroviral meningoencephalitis.[62]
High-dose immunomodulatory and anti-inflammatory therapy: For immunomodulation, higher doses of IVIG are necessary, ranging from 1000 to 3000 mg/kg of body weight to achieve peak plasma concentrations of 2500 to 3500 mg/dL. The optimal dosage, duration, and frequency are usually determined based on the indication, response to treatment, adverse effects, relapse rate, infectious episodes, patient preferences, and affordability. In general, a high-dose IVIG protocol, usually but not always, involves an initial dose, maintenance dose, tapering/intensifying dose, and discontinuation.[63] In general, a protocol of 2 mg/kg/course divides into 400 mg/day for five days and is a universally employed administration strategy for autoimmune diseases.[64] It is modifiable in certain conditions; for instance, in the case of ITP, a dose of 1000 mg/kg is given for 1 to 2 days.[65] Weekly regimens may also be employed depending on the clinical situation and the particular patient.[63]
Hyperimmune therapy: The dosage of hyperimmune IVIG varies with every indication, but some generalizations are possible. The most common means of administration is as a single intramuscular dose after the suspected exposure to a particular pathogen, and the earlier it is administered after exposure, the better the outcome. In addition to the intramuscular route, some of these immune sera are given as IVIG therapy. They may also be administered in a multi-dose regimen; for example, 750 mg/kg of respiratory syncytial virus (RSV) IVIG is given every month to infants in RSV season. The dosage can also increase in cases of immunocompromised and immunosuppressed patients.[18]
Infusion rate: is another important parameter to consider in IVIG therapy, especially in patients new to IVIG therapy. Infusions are started at a rate of 0.5 to 1 mL/kg/hour for the first 15 to 30 minutes, and if no adverse reaction occurs, then the rate can be increased subsequently every 15 to 30 minutes to a maximum of 3 to 6 mL/kg/hour.[66] Dose fractionation should also be considered to decrease the possibility of any adverse reaction.
Pharmacokinetic profiles of IVIG products show a considerable interindividual variability and also vary in different disease states; for example, in bone marrow recipients, the reported half-life of IVIG is around 2 to 6 days.[67] Due to the presence of FcRn, IVIG preparations have a similar half-life as that of endogenous IgG, which has a median half-life of about 23 days.[41][68] In immunodeficient patients, the reported half-life of IVIG is 33 to 36 days.[69]
SCIG therapy can be a consideration in the event of any systemic adverse effects and poor venous access. SCIG is usually administered at a lower dose of 100 to 200 mg/kg and is administered much more frequently, i.e., every week or two weeks, and achieves a better nadir (trough level) than IVIG that achieves higher peak levels.[70][71] SCIG, which typically contains a small transfusion volume of, on average, 20 to 60 mL, also has the advantage that it can be administered at home and has better patient compliance.[72][73]
Adverse Effects
Adverse reactions can be either immediate or delayed and can be of different severity. Immediate reactions can be mild, moderate, and serious. These occur within 30 to 60 minutes of the start of IVIG infusion and are reported in 5% of patients. The most common generalized side effects are mild in severity and include headache, fever, chills, and fatigue. These effects are attributable to the excipients and stabilizers contained in the preparation. Sugar-depleted preparations are now available with only amino acids as a stabilizing agents. Most of these reactions are mild and transient and attributed to a particular IVIG product and its infusion rate.[74] A ten-year retrospective study on the adverse effects of IVIG concluded that most of the adverse events are due to the fast infusion rates.[75] Vomiting, chest pain, and headache classify as moderate reactions. Mild-to-moderate reactions can be mitigated with symptomatic treatment, premedication, slowing the transfusion rate, lowering the dose or withdrawing, and replacing with a different IVIG preparation.
Serious adverse reactions are more common in geriatric patients than in any other age group due to pre-existing co-morbidities.[76] The most common of these include headache, myalgia, back pain, nausea, vomiting, rash, fatigue, malaise, tachycardia, erythema, flushing, fever, hypotension or hypertension, and fluid overload. Severe uncommon side effects include urticaria, severe headaches, dyspnea, pruritus, thromboembolic events, and hemolytic reactions. Serious rare effects are transfusion-related acute lung injury (TRALI), acute renal failure, anaphylaxis to IgE or IgG antibodies to IgA (in IgA deficiency), arrhythmias, aseptic meningitis, arthritis, hepatitis, pleural effusion, or other dermatological manifestations.
Less than 1% of patients may have a delayed reaction, including renal impairment, transfusion-related infection, and hematological and neurological disorders. Solvent-related adverse effects such as the high volume of infusions as needed in some liquid formulations can lead to volume overload in patients with cardiac or renal conditions and require appropriate attention using concentrated IVIG preparations or subcutaneous immunoglobulin (SCIG) therapy.[77] Adverse effects are preventable with certain premedications, including non-steroidal anti-inflammatory drugs, antihistamines, corticosteroids, or saline for pre-hydration.
There are certain reported drawbacks or risks, but due to stringent regulation of IVIG product approval, there are no recently reported cases. These include:
- As a blood product, IVIG has the potential risk of transmission of viral infectious agents (HIV, hepatitis B, etc.), and there are only a few reported cases of hepatitis C transmission in the distant past.[24]
- Drawbacks of IVIG therapy are possible when the IgG pool is from the donors with no exposure to specific pathogens, and these microorganisms can still cause infection in the patient. Though these are significantly mitigated in the current scenario, this is still a distinct possibility in case of infection by a rare pathogen.
- IgA or IgM have unique functions, and if an IVIG product lacks these immunoglobulins, then those functions may not be substituted.[78]
Contraindications
Although there are no absolute contraindications for IVIG products as they are not generic drugs and are not interchangeable, their use in high-risk patients must proceed with caution. Every IVIG product is different, and a patient with a life-threatening reaction to one product may not have any reactions with a different preparation. Thus the contraindications are related to the particular component of the IVIG product.
Noteworthy Caveats
- Sugar-stabilized IVIG products should be avoided in patients with renal failure or diabetes.
- Hyperosmolar IVIG products are not for post-transplantation patients due to the risk of renal failure and osmotic nephropathy.
- High sodium-containing products should be used cautiously for individuals with cardiac conditions and hypertension.
- Severe anaphylactic reactions are rare and have been reported when using IVIG products due to the presence of IgG or IgE anti-IgA antibodies in patients with IgA deficiency.[79][80] Paradoxical use of IgA-depleted IVIG or a SCIG preparation is suggested in the treatment of patients with IgA deficiency.[81]
- Measles, mumps, and rubella (MMR) vaccine should not be administered in children receiving IVIG therapy, as the IgG could counter the attenuated virus in the vaccine preparation and render them inactive. Thus vaccines should be delayed for at least nine months after the IVIG therapy or vice versa.[82]
Monitoring
Patients receiving initial (first-time) IVIG infusions and high-risk categories should be monitored carefully for any infusion-related reactions or adverse effects. The slowest initial infusion rates are necessary to prevent any undesirable situation. The infusion should be carried out in the presence of an expert physician and in a setting well equipped to tackle any adverse reaction swiftly. Concomitant medications of the patients should be reviewed before starting IVIG therapy, for example, inhibitors of the renin-angiotensin system (RAS).[83]
IgG levels in blood serve as an essential yardstick to guide IVIG therapy. It is also used to assess the effectiveness of the treatment and helps to modify the IVIG course and frequency. Measuring IgG levels at different times to evaluate the peak plasma levels and trough levels can assess response to therapy. Trough levels are crucial in replacement therapy, as maintaining constant plasma level are necessary for this life-long therapeutic strategy. However, the clinical scenario dictates the true response to IVIG therapy in all circumstances.[84] Clinical response is the most important variable on which to base a course of IVIG treatment, and doses are repeated every month over several months to years (for immunomodulation) or indefinitely (for immunodeficiencies) depending on the condition of the patient and undergo reevaluation as needed.
Patients with blood types A, B, or AB should be monitored carefully for hemolytic transfusion reaction during high-dose therapy as they may contain anti-A or anti-B blood group antibodies. These patients should be followed up with a hemoglobin workup two days after the therapy.[85]
Toxicity
Various stringent quality-control measures are employed to ensure the safety of a typical IVIG product, which includes virus inactivation, removal of coagulation factors, and depletion of IgG aggregates. Though IVIG is generally considered to be a safe, well-tolerated, and efficacious therapeutic modality, reports exist in the literature of reported toxicities.[22]
There are reports of renal toxicity with sucrose-containing products, in patients greater than or equal to 65 years, patients receiving concomitant nephrotoxic agents, patients with diabetes mellitus, those with pre-existing renal disease, hypovolemia, and sepsis. These patients are all at increased risk for acute renal failure and renal insufficiency.[86] Urine output, blood urea nitrogen, and creatinine require assessment in patients with an increased risk of developing acute renal failure. There is also a report of cardiac toxicity after IVIG therapy in a patient with scleromyxedema, where it resulted in myocardial infarction.[87] Hematological toxicities, including various cytopenias and thrombotic complications, have also been reported and should be considered in patients with an increased risk of thrombosis.[88]
Enhancing Healthcare Team Outcomes
The onus for IVIG therapy's success lies mainly in the treating clinician to achieve treatment goals, as every patient needs a unique and tailored infusion regime. The first and primary means of achieving this is by having a correct diagnosis, and this occurs through efficient interprofessional communication between specialists. More often than not, the diagnosis may fall on the category where off-label use of IVIG is required (as evidenced by a large number of such conditions), and assessing the appropriateness of IVIG therapy must be balanced against the morbidity of the condition. Clinicians and other providers can accomplish this by staying up-to-date on the current guidelines from the authorities such as the American Academy of Allergy, Asthma & Immunology, the European Academy of Allergy and Clinical Immunology, and the World Allergy Organization. Recently available scientific evidence of IVIG use in specific disease trials or studies should always be used along with clinician's experience to guide the decision on dosage, targeted optimal IgG levels, choice of IVIG product, and course of treatment and should be modified in a patient-centric setting. The choice of IVIG products needs special attention as the products are not generic and only a particular product, but not the other may meet the patient's needs.
IVIG therapy is a consistently evolving practice. More large-scale clinical trials are needed to assess the efficacy of treatment in specific conditions that may not be feasible in other rare conditions. In such situations, a treating clinician is responsible for publishing individual case studies and being part of a collective framework in establishing guidelines for IVIG therapy. Nurses play an evident role in patient care during IVIG infusion and promptly report any adverse reaction to the clinical team to tackle the situation immediately. The government and health authorities can influence the type of product approved in the local market and serve a fundamental role. The cost of IVIG and the availability in low and middle-income countries is challenging, and governmental/national measures to reduce the costs will bring the IVIG therapy to the doorstep of the poor. Finally, the hospital authorities must be well-equipped to handle the transfusion and play a role in establishing standard IVIG protocols for well-established diseases. The hospital's research office should encourage more projects to address the off-label use in rare conditions. WIth appropriate interprofessional teamwork involving clinicians (MDs, DOs, PAs, and NPs), specialists, nurses, and pharmacists, IVIG therapy can produce better patient outcomes, be expanded to cover more conditions, and result in fewer adverse events. [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Godwin L, Sinawe H, Crane JS. Biochemistry, Immunoglobulin E. StatPearls. 2023 Jan:(): [PubMed PMID: 31082102]
Justiz Vaillant AA, Jamal Z, Patel P, Ramphul K. Immunoglobulin. StatPearls. 2023 Jan:(): [PubMed PMID: 30035936]
Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. Journal of immunology (Baltimore, Md. : 1950). 2015 Jan 1:194(1):13-20. doi: 10.4049/jimmunol.1400844. Epub [PubMed PMID: 25527792]
Level 3 (low-level) evidenceLoh RK, Vale S, McLean-Tooke A. Quantitative serum immunoglobulin tests. Australian family physician. 2013 Apr:42(4):195-8 [PubMed PMID: 23550242]
Dati F, Schumann G, Thomas L, Aguzzi F, Baudner S, Bienvenu J, Blaabjerg O, Blirup-Jensen S, Carlström A, Petersen PH, Johnson AM, Milford-Ward A, Ritchie RF, Svendsen PJ, Whicher J. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP Reference Material (CRM 470). International Federation of Clinical Chemistry. Community Bureau of Reference of the Commission of the European Communities. College of American Pathologists. European journal of clinical chemistry and clinical biochemistry : journal of the Forum of European Clinical Chemistry Societies. 1996 Jun:34(6):517-20 [PubMed PMID: 8831057]
Level 1 (high-level) evidenceBarahona Afonso AF, João CM. The Production Processes and Biological Effects of Intravenous Immunoglobulin. Biomolecules. 2016 Mar 9:6(1):15. doi: 10.3390/biom6010015. Epub 2016 Mar 9 [PubMed PMID: 27005671]
Radosevich M, Burnouf T. Intravenous immunoglobulin G: trends in production methods, quality control and quality assurance. Vox sanguinis. 2010 Jan:98(1):12-28. doi: 10.1111/j.1423-0410.2009.01226.x. Epub 2009 Jul 29 [PubMed PMID: 19660029]
Level 2 (mid-level) evidencePerez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, El-Gamal Y, Harville TO, Hossny E, Mazer B, Nelson R, Secord E, Jordan SC, Stiehm ER, Vo AA, Ballow M. Update on the use of immunoglobulin in human disease: A review of evidence. The Journal of allergy and clinical immunology. 2017 Mar:139(3S):S1-S46. doi: 10.1016/j.jaci.2016.09.023. Epub 2016 Dec 29 [PubMed PMID: 28041678]
Modell V, Knaus M, Modell F, Roifman C, Orange J, Notarangelo LD. Global overview of primary immunodeficiencies: a report from Jeffrey Modell Centers worldwide focused on diagnosis, treatment, and discovery. Immunologic research. 2014 Oct:60(1):132-44. doi: 10.1007/s12026-014-8498-z. Epub [PubMed PMID: 24668296]
Level 3 (low-level) evidenceReda SM, El-Ghoneimy DH, Afifi HM. Clinical predictors of primary immunodeficiency diseases in children. Allergy, asthma & immunology research. 2013 Mar:5(2):88-95. doi: 10.4168/aair.2013.5.2.88. Epub 2012 Nov 2 [PubMed PMID: 23450209]
BRUTON OC. Agammaglobulinemia. Pediatrics. 1952 Jun:9(6):722-8 [PubMed PMID: 14929630]
El-Sayed ZA, Abramova I, Aldave JC, Al-Herz W, Bezrodnik L, Boukari R, Bousfiha AA, Cancrini C, Condino-Neto A, Dbaibo G, Derfalvi B, Dogu F, Edgar JDM, Eley B, El-Owaidy RH, Espinosa-Padilla SE, Galal N, Haerynck F, Hanna-Wakim R, Hossny E, Ikinciogullari A, Kamal E, Kanegane H, Kechout N, Lau YL, Morio T, Moschese V, Neves JF, Ouederni M, Paganelli R, Paris K, Pignata C, Plebani A, Qamar FN, Qureshi S, Radhakrishnan N, Rezaei N, Rosario N, Routes J, Sanchez B, Sediva A, Seppanen MR, Serrano EG, Shcherbina A, Singh S, Siniah S, Spadaro G, Tang M, Vinet AM, Volokha A, Sullivan KE. X-linked agammaglobulinemia (XLA):Phenotype, diagnosis, and therapeutic challenges around the world. The World Allergy Organization journal. 2019:12(3):100018. doi: 10.1016/j.waojou.2019.100018. Epub 2019 Mar 22 [PubMed PMID: 30937141]
De Ranieri D, Fenny NS. Intravenous Immunoglobulin in the Treatment of Primary Immunodeficiency Diseases. Pediatric annals. 2017 Jan 1:46(1):e8-e12. doi: 10.3928/19382359-20161213-03. Epub [PubMed PMID: 28079912]
Chinen J, Shearer WT. Secondary immunodeficiencies, including HIV infection. The Journal of allergy and clinical immunology. 2010 Feb:125(2 Suppl 2):S195-203. doi: 10.1016/j.jaci.2009.08.040. Epub 2009 Dec 29 [PubMed PMID: 20042227]
Cunningham-Rundles C. How I treat common variable immune deficiency. Blood. 2010 Jul 8:116(1):7-15. doi: 10.1182/blood-2010-01-254417. Epub 2010 Mar 23 [PubMed PMID: 20332369]
Tjon AS, van Gent R, Geijtenbeek TB, Kwekkeboom J. Differences in Anti-Inflammatory Actions of Intravenous Immunoglobulin between Mice and Men: More than Meets the Eye. Frontiers in immunology. 2015:6():197. doi: 10.3389/fimmu.2015.00197. Epub 2015 Apr 28 [PubMed PMID: 25972869]
Cooper N, Ghanima W. Immune Thrombocytopenia. The New England journal of medicine. 2019 Sep 5:381(10):945-955. doi: 10.1056/NEJMcp1810479. Epub [PubMed PMID: 31483965]
Hemming VG. Use of intravenous immunoglobulins for prophylaxis or treatment of infectious diseases. Clinical and diagnostic laboratory immunology. 2001 Sep:8(5):859-63 [PubMed PMID: 11527792]
Level 3 (low-level) evidenceForthal DN. Functions of Antibodies. Microbiology spectrum. 2014 Aug 15:2(4):1-17 [PubMed PMID: 25215264]
Bournazos S, Ravetch JV. Diversification of IgG effector functions. International immunology. 2017 Jul 1:29(7):303-310. doi: 10.1093/intimm/dxx025. Epub [PubMed PMID: 28472280]
Bournazos S, Ravetch JV. Fcγ receptor pathways during active and passive immunization. Immunological reviews. 2015 Nov:268(1):88-103. doi: 10.1111/imr.12343. Epub [PubMed PMID: 26497515]
Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nature reviews. Immunology. 2013 Mar:13(3):176-89. doi: 10.1038/nri3401. Epub 2013 Feb 15 [PubMed PMID: 23411799]
Level 3 (low-level) evidenceBasta M. Ambivalent effect of immunoglobulins on the complement system: activation versus inhibition. Molecular immunology. 2008 Oct:45(16):4073-9. doi: 10.1016/j.molimm.2008.07.012. Epub 2008 Aug 15 [PubMed PMID: 18706699]
Level 3 (low-level) evidenceChaigne B, Mouthon L. Mechanisms of action of intravenous immunoglobulin. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2017 Feb:56(1):45-49. doi: 10.1016/j.transci.2016.12.017. Epub 2016 Dec 30 [PubMed PMID: 28161150]
Kaveri SV, Maddur MS, Hegde P, Lacroix-Desmazes S, Bayry J. Intravenous immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clinical and experimental immunology. 2011 Jun:164 Suppl 2(Suppl 2):2-5. doi: 10.1111/j.1365-2249.2011.04387.x. Epub [PubMed PMID: 21466545]
Paquin-Proulx D, Santos BA, Carvalho KI, Toledo-Barros M, Barreto de Oliveira AK, Kokron CM, Kalil J, Moll M, Kallas EG, Sandberg JK. IVIg immune reconstitution treatment alleviates the state of persistent immune activation and suppressed CD4 T cell counts in CVID. PloS one. 2013:8(10):e75199. doi: 10.1371/journal.pone.0075199. Epub 2013 Oct 9 [PubMed PMID: 24130688]
Level 1 (high-level) evidenceBayry J, Fournier EM, Maddur MS, Vani J, Wootla B, Sibéril S, Dimitrov JD, Lacroix-Desmazes S, Berdah M, Crabol Y, Oksenhendler E, Lévy Y, Mouthon L, Sautès-Fridman C, Hermine O, Kaveri SV. Intravenous immunoglobulin induces proliferation and immunoglobulin synthesis from B cells of patients with common variable immunodeficiency: a mechanism underlying the beneficial effect of IVIg in primary immunodeficiencies. Journal of autoimmunity. 2011 Feb:36(1):9-15. doi: 10.1016/j.jaut.2010.09.006. Epub 2010 Dec 9 [PubMed PMID: 20970960]
Quinti I, Mitrevski M. Modulatory Effects of Antibody Replacement Therapy to Innate and Adaptive Immune Cells. Frontiers in immunology. 2017:8():697. doi: 10.3389/fimmu.2017.00697. Epub 2017 Jun 16 [PubMed PMID: 28670314]
Ben Mkaddem S, Benhamou M, Monteiro RC. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Frontiers in immunology. 2019:10():811. doi: 10.3389/fimmu.2019.00811. Epub 2019 Apr 12 [PubMed PMID: 31057544]
Level 3 (low-level) evidenceTakai T. Roles of Fc receptors in autoimmunity. Nature reviews. Immunology. 2002 Aug:2(8):580-92 [PubMed PMID: 12154377]
Level 3 (low-level) evidenceNimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. The Journal of experimental medicine. 2007 Jan 22:204(1):11-5 [PubMed PMID: 17227911]
Level 3 (low-level) evidenceLudwig RJ, Vanhoorelbeke K, Leypoldt F, Kaya Z, Bieber K, McLachlan SM, Komorowski L, Luo J, Cabral-Marques O, Hammers CM, Lindstrom JM, Lamprecht P, Fischer A, Riemekasten G, Tersteeg C, Sondermann P, Rapoport B, Wandinger KP, Probst C, El Beidaq A, Schmidt E, Verkman A, Manz RA, Nimmerjahn F. Mechanisms of Autoantibody-Induced Pathology. Frontiers in immunology. 2017:8():603. doi: 10.3389/fimmu.2017.00603. Epub 2017 May 31 [PubMed PMID: 28620373]
Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annual review of immunology. 2008:26():513-33. doi: 10.1146/annurev.immunol.26.021607.090232. Epub [PubMed PMID: 18370923]
Level 3 (low-level) evidenceSultan Y, Kazatchkine MD, Maisonneuve P, Nydegger UE. Anti-idiotypic suppression of autoantibodies to factor VIII (antihaemophilic factor) by high-dose intravenous gammaglobulin. Lancet (London, England). 1984 Oct 6:2(8406):765-8 [PubMed PMID: 6148519]
Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Köhl J. The role of the anaphylatoxins in health and disease. Molecular immunology. 2009 Sep:46(14):2753-66. doi: 10.1016/j.molimm.2009.04.027. Epub 2009 May 28 [PubMed PMID: 19477527]
Level 3 (low-level) evidenceBasta M, Van Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, Szebeni J, Alving CR, Carroll MC, Berkower I, Stojilkovic SS, Metcalfe DD. F(ab)'2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nature medicine. 2003 Apr:9(4):431-8 [PubMed PMID: 12612546]
Level 3 (low-level) evidenceBasta M, Dalakas MC. High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. The Journal of clinical investigation. 1994 Nov:94(5):1729-35 [PubMed PMID: 7962520]
Level 1 (high-level) evidenceNagelkerke SQ, Kuijpers TW. Immunomodulation by IVIg and the Role of Fc-Gamma Receptors: Classic Mechanisms of Action after all? Frontiers in immunology. 2014:5():674. doi: 10.3389/fimmu.2014.00674. Epub 2015 Jan 21 [PubMed PMID: 25653650]
Li X, Kimberly RP. Targeting the Fc receptor in autoimmune disease. Expert opinion on therapeutic targets. 2014 Mar:18(3):335-50. doi: 10.1517/14728222.2014.877891. Epub [PubMed PMID: 24521454]
Level 3 (low-level) evidenceBussel JB. Fc receptor blockade and immune thrombocytopenic purpura. Seminars in hematology. 2000 Jul:37(3):261-6 [PubMed PMID: 10942220]
Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proceedings of the National Academy of Sciences of the United States of America. 1996 May 28:93(11):5512-6 [PubMed PMID: 8643606]
Level 3 (low-level) evidenceAnthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011 Jun 19:475(7354):110-3. doi: 10.1038/nature10134. Epub 2011 Jun 19 [PubMed PMID: 21685887]
Level 3 (low-level) evidenceKozicky LK, Zhao ZY, Menzies SC, Fidanza M, Reid GS, Wilhelmsen K, Hellman J, Hotte N, Madsen KL, Sly LM. Intravenous immunoglobulin skews macrophages to an anti-inflammatory, IL-10-producing activation state. Journal of leukocyte biology. 2015 Dec:98(6):983-94. doi: 10.1189/jlb.3VMA0315-078R. Epub 2015 Jul 27 [PubMed PMID: 26216934]
Park-Min KH, Serbina NV, Yang W, Ma X, Krystal G, Neel BG, Nutt SL, Hu X, Ivashkiv LB. FcgammaRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007 Jan:26(1):67-78 [PubMed PMID: 17239631]
Level 3 (low-level) evidenceBayry J, Lacroix-Desmazes S, Carbonneil C, Misra N, Donkova V, Pashov A, Chevailler A, Mouthon L, Weill B, Bruneval P, Kazatchkine MD, Kaveri SV. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003 Jan 15:101(2):758-65 [PubMed PMID: 12393386]
Bayry J, Lacroix-Desmazes S, Delignat S, Mouthon L, Weill B, Kazatchkine MD, Kaveri SV. Intravenous immunoglobulin abrogates dendritic cell differentiation induced by interferon-alpha present in serum from patients with systemic lupus erythematosus. Arthritis and rheumatism. 2003 Dec:48(12):3497-502 [PubMed PMID: 14674000]
Casulli S, Topçu S, Fattoum L, von Gunten S, Simon HU, Teillaud JL, Bayry J, Kaveri SV, Elbim C. A differential concentration-dependent effect of IVIg on neutrophil functions: relevance for anti-microbial and anti-inflammatory mechanisms. PloS one. 2011:6(10):e26469. doi: 10.1371/journal.pone.0026469. Epub 2011 Oct 31 [PubMed PMID: 22065996]
von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, Simon HU. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. The Journal of allergy and clinical immunology. 2007 Apr:119(4):1005-11 [PubMed PMID: 17337295]
Prasad NK, Papoff G, Zeuner A, Bonnin E, Kazatchkine MD, Ruberti G, Kaveri SV. Therapeutic preparations of normal polyspecific IgG (IVIg) induce apoptosis in human lymphocytes and monocytes: a novel mechanism of action of IVIg involving the Fas apoptotic pathway. Journal of immunology (Baltimore, Md. : 1950). 1998 Oct 1:161(7):3781-90 [PubMed PMID: 9759905]
Level 3 (low-level) evidenceMaddur MS, Sharma M, Hegde P, Lacroix-Desmazes S, Kaveri SV, Bayry J. Inhibitory effect of IVIG on IL-17 production by Th17 cells is independent of anti-IL-17 antibodies in the immunoglobulin preparations. Journal of clinical immunology. 2013 Jan:33 Suppl 1():S62-6. doi: 10.1007/s10875-012-9752-6. Epub 2012 Aug 7 [PubMed PMID: 22864643]
Massoud AH, Guay J, Shalaby KH, Bjur E, Ablona A, Chan D, Nouhi Y, McCusker CT, Mourad MW, Piccirillo CA, Mazer BD. Intravenous immunoglobulin attenuates airway inflammation through induction of forkhead box protein 3-positive regulatory T cells. The Journal of allergy and clinical immunology. 2012 Jun:129(6):1656-65.e3. doi: 10.1016/j.jaci.2012.02.050. Epub 2012 May 5 [PubMed PMID: 22564681]
Level 3 (low-level) evidenceEphrem A, Chamat S, Miquel C, Fisson S, Mouthon L, Caligiuri G, Delignat S, Elluru S, Bayry J, Lacroix-Desmazes S, Cohen JL, Salomon BL, Kazatchkine MD, Kaveri SV, Misra N. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008 Jan 15:111(2):715-22 [PubMed PMID: 17932250]
Level 3 (low-level) evidenceHori A, Fujimura T, Kawamoto S. Anti-inflammatory intravenous immunoglobulin (IVIg) suppresses homeostatic proliferation of B cells. Cytotechnology. 2018 Jun:70(3):921-927. doi: 10.1007/s10616-017-0176-2. Epub 2018 Apr 2 [PubMed PMID: 29611058]
Le Pottier L, Sapir T, Bendaoud B, Youinou P, Shoenfeld Y, Pers JO. Intravenous immunoglobulin and cytokines: focus on tumor necrosis factor family members BAFF and APRIL. Annals of the New York Academy of Sciences. 2007 Sep:1110():426-32 [PubMed PMID: 17911457]
Xu C, Poirier B, Duong Van Huyen JP, Lucchiari N, Michel O, Chevalier J, Kaveri S. Modulation of endothelial cell function by normal polyspecific human intravenous immunoglobulins: a possible mechanism of action in vascular diseases. The American journal of pathology. 1998 Oct:153(4):1257-66 [PubMed PMID: 9777957]
Cho YT, Chu CY. Treatments for Severe Cutaneous Adverse Reactions. Journal of immunology research. 2017:2017():1503709. doi: 10.1155/2017/1503709. Epub 2017 Dec 27 [PubMed PMID: 29445753]
Washburn N, Schwab I, Ortiz D, Bhatnagar N, Lansing JC, Medeiros A, Tyler S, Mekala D, Cochran E, Sarvaiya H, Garofalo K, Meccariello R, Meador JW 3rd, Rutitzky L, Schultes BC, Ling L, Avery W, Nimmerjahn F, Manning AM, Kaundinya GV, Bosques CJ. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proceedings of the National Academy of Sciences of the United States of America. 2015 Mar 17:112(11):E1297-306. doi: 10.1073/pnas.1422481112. Epub 2015 Mar 2 [PubMed PMID: 25733881]
Level 3 (low-level) evidenceHsu JL, Safdar N. Polyclonal immunoglobulins and hyperimmune globulins in prevention and management of infectious diseases. Infectious disease clinics of North America. 2011 Dec:25(4):773-88. doi: 10.1016/j.idc.2011.07.005. Epub [PubMed PMID: 22054755]
Toubi E, Etzioni A. Intravenous immunoglobulin in immunodeficiency states: state of the art. Clinical reviews in allergy & immunology. 2005 Dec:29(3):167-72 [PubMed PMID: 16391391]
Notarangelo LD. Primary immunodeficiencies. The Journal of allergy and clinical immunology. 2010 Feb:125(2 Suppl 2):S182-94. doi: 10.1016/j.jaci.2009.07.053. Epub 2009 Dec 29 [PubMed PMID: 20042228]
Level 3 (low-level) evidenceRojavin MA, Hubsch A, Lawo JP. Quantitative Evidence of Wear-Off Effect at the End of the Intravenous IgG (IVIG) Dosing Cycle in Primary Immunodeficiency. Journal of clinical immunology. 2016 Apr:36(3):210-9. doi: 10.1007/s10875-016-0243-z. Epub 2016 Feb 24 [PubMed PMID: 26910102]
Erlendsson K, Swartz T, Dwyer JM. Successful reversal of echovirus encephalitis in X-linked hypogammaglobulinemia by intraventricular administration of immunoglobulin. The New England journal of medicine. 1985 Feb 7:312(6):351-3 [PubMed PMID: 4038544]
Level 3 (low-level) evidencePatwa HS. Dosing and individualized treatment - patient-centric treatment: changing practice guidelines. Clinical and experimental immunology. 2014 Dec:178 Suppl 1(Suppl 1):36-8. doi: 10.1111/cei.12503. Epub [PubMed PMID: 25546754]
Level 3 (low-level) evidenceZandman-Goddard G, Krauthammer A, Levy Y, Langevitz P, Shoenfeld Y. Long-term therapy with intravenous immunoglobulin is beneficial in patients with autoimmune diseases. Clinical reviews in allergy & immunology. 2012 Apr:42(2):247-55. doi: 10.1007/s12016-011-8278-7. Epub [PubMed PMID: 21732045]
Level 2 (mid-level) evidenceKhan AM, Mydra H, Nevarez A. Clinical Practice Updates in the Management Of Immune Thrombocytopenia. P & T : a peer-reviewed journal for formulary management. 2017 Dec:42(12):756-763 [PubMed PMID: 29234214]
Kareva L, Mironska K, Stavric K, Hasani A. Adverse Reactions to Intravenous Immunoglobulins - Our Experience. Open access Macedonian journal of medical sciences. 2018 Dec 20:6(12):2359-2362. doi: 10.3889/oamjms.2018.513. Epub 2018 Dec 17 [PubMed PMID: 30607191]
Koleba T, Ensom MH. Pharmacokinetics of intravenous immunoglobulin: a systematic review. Pharmacotherapy. 2006 Jun:26(6):813-27 [PubMed PMID: 16716135]
Level 1 (high-level) evidenceBonilla FA. Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes. Immunology and allergy clinics of North America. 2008 Nov:28(4):803-19, ix. doi: 10.1016/j.iac.2008.06.006. Epub [PubMed PMID: 18940575]
Wasserman RL, Church JA, Peter HH, Sleasman JW, Melamed I, Stein MR, Bichler J, IgPro10 in PID Study group. Pharmacokinetics of a new 10% intravenous immunoglobulin in patients receiving replacement therapy for primary immunodeficiency. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2009 Jun 28:37(3-4):272-8. doi: 10.1016/j.ejps.2009.02.014. Epub 2009 Mar 6 [PubMed PMID: 19491015]
Shehata N,Palda V,Bowen T,Haddad E,Issekutz TB,Mazer B,Schellenberg R,Warrington R,Easton D,Anderson D,Hume H, The use of immunoglobulin therapy for patients with primary immune deficiency: an evidence-based practice guideline. Transfusion medicine reviews. 2010 Jan; [PubMed PMID: 19962579]
Level 1 (high-level) evidenceBonagura VR. Using intravenous immunoglobulin (IVIG) to treat patients with primary immune deficiency disease. Journal of clinical immunology. 2013 Jan:33 Suppl 2():S90-4. doi: 10.1007/s10875-012-9838-1. Epub 2012 Dec 28 [PubMed PMID: 23271459]
Gardulf A, Björvell H, Andersen V, Björkander J, Ericson D, Frøland SS, Gustafson R, Hammarström L, Nyström T, Søeberg B. Lifelong treatment with gammaglobulin for primary antibody deficiencies: the patients' experiences of subcutaneous self-infusions and home therapy. Journal of advanced nursing. 1995 May:21(5):917-27 [PubMed PMID: 7541407]
Kobayashi RH, Gupta S, Melamed I, Mandujano JF, Kobayashi AL, Ritchie B, Geng B, Atkinson TP, Rehman S, Turpel-Kantor E, Litzman J. Clinical Efficacy, Safety and Tolerability of a New Subcutaneous Immunoglobulin 16.5% (Octanorm [Cutaquig®]) in the Treatment of Patients With Primary Immunodeficiencies. Frontiers in immunology. 2019:10():40. doi: 10.3389/fimmu.2019.00040. Epub 2019 Feb 4 [PubMed PMID: 30778345]
Guo Y, Tian X, Wang X, Xiao Z. Adverse Effects of Immunoglobulin Therapy. Frontiers in immunology. 2018:9():1299. doi: 10.3389/fimmu.2018.01299. Epub 2018 Jun 8 [PubMed PMID: 29951056]
Palabrica FR, Kwong SL, Padua FR. Adverse events of intravenous immunoglobulin infusions: a ten-year retrospective study. Asia Pacific allergy. 2013 Oct:3(4):249-56. doi: 10.5415/apallergy.2013.3.4.249. Epub 2013 Oct 31 [PubMed PMID: 24260730]
Level 2 (mid-level) evidenceSiegel J. The product: All intravenous immunoglobulins are not equivalent. Pharmacotherapy. 2005 Nov:25(11 Pt 2):78S-84S [PubMed PMID: 16229678]
Stein MR. The new generation of liquid intravenous immunoglobulin formulations in patient care: a comparison of intravenous immunoglobulins. Postgraduate medicine. 2010 Sep:122(5):176-84. doi: 10.3810/pgm.2010.09.2214. Epub [PubMed PMID: 20861601]
Taneja A, Muco E, Chhabra A. Bruton Agammaglobulinemia. StatPearls. 2023 Jan:(): [PubMed PMID: 28846295]
Burks AW, Sampson HA, Buckley RH. Anaphylactic reactions after gamma globulin administration in patients with hypogammaglobulinemia. Detection of IgE antibodies to IgA. The New England journal of medicine. 1986 Feb 27:314(9):560-4 [PubMed PMID: 3945295]
Level 3 (low-level) evidenceRachid R, Castells M, Cunningham-Rundles C, Bonilla FA. Association of anti-IgA antibodies with adverse reactions to γ-globulin infusion. The Journal of allergy and clinical immunology. 2011 Jul:128(1):228-230.e1. doi: 10.1016/j.jaci.2011.01.061. Epub 2011 Mar 11 [PubMed PMID: 21397310]
Level 3 (low-level) evidenceBjörkander J, Hammarström L, Smith CI, Buckley RH, Cunningham-Rundles C, Hanson LA. Immunoglobulin prophylaxis in patients with antibody deficiency syndromes and anti-IgA antibodies. Journal of clinical immunology. 1987 Jan:7(1):8-15 [PubMed PMID: 3494039]
Tacke CE, Smits GP, van der Klis FR, Kuipers IM, Zaaijer HL, Kuijpers TW. Reduced serologic response to mumps, measles, and rubella vaccination in patients treated with intravenous immunoglobulin for Kawasaki disease. The Journal of allergy and clinical immunology. 2013 Jun:131(6):1701-3. doi: 10.1016/j.jaci.2013.01.045. Epub 2013 Mar 14 [PubMed PMID: 23498596]
Level 3 (low-level) evidenceMoulis G, Sailler L, Sommet A, Lapeyre-Mestre M, Montastruc JL, French Association of Regional Pharmacovigilance Centers. Exposure to inhibitors of the renin-angiotensin system is a major independent risk factor for acute renal failure induced by sucrose-containing intravenous immunoglobulins: a case-control study. Pharmacoepidemiology and drug safety. 2012 Mar:21(3):314-9. doi: 10.1002/pds.2253. Epub 2011 Sep 28 [PubMed PMID: 21953992]
Level 2 (mid-level) evidenceJolles S, Orange JS, Gardulf A, Stein MR, Shapiro R, Borte M, Berger M. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clinical and experimental immunology. 2015 Feb:179(2):146-60. doi: 10.1111/cei.12485. Epub [PubMed PMID: 25384609]
Kahwaji J, Barker E, Pepkowitz S, Klapper E, Villicana R, Peng A, Chang R, Jordan SC, Vo AA. Acute hemolysis after high-dose intravenous immunoglobulin therapy in highly HLA sensitized patients. Clinical journal of the American Society of Nephrology : CJASN. 2009 Dec:4(12):1993-7. doi: 10.2215/CJN.04540709. Epub 2009 Oct 15 [PubMed PMID: 19833910]
Dantal J. Intravenous immunoglobulins: in-depth review of excipients and acute kidney injury risk. American journal of nephrology. 2013:38(4):275-84. doi: 10.1159/000354893. Epub 2013 Sep 14 [PubMed PMID: 24051350]
Binitha MP, Nandakumar G, Thomas D. Suspected cardiac toxicity to intravenous immunoglobulin used for treatment of scleromyxedema. Indian journal of dermatology, venereology and leprology. 2008 May-Jun:74(3):248-50 [PubMed PMID: 18583794]
Level 3 (low-level) evidenceBaxley A, Akhtari M. Hematologic toxicities associated with intravenous immunoglobulin therapy. International immunopharmacology. 2011 Nov:11(11):1663-7. doi: 10.1016/j.intimp.2011.07.024. Epub 2011 Aug 16 [PubMed PMID: 21843660]