Introduction
Laryngeal cancers represent one-third of all head and neck cancers and are a significant source of morbidity and mortality. These cancers primarily originate from any of the 3 subdivisions of the larynx—the supraglottis, glottis, and subglottis—and each maintains its own staging system. Squamous cell carcinoma is the most common histologic subtype, with nearly all squamous cell carcinoma variants described in this anatomic location (see Image. Anatomy of the Larynx).[1][2] Other very rare histologies include sarcomas of the laryngeal skeleton, minor salivary gland carcinomas, melanoma, and lymphomas.[3][4][5] Laryngeal cancers are most often diagnosed in patients with a significant smoking history, who are also at risk for cancers in the remainder of the aerodigestive tract. Confounding associations with ethanol consumption (supraglottis) and various environmental exposures, such as Agent Orange, asbestos, or metal-working occupational fumes (all subsites), also exist.[6][7][8] Primary subglottic cancer is quite rare and portends a bleak prognosis.[2] Unlike areas of the oropharynx, such as Waldeyer's ring, the association with human papillomavirus (HPV) is not nearly as robust. While HPV-related oncogenetic pathways, such as p16, have been described in laryngeal carcinomas, the etiologic and prognostic significance of these viral-related pathways remains to be elucidated.[9][10]
Each primary subsite of laryngeal carcinoma carries different implications in symptomatic presentation, patterns of spread, prognosis, and treatment paradigms. Early-stage disease is often highly treatable or curable in the supraglottis and glottis, although the prognosis remains poor in the subglottis. Early-stage (stage I or II) laryngeal cancer can be successfully treated with monotherapy, meaning a single-modality of treatment, either surgical or radiation therapy, which typically preserves the larynx.[11] In contrast, advanced-stage disease (stage III or IV) carries a significantly poorer prognosis across all subsites, although the pattern is maintained. Glottic primaries often yield the best outcomes, followed by supraglottic and then subglottic tumors.[12] The treatment for advanced-stage laryngeal cancer warrants multimodal therapy, which may include surgery followed by radiation therapy, primary chemoradiation therapy, or a combination of all 3 methodologies (see Image. Laryngeal Cancer).[13]
All the aforementioned generalizations pertain specifically to laryngeal squamous cell carcinoma. Minor salivary gland carcinoma of the larynx, laryngeal melanoma, and other rare carcinomas are primarily managed within clinical trials due to their rarity, precluding standardized treatment recommendations.[14] Sarcoma of the larynx, particularly chondrosarcoma (the most common subtype), is best treated surgically. Notably, it is a rare case where the overall prognosis is quite good, although the treatment often requires a total laryngectomy, which has severe consequences.[15]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Smoking is the most significant risk factor for laryngeal cancer, accounting for over 70% of all cases.[16] Any history of smoking increases risk, with current smokers having a higher relative risk compared to ex-smokers overall, and a higher relative risk for supraglottic versus glottic cancers. Heavy alcohol consumption is also a risk factor, though its independent effect is unclear due to its frequent combination with tobacco use. Marijuana smoking may be a risk factor in younger patients.[17]
Other risk factors for laryngeal cancer include:
- Long-term secondhand smoke exposure (odds ratio of 1.2 when lifelong secondhand exposure exceeds 20,000 hours).[18]
- Male sex (historically, laryngeal cancer is 4-5 times more common in men than women).
- White or Black race (North American data show a significantly higher incidence of tobacco-related cancers in the White and Black populations than Asian-Americans and Hispanics).[19]
- Genetic and other syndromes (such as Fanconi anemia, Plummer-Vinson syndrome, and dyskeratosis congenita) predispose individuals to laryngeal cancer.[2][20][21]
- Occupational and environmental exposures (such as Agent Orange, asbestos, nickel fumes, sulfuric acid mist, and wood dust increase the risk of laryngeal carcinoma.[2][22][23]
Epidemiology
In 2017, laryngeal cancer accounted for 13,150 new cases, roughly one-third of all head and neck cancers, and 3710 associated deaths. The mean age of patients is 65, with a higher proportion of males compared to females and Blacks compared to Whites. Recently, age-adjusted incidence rates have decreased by about 2% annually, attributed to declining tobacco use.[24] Approximately 98% of laryngeal cancers arise in either the supraglottic or glottic regions, with glottic cancers being 3 times more common than supraglottic cancers. Subglottic cancers represent about 2% of all cases.[25] Early-stage cancers are highly curable, with local control rates of 90% to 95% for T1 glottic cancers and 80% to 90% for early-stage supraglottic cancers. These early-stage cancers are generally amenable to vocal cord-sparing surgical therapy, offering curative options for surgical or radiation therapy.[26] In contrast, locally advanced cancers have control rates ranging from 40% to 70%, with bulky and/or T4 disease responding best to laryngectomy. However, advances over the years have increased laryngeal preservation and improved speech rehabilitation for patients receiving laryngectomy.[12]
Pathophysiology
The vast majority of laryngeal cancers are squamous cell carcinomas. A minority of cases are squamous cell variants, including verrucous, sarcomatoid, and neuroendocrine carcinomas. Verrucous and sarcomatoid carcinomas were historically regarded as radioresistant, although recent experience contradicts this notion.[27] Patterns of spread depend on the location of the primary tumor and the inherent lymphatic supply at that location. Laryngeal cancers are categorized into supraglottic, glottic, and subglottic subsites, with pathophysiology and treatment differing for each subsite.
Supraglottic Tumors
The supraglottis is subdivided into the suprahyoid epiglottis, infrahyoid epiglottis, false vocal cords, aryepiglottic folds, and arytenoids. Suprahyoid epiglottic tumors may grow exophytically and superiorly before inducing symptoms. In other cases, they may invade inferiorly into the tip of the epiglottis and destroy associated cartilage. Infrahyoid epiglottic tumors tend to grow circumferentially, involving the aryepiglottic folds, and can infiltrate inferiorly into the false vocal cords. They may also invade anteriorly into the pre-epiglottic fat space and, subsequently, the vallecula and base of the tongue.
Early lymphatic involvement is a hallmark of supraglottic cancers, with 55% of patients showing clinical evidence of nodal metastasis at presentation and 16% having contralateral involvement. Levels II, III, and IV of the cervical nodal basins are most commonly affected, with the ipsilateral level II nodes (jugulodigastric nodes) at the highest risk.[28] Locally advanced tumors present a higher risk of nodal metastasis due to bilateral tumor involvement, which increases the risk of lymphatic spread in the bilateral neck, and/or superior extension and invasion into the base of the tongue, vallecula, and pyriform sinus.
Glottic Tumors
The apex of the ventricle marks the transition from the supraglottic to the glottic larynx. The vocal cords are typically 3 mm to 5 mm thick and terminate posteriorly at a commissure with the vocal process. Due to their sparse lymphatic supply, glottic tumors do not pose a risk of lymphatic involvement unless there is a supraglottic or glottic extension. Glottic cancers typically present confined to the anterior portion of the upper free margin of a vocal cord. They can induce vocal cord fixation due to pure bulk, involvement of intrinsic muscles and ligaments, or, rarely, the involvement of the recurrent laryngeal nerve.[29]
Subglottic Tumors
The subglottis extends from 5 mm below the free margin of the vocal cord superiorly to the inferior border of the cricoid cartilage (or 10 mm below the apex of the ventricle). Like glottic tumors, they have sparse lymphatic drainage, primarily to levels IV and VI of the cervical nodal chain.[30][31]
Histopathology
The vast majority of laryngeal cancers are squamous cell carcinomas. Rarely, other malignancies such as adenocarcinomas, sarcomas, lymphomas, and neuroendocrine tumors are involved.
History and Physical
Historically, patients are predominantly male with a history of current or past tobacco smoking, and chronic alcohol abuse is an additional risk factor. Hoarseness is the most common initial symptom of glottic cancers due to vocal cord immobility or fixation, with pain while swallowing and referred ear pain potentially indicating advanced disease.[32] In contrast, pain with swallowing is the most common early symptom of supraglottic cancer, with hoarseness presenting later and indicating more advanced disease extending into the glottis.[33] Nodal metastases often present as fixed, firm, painless masses in the neck. Late symptoms across all subsites include weight loss, dysphagia, aspiration and its sequelae, and airway compromise.[34]
Establishing the timing and duration of symptom onset, particularly noting any aggravating or alleviating factors, is crucial for accurately diagnosing laryngeal malignancy. Viral infections and other upper respiratory tract conditions, as well as laryngopharyngeal reflux (LPR), often mimic laryngeal malignancy in high-risk patients. While gastroesophageal reflux (GERD)/LPR was historically considered a potential predisposing condition for laryngeal malignancy, this association has not been consistently supported by multiple studies.[35] As LPR/GERD is highly prevalent and exacerbated by smoking, it represents a frequent red herring in evaluating potential laryngeal cancer patients.[36] A comprehensive head and neck examination and upper aerodigestive endoscopy are essential for screening individuals at risk for laryngeal malignancy.[37] Routine population-based screening has not proven effective; however, evidence suggests that screening for the highest-risk patients may be effective.[38] The most crucial physical examination component is the observation of the primary lesion, which can occur via direct or mirror laryngoscopy techniques or, most often, via fiberoptic endoscopy. The objective is to assess the local extent of the tumor, determine its size and involvement with nearby structures, and evaluate vocal cord mobility.[37] Direct laryngoscopy enhances the ability to delineate the extent of disease and obtain tissue specimens. A comprehensive neck examination is essential to evaluate nodal metastasis and primary lesion extension. Tenderness of the thyroid cartilage indicates direct tumor extension, while firm fullness palpated just superior to the thyroid notch classically indicates pre-epiglottic space invasion. However, as with all physical signs in laryngeal cancer, physical examination findings alone carry a significant false-positive rate.[39] Therefore, imaging is a crucial component of the workup for a laryngeal mass. A detailed cranial nerve examination is also important. Immobility of the vocal fold may suggest involvement of the recurrent and/or superior laryngeal nerves or direct vagus nerve involvement, depending on the position of the fold. Weakness of the accessory nerve indicates involvement through primary extension or extranodal spread, while weakness of the lingual or hypoglossal nerve may suggest extranodal spread or extensive local tumor burden.
Evaluation
In addition to history, physical examination, and laryngeal visualization, other studies are necessary to formally diagnose and stage the cancer. Multiple methods for obtaining tissue are feasible, including biopsy during direct laryngoscopy of the suspected primary lesion and fine-needle aspiration cytology for suspected nodal disease.
For all laryngeal cancers, imaging of the primary lesion and draining lymph nodes is essential, typically performed with contrast-enhanced computed tomography (CT) of the neck. This study visualizes neck lymphatics and structures not adequately assessed by direct laryngoscopy, such as the subglottic region, pre-epiglottic space, inner table of the thyroid cartilage, paralaryngeal space, and extranodal extension, crucial for accurate staging.[34][40] If the cancer suggests an advanced stage, contrast-enhanced CT of the chest and positron emission tomography (PET)/CT are indicated to rule out distant metastases. Suspected invasion into the hypopharynx or cervical esophagus may necessitate esophagogastroduodenoscopy (EGD) and/or barium swallow to identify the precise aerodigestive tissue of cancer origin.[40]
Additional hematologic studies are necessary before initiating any definitive treatment. These include a complete blood count, platelet count, liver and renal function panel, blood type, thyroid function, electrolytes, and albumin levels.
Certain factors are considered during the workup of laryngeal cancer and in determining whether the patient is a surgical candidate. These factors help define the extent of oncologic surgery required and if any reconstructive surgery is expected to be needed, which include:
- Vocal cord mobility/function of the larynx
- Presence of cervical or distant metastatic lesions
- Involvement of the base of the tongue
- Involvement of the paraglottic and pre-epiglottic space
- Involvement of the thyroid cartilage
- Involvement of the carotid artery and sheath
- Invasion of the esophagus
- Invasion of soft tissue and adjacent laryngeal muscles
- Involvement of neck lymph nodes
Treatment / Management
The management of laryngeal cancer in patients depends on the stage of the disease and the subsite of the tumor at the time of treatment.
Early-Stage Laryngeal Cancer
Early-stage laryngeal cancers, including T1-2N0 (stages I and II) disease, are successfully treated with a single treatment modality—either radiation therapy or surgery.
T1-2N0 glottic cancer: Local radiation therapy or surgery is recommended for T1-2N0 glottic cancer, with the choice of modality highly dependent on provider experience and patient preference. Given the sparse lymphatic drainage of the true glottis, these modalities share a common fundamental principle: they only address the primary tumor. Local control rates from retrospective experience are historically comparable between surgical approaches and radiation therapy. Voice-sparing surgery is an option in many, but not all, of these cancers. Patient and anatomic considerations may affect a patient's candidacy for any surgical intervention, whereas most patients are potential candidates for primary radiation therapy. If the larynx is functional and the cancer can be adequately resected via endoscopic techniques (either transoral laser microsurgery [TLM] or transoral robotic surgery [TORS]), the outcomes (disease-specific survival and larynx preservation) have been shown to be superior with surgery in the sole trial that directly compared these 2 patient populations.[41] In patients whose cancer cannot be oncologically resected endoscopically and would therefore require open laryngofissure or total laryngectomy, primary radiation therapy is a vastly superior option due to the local morbidity and mortality associated with the surgical options in these cases.(A1)
T1-2N0, selected T1-2N1/T3N0-1 supraglottic cancer: Similar to early-stage glottic cancers, early supraglottic cancers can be managed with either larynx-sparing surgery or radiation therapy monotherapy, with demonstrated overall comparable efficacy. The major difference between glottic and supraglottic cancers is the management of the neck. Given the significant risk of nodal metastases in supraglottic tumors, it is necessary to address bilateral neck nodal basins.[42] Surgical approaches include endoscopic resection (TLM or TORS) or open partial laryngectomy (supraglottic or supracricoid laryngectomy) for T1-2 and low-volume T3 disease, with neck dissections often indicated in T2 or T3 lesions. Adjuvant radiation therapy is given to many patients, with common indications including positive nodal disease, extracapsular extension, and positive surgical margins. Definitive radiation therapy often includes at-risk cervical nodal stations, generally levels II to IV bilaterally.
Locally Advanced Laryngeal Cancers
Locally advanced cancers, inclusive of T3-4N1-3 disease, are more complex to treat and involve multimodality therapy (either surgery followed by radiation therapy or a combination of chemotherapy and radiation therapy). These cancers, if surgically resectable, are not typically amenable to laryngeal preservation surgery, while definitive radiation concurrent with cisplatin chemotherapy remains an option for laryngeal preservation. In contrast to early-stage disease, the therapeutic approach to locally advanced disease is based on level I evidence, with combined chemotherapy and radiation demonstrating improved locoregional control and larynx preservation in patients with a functional larynx and no erosion of laryngeal cartilages. In T4 disease, laryngectomy and adjuvant radiation therapy have demonstrated similar locoregional control rates compared to chemoradiation and salvage surgery but improved overall survival. Larynx-preserving chemoradiation is therefore not recommended for T4 disease and is associated with inferior survival unless the patient is not a surgical candidate for overall medical reasons.[43][44][45](A1)
Postoperative radiation therapy is relatively indicated in the case of advanced tumor and nodal stage on surgical pathology (pT3-4, pN2-3), as well as other high-risk pathologic features, including close margins (<5 mm), perineural invasion, lymphovascular space invasion, extracapsular extension, and emergent tracheostomy due to morbid tumor invasion (to reduce the risk of tumor spread into the tracheostomy). Moreover, close or involved margins, multiple positive nodes, and extracapsular extension necessitate the addition of chemotherapy concurrent with radiation therapy, although induction, adjuvant, and neoadjuvant protocols have all been used with varying degrees of success when concurrent therapy is not possible. This scenario is often based on the physiological factors of a patient and may be somewhat predicted based on the patient's Eastern Cooperative Oncology Group (ECOG) status.[46][47][48][49]
Metastatic Laryngeal Cancers
Laryngeal cancer most often metastasizes to the cervical lymph nodes regionally, but the most frequent site of distant metastasis is the lung (followed by the liver and bones). If a solitary lung lesion is present, obtaining a histological diagnosis is crucial to confirm it is not a concurrent primary lung cancer, owing to the overlapping risk factors, as the treatment goals, options, and prognosis differ significantly between M1 lung cancer versus M0 lung cancer and primary lung cancer. With the presence of distant metastases, treatment for laryngeal cancer becomes systemic (chemotherapy and/or immunotherapy) and is largely palliative in nature.[50] Some centers may recommend surgical resection of a single pulmonary metastasis as part of the treatment plan with curative intent in selected patients.[51] Experience with this approach varies among centers and treatment teams, but in experienced settings, it has shown promising results. Managing advanced-stage laryngeal cancer alongside a new primary lung cancer benefits significantly from a tumor board or multidisciplinary team of healthcare providers, relying on considerations of the stage, histology, and genetic characteristics of both tumors.(A1)
Non-Squamous Cell Laryngeal Malignancies
Squamous cell carcinoma and its variants comprise the vast majority of laryngeal malignancies. However, rarer tumors include minor salivary gland malignancies, such as adenoid cystic carcinoma, as well as mesenchymal tumors, such as sarcomas and melanomas. Treatment for these rare tumors is dictated by their histology, with protocols tailored to include airway protection. Mucosal melanoma is invariably classified as stage IV disease and necessitates systemic therapy, with surgical interventions reserved for exceptional cases or to facilitate respiration through tracheostomy.[52] Adenoid cystic carcinoma poses a significant treatment challenge, typically requiring primary surgical resection with potential adjuvant radiation therapy as the cornerstone of treatment. Likewise, sarcomas, particularly chondrosarcoma, the most prevalent laryngeal variant, are primarily managed surgically. Unfortunately, this often necessitates extensive surgical approaches, including total laryngectomy, to achieve adequate oncologic control, even in cases of early-stage laryngeal tumors with these rare histologies.[53](B2)
Differential Diagnosis
Differential diagnoses to consider and rule out regarding laryngeal cancer include:
- Acute sialadenitis
- Reactive lymphadenitis
- Benign tumors
- Branchial cleft cyst
- Fungal laryngitis
- Chronic sialadenitis
- Contact granuloma
- Hemangioma
- Laryngeal papilloma
- Laryngocele
- Vocal cord polyp
- Vocal cord nodule (singer's nodule)
- Reinke edema
- Thyroglossal duct cyst
- GERD
- Granulomatous disease (ie, Wegener granulomatosis)
- Sarcoidosis
- Laryngeal tuberculosis
- Syphilis
Surgical Oncology
Laryngeal cancer treatment uses 3 categories of surgery—endoscopic/transoral surgery, open framework surgery (partial laryngectomy), and total laryngectomy.
Endoscopic and Transoral Surgery
Endoscopic and transoral surgeries are conducted through the mouth or using various laryngoscopes, although they may not be feasible for all patients due to the requirement for adequate line-of-sight exposure. Laser ablation via flexible laryngoscopy, primarily used for premalignant lesions, is not included in this review, although some authors are exploring its application for T1 disease.[54]
Endoscopic cordectomy: This technique is suitable for T1 and select T2 glottic cancers and is most commonly performed using a CO2 laser, although it can also be performed using cold micro-laryngeal instruments. This technique involves removing the affected vocal fold. The European Laryngological Society has classified different types of cordectomy based on the extent of resection (see Table 1. European Laryngological Society Endoscopic Cordectomy Classification).[55] Tumors extending onto the laryngeal surface of the false vocal fold can also be addressed. However, tumors that extend into the anterior commissure or onto the contralateral cord are associated with a higher recurrence rate at the anterior commissure. As a result, these cases are likely better treated with radiation therapy or open surgery. This recurrence is presumed to occur due to tumor tracking along the insertion of the ligament into the thyroid cartilage.[56]
Table 1. European Laryngological Society Endoscopic Cordectomy Classification
| Cordectomy Types | Description | Tissue Removed |
| I | Subepithelial | Epithelium only |
| II | Subligamental | Epithelium, Reinke space, and vocal ligament |
| III | Transmuscular | Epithelium, Reinke space, vocal ligament, and part of vocalis |
| IV | Total cordectomy | Extends from the vocal process up to anterior commissure up to and with, or without, the perichondrium |
| Va | Extended cordectomy encompassing contralateral cord | Total cordectomy, anterior portion contralateral vocal cord, and Broyles ligament, with or without petiole of epiglottis |
| Vb | Extended cordectomy encompassing arytenoid | Total cordectomy, arytenoid cartilage partially or totally removed, and preservation of the postarytenoid mucosa |
| Vc | Extended cordectomy encompassing ventricular fold | Total cordectomy, Morgani ventricle, and ventricular fold |
| Vd | Extended cordectomy encompassing subglottis | Total cordectomy, subglottic tissue up to a maximum of 1 cm inferior to the glottis |
Endoscopic supraglottic laryngectomy: This operation is performed for T1 and T2 cancers of the supraglottis and can also be performed transorally using a laser or a robot; it is also known as a horizontal partial laryngectomy. This operation was initially developed as an open, transcervical surgery and then modified for endoscopic approaches. The open technique and tissue resected differs slightly from the endoscopic approach, and the open operation will be discussed later in this review. The European Laryngological Society has also published a classification system for endoscopic supraglottic laryngectomy based on the tissues removed (see Table 2. European Laryngological Society Endoscopic Supraglottic Laryngectomy Classification).[57] All procedures involve the removal of supraglottic tumors, making the patient's baseline lung function crucial to consider due to the relatively common occurrence of postoperative microaspiration. However, outcomes are favorable in appropriately selected candidates.[58] Extension of the tumor onto the true glottis or transglottic extension represents the only absolute contraindications to supraglottic laryngectomy.[59]
Table 2. European Laryngological Society Endoscopic Supraglottic Laryngectomy Classification
| Laryngectomy Types | Tissue Removed | Suitable Tumor Location |
| I | Limited excision for small superficial tumors | Free edge of the epiglottis, aryepiglottic fold, arytenoid, ventricular fold, or any other part of the supraglottis |
| IIa | Medial supraglottic laryngectomy without excision of pre-epiglottic space (superior hemi-epiglottectomy) | Suprahyoid supraglottis |
| IIb | Medial supraglottic laryngectomy without excision of pre-epiglottic space (total epiglottectomy) | Involving the laryngeal surface of epiglottis or other infrahyoid supraglottis |
| IIIa | Medial supraglottic laryngectomy with excision of pre-epiglottic space without resection of ventricular fold | T1-2 tumors of the infrahyoid endolaryngeal epiglottis without extension to ventricular fold |
| IIIb | Medial supraglottic laryngectomy with excision of pre-epiglottic space with resection of ventricular fold | T1-2 tumors of the infrahyoid endolaryngeal epiglottis with extension to ventricular fold |
| IVa | Lateral supraglottic laryngectomy with excision of ventricular fold | A 3-fold region with ventricular fold involvement |
| IVb | Lateral supraglottic laryngectomy with excision of arytenoid | A 3-fold region with arytenoid involvement |
Open Framework Surgery (Partial Laryngectomy)
Vertical partial laryngectomy: This procedure is used for T1, T2, and select T3 glottic tumors and is the open counterpart to endoscopic cordectomy. However, with the expanding use and availability of endoscopic resection, it is used far less commonly today. The classic operation removes the entire vocal cord en bloc with the underlying portion of thyroid cartilage from the anterior commissure to the ipsilateral arytenoid, sparing it. An extended vertical partial laryngectomy can encompass resection of part of the arytenoid or the entire anterior commissure, although this often necessitates resecting a portion of the contralateral vocal fold as well.[60] Subglottic extension, or involvement of more than one-third of the contralateral vocal fold, is a contraindication to vertical partial laryngectomy.[61]
Open supraglottic laryngectomy: This procedure is used for T1, T2, and select T3 tumors of the supraglottis. This operation involves the removal of all supraglottic structures, including the epiglottis, upper portion of the thyroid cartilage, hyoid, and false vocal folds. Contraindications include transglottic tumors, extensive tongue base involvement, cricoid cartilage involvement, pyriform sinus involvement beyond the uppermost edge, poor swallowing function or pulmonary reserve (as much of the protective function of the supraglottis will be compromised by resection and microaspiration is common postoperatively).[62] A temporary tracheostomy is typically required, but most patients are decannulated before discharge home from the hospital.
Supracricoid laryngectomy: This procedure is used for T3 and select T4 tumors of the glottis or supraglottis that are not amenable to supraglottic laryngectomy due to involvement of 1 or both vocal folds but spare at least 1 arytenoid. Resection includes the thyroid cartilage, both false vocal folds, and can include the ipsilateral true cord and arytenoid but must spare at least a functioning arytenoid.[63] Once the resection is performed, the remaining larynx is then suspended to the hyoid (cricohyoidopexy) or epiglottis (unless this was resected-cricohyoepiglottopexy) to maximize postoperative swallowing function.[63][64] A tracheostomy is required at the time of surgery and may also be required permanently. The voice outcomes are commonly described as poor after supracricoid laryngectomy, although they may be quite similar to their immediate preoperative voice. Most patients can phonate intelligibly and use a telephone.[65]
Total Laryngectomy
This surgery is employed for T3 and T4 diseases when cartilage invasion exists, the larynx is nonfunctional, or as a salvage option in cases of recurrence after prior radiation therapy. Total laryngectomy involves the removal of all supraglottic, glottic, and subglottic tissues from the tongue base to the superior trachea and the creation of a permanent tracheostoma in the anterior neck for respiration.[12] This surgery can be extended to include portions of the tongue base or lateral pharynx if there is tumor involvement (extended total laryngectomy) or can include the entire pharynx as well (laryngopharyngectomy). This completely separates the lower airways from the oral and nasal cavities, making intubation via the mouth or nose impossible, and the patient becomes an obligate "neck breather."
Reconstruction of the neopharynx will depend on the extent of the tumor and the amount of additional pharynx resected, as well as prior treatment. Primary closure is often possible if the remaining pharyngeal mucosa can be closed over a 10-mm Montgomery tube without tension. If this is not possible, a reconstructive flap (regional or free flap) should be considered.[66] The use of a vascularized flap for closure has been shown to decrease the rate of pharyngocutaneous fistula in those at high risk (prior radiation, extra-laryngeal extension, and extended laryngectomy), as well as decrease the duration of the fistula in those who develop it, and so should be routinely used in such patients.[67]
Radiation Oncology
Radiation therapy for laryngeal cancer is administered as external beam radiation therapy (as opposed to brachytherapy, where radiation-emitting implants are surgically placed into a tumor). A cumulative dose of ≥60 Gy (average 60-66 Gy) is considered curative for laryngeal carcinoma.[68][48] This total radiation dose is fractionated into smaller doses and administered at each treatment session to minimize adverse effects. A typical schedule is 1 fraction daily, Monday to Friday, for 6 weeks.[48] The radiation fields (volumes) can be further tailored to the tumor's shape and at-risk lymphatic beds (conformal radiotherapy) while minimizing radiation sequelae to surrounding structures. The radiation dose given at each fraction and the time between fractions can also be adjusted to minimize toxicity and maximize tumor effect.[69]
Intensity-Modulated Radiation Therapy
Intensity-modulated radiation therapy (IMRT) is the primary type of radiation therapy used in the treatment of laryngeal cancer (and in head and neck cancers in general). IMRT is a form of conformal radiation therapy that uses computers to generalize a map of the tumor and uses multiple external beam sources of varying intensities to maximize the radiation dose given to the tumor itself while minimizing exposure to surrounding structures. Despite its precision, IMRT can cause significant adverse effects, including dry mouth (xerostomia), dysphagia (which may be very delayed-onset, even developing years after treatment) [70], odynophagia, lymphedema of the neck, hypothyroidism, dysgeusia, and radiation dermatitis.[71] The most bothersome adverse effects during treatment include mucositis and dermatitis, as epithelial cells are very radiosensitive. The most significant long-term effects include hypothyroidism, xerostomia (the thyroid and salivary glands are also extremely radiosensitive), and dysphagia.[72][73]
Volumetric Modulated Arc Therapy
Volumetric modulated arc therapy is a variant of IMRT where a constant dose of radiation is given while the machine source rotates 360° (or 180° in half-arc planes) around the patient's body. This technique minimizes the exposure of non-tumor structures, particularly the carotid arteries, to radiation therapy doses.[74] Such protocols are sometimes used in treating laryngeal cancer, although not as frequently as static, opposed-wedge field IMRT plans.[75]
Hyperfractionated Radiation Therapy
Hyperfractionated radiation therapy involves administering a lower dose of radiation more frequently, typically twice daily, over the standard treatment course while keeping the overall radiation dosage the same. This approach may mitigate some radiation therapy toxicities but can be extremely inconvenient for patients.[76]
Hypofractionated Radiation Therapy
Hypofractionated radiation therapy is a treatment protocol where a higher dose (fraction) of radiation is administered each day over a shortened treatment course while maintaining the overall radiation dosage.[77]
Accelerated Fractionation Radiation Therapy
Accelerated fractionation radiation therapy is a treatment protocol where the standard dose (fraction) of radiation is administered each day over a shortened overall treatment course. For example, this might involve treating 6 days per week instead of 5, while maintaining the same overall radiation dosage.[78]
Treatment Planning
The basic tenet of laryngeal cancer treatment is that early-stage cancer (stages I and II) can often be managed with single-modality therapy, such as surgery or radiation therapy. Chemotherapy or immunotherapy alone is not typically used for curative treatment.[11] In contrast, late-stage cancer (stages III and IV) necessitates multimodal therapy, which may include surgery followed by radiation therapy, or primary radiation therapy combined with chemotherapy or immunotherapy, administered either concurrently or adjuvantly.[79] In early-stage cancer, survival outcomes are comparable whether treated with radiation therapy or surgery. Therefore, treatment decisions hinge on patient preferences and anatomical considerations that may preclude endoscopic or robotic surgery, with the secondary goal being laryngeal function preservation.[11]
Patients with advanced-stage cancer can achieve comparable overall survival with either treatment regimen, except in specific scenarios. If the larynx is nonfunctional or at a high risk after nonsurgical treatment, laryngectomy is preferred to prevent chronic aspiration and pneumonia risks.[80] Additionally, for patients with T4 laryngeal cancer or T3 due to cartilage invasion, primary laryngectomy offers superior oncologic outcomes compared to nonsurgical treatments.[12]
Medical Oncology
Chemotherapy and immunotherapy are integral components of combined-modality treatment for advanced-stage laryngeal cancer. Platinum-based chemotherapeutic agents (cisplatin and carboplatin) are first-line treatment for most laryngeal cancer, often combined with drugs such as 5-fluorouracil, paclitaxel, docetaxel, or methotrexate.[81] If patients are unable to receive chemotherapy often due to comorbid medical conditions, immunotherapy with agents such as cetuximab, pembrolizumab, and nivolumab is considered.[82] However, neither chemotherapy nor immunotherapy is administered alone with curative intent; they are primarily used for palliation purposes.
Most chemotherapy and immunotherapy are typically administered intravenously, often requiring long-term intravenous access such as an indwelling port. The timing of chemotherapy or immunotherapy administration is determined by the treating oncologist who considers the patient's overall medical condition and the concurrent treatment modalities.[83]
Concurrent chemotherapy: Concurrent chemotherapy involves administering chemotherapy simultaneously with radiation therapy. Typical schedules for cisplatin include weekly infusions for 7 cycles or slightly higher doses administered once every 3 weeks for a total of 3 cycles.[84] Concurrent chemoradiation therapy has been noted for its superior oncologic response, but it also comes with the most severe adverse effects, often causing challenges for patients to complete the concurrent treatment without any interruptions.[85]
Induction (neoadjuvant) chemotherapy: Induction chemotherapy involves administering chemotherapy before either surgery or definitive radiation therapy. The aim is to assess tumor response, potentially shrinking an unresectable tumor to make it resectable or enhancing the effects of definitive radiation therapy. Some studies have suggested an overall survival benefit in using induction chemotherapy for patients with resectable laryngeal cancer,[86] while several ongoing clinical trials are exploring its use followed by combined chemoradiation therapy or radiation therapy with a biologic agent.[87][88]
Adjuvant chemotherapy: Adjuvant chemotherapy involves administering chemotherapy, typically in combination with radiation therapy, after surgical resection of the tumor. It is used in advanced-stage laryngeal cancer with specific poor prognostic features observed on histology, including positive surgical margins, perineural invasion, lymphovascular invasion, and extracapsular spread or matted nodal disease, based on comparative trials from the European Organisation for Research and Treatment Of Cancer (EORTC) and the Radiation Therapy Oncology Group (RTOG).[89]
Staging
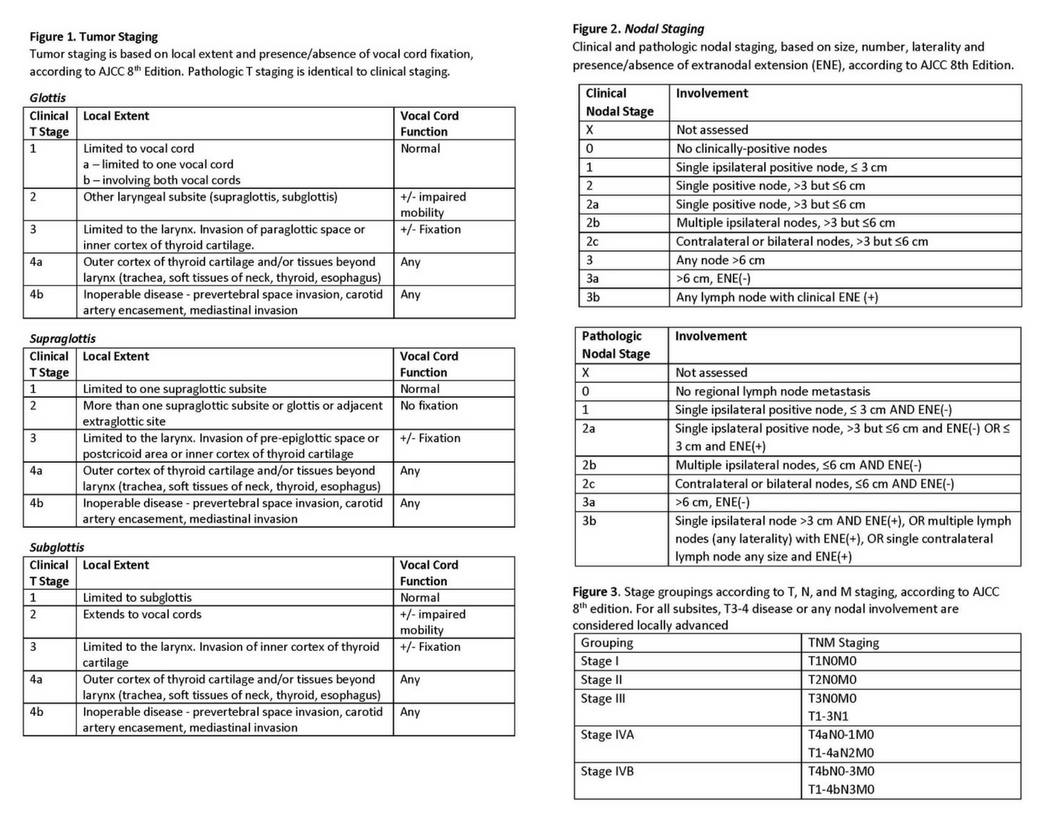
The staging of laryngeal cancer is critical for prognosis and treatment decisions, considering the specific subsite within the larynx affected, each carrying distinct implications. The American Joint Committee on Cancer (AJCC), Cancer Staging Manual, 8th ed. stages primary tumors in glottic and supraglottic cancers based on the local extent and whether vocal cord fixation is present or absent. In this staging system, pathological T staging is identical to clinical staging. Clinical and pathological nodal staging is based on factors such as size, number, laterality, and the presence or absence of extranodal extension.
Stage groupings according to T, N, and M staging consider the size and extent of the tumor (T), involvement of regional lymph nodes (N), and the presence of distant metastasis (M). Laryngeal cancers are staged from I to IV, with higher stages indicating more advanced disease. Stage I tumors are limited to the larynx, while stage IV tumors have spread extensively. T3 to T4 disease or any nodal involvement is considered locally advanced for all subsites (see Image. Tumor and Nodal Staging of Laryngeal Cancer).
Prognosis
The overall prognosis for laryngeal cancer is intimately linked to the stage of disease at the time of treatment. However, it is also affected by the patient's overall medical health and discontinuation of smoking. The overall 5-year survival rate for laryngeal cancer in the United States is 61%.[90]
If the cancer is confined to the larynx (stages I and II disease), the overall 5-year survival is 78%. Once positive nodal metastases are present (stage III disease), the 5-year survival drops to 46%, and if distant metastases are present, the 5-year survival is 34%.[91] Survivorship can be further examined by laryngeal subsite, with the overall prognosis being the best for glottic cancer, followed by supraglottic and then subglottic tumors.[92]
Glottis
The overall 5-year survival rate for glottic squamous cell carcinoma is 77%. If the disease is confined to the larynx (stages I and II), the 5-year survival rate is 84%. This drops to 52% if lymph node metastases are present (stage III disease) and further decreases to 45% in the setting of distant metastases.[93]
Supraglottis
The overall 5-year survival rate for supraglottic squamous cell carcinoma is 45%. If the disease is confined to the larynx (stages I and II), the 5-year survival rate is 61%. This drops to 46% if lymph node metastases are present (stage III disease) and further decreases to 30% in the setting of distant metastases.[94][95]
Subglottis
The overall 5-year survival rate for subglottic squamous cell carcinoma is 49%. If the disease is confined to the larynx (stages I and II), the 5-year survival rate is 59%. This drops to 38% if lymph node metastases are present (stage III disease) and further decreases to 44% in the setting of distant metastases.[96][97]
Complications
Complications of laryngeal cancer treatment will depend on the modality used and have been detailed in the respective treatment sections of this review. Common complications from radiation therapy include dysphonia, dysphagia, dysgeusia, mucositis, dermatitis, hypothyroidism, and persistent dry mouth. Surgical complications vary with the type of surgery performed and can include dysphonia, aphonia, aspiration, pharyngocutaneous fistula, shoulder weakness, bleeding, and infection.
Deterrence and Patient Education
Deterrence and prevention of laryngeal cancer primarily revolve around mitigating risk factors and promoting healthy lifestyle choices. Chief among these is the cessation of tobacco use, as smoking is the leading cause of laryngeal cancer. Encouraging smoking cessation programs and providing support for those looking to quit can significantly reduce the incidence of this disease. Similarly, limiting alcohol consumption, as well as avoiding excessive exposure to environmental carcinogens such as asbestos and certain industrial chemicals, can help decrease the risk of laryngeal cancer. Promoting a diet rich in fruits and vegetables, which are high in antioxidants and protective nutrients, may offer some protective benefits. Education campaigns highlighting the importance of regular screening and early detection can also aid in identifying precancerous lesions or early-stage tumors, potentially leading to more successful treatment outcomes.
Enhancing Healthcare Team Outcomes
Managing laryngeal cancer requires an interprofessional team of healthcare providers comprising an ENT surgeon, oncologist, dietitian, pulmonologist, speech therapist, intensivist, radiation therapist, advanced care practitioner, nurses, pharmacists, and other healthcare professionals. Most patients initially present with symptoms such as hoarseness, otalgia, dysphagia, and weight loss to their primary care clinician. Typically, these patients are male with a history of current or past tobacco smoking. A referral to an ENT surgeon should be considered if hoarseness persists and is accompanied by other signs suggestive of malignancy.
The most crucial aspect of the physical examination involves an invasive assessment of the primary lesion, which includes indirect laryngoscopy, mirror examination, and often fiberoptic endoscopy. Given the complexity of laryngeal cancer, a multidisciplinary approach is essential for evaluation and follow-up therapy. Collaborative strategic planning is crucial to coordinate care effectively, requiring healthcare professionals to collaborate closely to develop comprehensive treatment plans tailored to each patient's unique needs and preferences.
Post-treatment complications are common, necessitating close monitoring for airway patency. While outcomes for early-stage laryngeal cancer are generally favorable, those with advanced cancer face a more challenging prognosis.[98][99] Patient and family education is crucial, covering procedures and follow-up care to optimize outcomes. Care coordination also includes connecting patients with support services such as speech therapy, nutritional counseling, or palliative care, addressing their holistic needs to enhance overall quality of life.
Media
(Click Image to Enlarge)
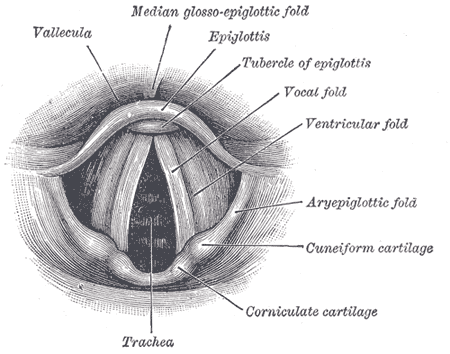
Anatomy of the Larynx. Laryngoscopic view showing the interior structures of the larynx, including the vallecula, epiglottis, tubercle of the epiglottis, vocal fold, ventricular fold, aryepiglottic fold, cuneiform cartilage, corniculate cartilage, and trachea.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Ciolofan MS, Vlăescu AN, Mogoantă CA, Ioniță E, Ioniță I, Căpitănescu AN, Mitroi MR, Anghelina F. Clinical, Histological and Immunohistochemical Evaluation of Larynx Cancer. Current health sciences journal. 2017 Oct-Dec:43(4):367-375. doi: 10.12865/CHSJ.43.04.14. Epub 2017 Dec 28 [PubMed PMID: 30595905]
Mowery A, Conlin M, Clayburgh D. Increased risk of head and neck cancer in Agent Orange exposed Vietnam Era veterans. Oral oncology. 2020 Jan:100():104483. doi: 10.1016/j.oraloncology.2019.104483. Epub 2019 Dec 3 [PubMed PMID: 31810040]
Astl J, Holy R, Tuckova I, Belsan T, Pala M, Rotnagl J. Sarcomas of the Larynx: One Institution's Experience and Treatment Protocol Analyses. Medicina (Kaunas, Lithuania). 2021 Feb 25:57(3):. doi: 10.3390/medicina57030192. Epub 2021 Feb 25 [PubMed PMID: 33668739]
Doğan S, Vural A, Kahriman G, İmamoğlu H, Abdülrezzak Ü, Öztürk M. Non-squamous cell carcinoma diseases of the larynx: clinical and imaging findings. Brazilian journal of otorhinolaryngology. 2020 Jul-Aug:86(4):468-482. doi: 10.1016/j.bjorl.2019.02.003. Epub 2019 Mar 16 [PubMed PMID: 30956151]
Aggarwal S, Kaushal V, Singla S, Sen R. Primary glottic malignant melanoma of the larynx (PGMML): a very rare entity. BMJ case reports. 2015 Nov 20:2015():. doi: 10.1136/bcr-2015-211317. Epub 2015 Nov 20 [PubMed PMID: 26590185]
Level 3 (low-level) evidenceFerraguti G, Terracina S, Petrella C, Greco A, Minni A, Lucarelli M, Agostinelli E, Ralli M, de Vincentiis M, Raponi G, Polimeni A, Ceccanti M, Caronti B, Di Certo MG, Barbato C, Mattia A, Tarani L, Fiore M. Alcohol and Head and Neck Cancer: Updates on the Role of Oxidative Stress, Genetic, Epigenetics, Oral Microbiota, Antioxidants, and Alkylating Agents. Antioxidants (Basel, Switzerland). 2022 Jan 11:11(1):. doi: 10.3390/antiox11010145. Epub 2022 Jan 11 [PubMed PMID: 35052649]
Peng WJ, Mi J, Jiang YH. Asbestos exposure and laryngeal cancer mortality. The Laryngoscope. 2016 May:126(5):1169-74. doi: 10.1002/lary.25693. Epub 2015 Sep 29 [PubMed PMID: 26418833]
Kang DM, Kim JE, Kim YK, Lee HH, Kim SY. Occupational Burden of Asbestos-Related Diseases in Korea, 1998-2013: Asbestosis, Mesothelioma, Lung Cancer, Laryngeal Cancer, and Ovarian Cancer. Journal of Korean medical science. 2018 Aug 27:33(35):e226. doi: 10.3346/jkms.2018.33.e226. Epub 2018 Jul 19 [PubMed PMID: 30140191]
Wittekindt C, Wuerdemann N, Gattenlöhner S, Brobeil A, Wierzbicka M, Wagner S, Klußmann JP. The role of high-risk human papillomavirus infections in laryngeal squamous cell carcinoma. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2017 Nov:274(11):3837-3842. doi: 10.1007/s00405-017-4718-1. Epub 2017 Aug 31 [PubMed PMID: 28861601]
George P, Mani S, Abraham P, Michael RC. The Association of Human Papillomavirus in Benign and Malignant Laryngeal Lesions-a Pilot Study. Indian journal of surgical oncology. 2021 Jun:12(2):306-310. doi: 10.1007/s13193-020-01127-1. Epub 2020 Jun 13 [PubMed PMID: 34295074]
Level 3 (low-level) evidenceBaird BJ, Sung CK, Beadle BM, Divi V. Treatment of early-stage laryngeal cancer: A comparison of treatment options. Oral oncology. 2018 Dec:87():8-16. doi: 10.1016/j.oraloncology.2018.09.012. Epub 2018 Oct 16 [PubMed PMID: 30527248]
Department of Veterans Affairs Laryngeal Cancer Study Group, Wolf GT, Fisher SG, Hong WK, Hillman R, Spaulding M, Laramore GE, Endicott JW, McClatchey K, Henderson WG. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The New England journal of medicine. 1991 Jun 13:324(24):1685-90 [PubMed PMID: 2034244]
Level 1 (high-level) evidenceHoffmann TK. [Systemic therapy strategies for head-neck carcinomas: current status]. Laryngo- rhino- otologie. 2012 Mar:91 Suppl 1():S123-43. doi: 10.1055/s-0031-1297244. Epub 2012 Mar 28 [PubMed PMID: 22456917]
Chiesa-Estomba CM, Barillari MR, Mayo-Yáñez M, Maniaci A, Fakhry N, Cammaroto G, Ayad T, Lechien JR. Non-Squamous Cell Carcinoma of the Larynx: A State-of-the-Art Review. Journal of personalized medicine. 2023 Jun 30:13(7):. doi: 10.3390/jpm13071084. Epub 2023 Jun 30 [PubMed PMID: 37511697]
Zeitels SM, Baird BJ. Surgical Treatment Strategies for Laryngeal Chondrosarcomas: A Single Institution Investigation. The Laryngoscope. 2022 Jan:132(1):169-176. doi: 10.1002/lary.29762. Epub 2021 Jul 22 [PubMed PMID: 34291467]
Menach P, Oburra HO, Patel A. Cigarette smoking and alcohol ingestion as risk factors for laryngeal squamous cell carcinoma at kenyatta national hospital, kenya. Clinical medicine insights. Ear, nose and throat. 2012:5():17-24. doi: 10.4137/CMENT.S8610. Epub 2012 Oct 11 [PubMed PMID: 24179405]
Bhattacharyya S, Mandal S, Banerjee S, Mandal GK, Bhowmick AK, Murmu N. Cannabis smoke can be a major risk factor for early-age laryngeal cancer--a molecular signaling-based approach. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015 Aug:36(8):6029-36. doi: 10.1007/s13277-015-3279-4. Epub 2015 Mar 4 [PubMed PMID: 25736926]
Ramroth H, Dietz A, Becher H. Environmental tobacco smoke and laryngeal cancer: results from a population-based case-control study. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2008 Nov:265(11):1367-71. doi: 10.1007/s00405-008-0651-7. Epub 2008 Apr 1 [PubMed PMID: 18379814]
Level 2 (mid-level) evidenceGallaway MS, Henley SJ, Steele CB, Momin B, Thomas CC, Jamal A, Trivers KF, Singh SD, Stewart SL. Surveillance for Cancers Associated with Tobacco Use - United States, 2010-2014. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C. : 2002). 2018 Nov 2:67(12):1-42. doi: 10.15585/mmwr.ss6712a1. Epub 2018 Nov 2 [PubMed PMID: 30383737]
Igissin N, Zatonskikh V, Telmanova Z, Tulebaev R, Moore M. Laryngeal Cancer: Epidemiology, Etiology, and Prevention: A Narrative Review. Iranian journal of public health. 2023 Nov:52(11):2248-2259. doi: 10.18502/ijph.v52i11.14025. Epub [PubMed PMID: 38106821]
Level 3 (low-level) evidenceD'Souza AM, Mark J, Demarcantonio M, Leino D, Sisson R, Geller JI. Pediatric laryngeal carcinoma in a heterozygous carrier of Fanconi anemia. Pediatric blood & cancer. 2017 Aug:64(8):. doi: 10.1002/pbc.26463. Epub 2017 Jan 31 [PubMed PMID: 28139070]
Ferster APO, Schubart J, Kim Y, Goldenberg D. Association Between Laryngeal Cancer and Asbestos Exposure: A Systematic Review. JAMA otolaryngology-- head & neck surgery. 2017 Apr 1:143(4):409-416. doi: 10.1001/jamaoto.2016.3421. Epub [PubMed PMID: 27918783]
Level 1 (high-level) evidenceSeilkop SK, Lightfoot NE, Berriault CJ, Conard BR. Respiratory cancer mortality and incidence in an updated cohort of Canadian nickel production workers. Archives of environmental & occupational health. 2017 Jul 4:72(4):204-219. doi: 10.1080/19338244.2016.1199532. Epub 2016 Jun 9 [PubMed PMID: 27282555]
Zitricky F, Koskinen AI, Hemminki O, Försti A, Hemminki A, Hemminki K. Survival in oral and pharyngeal cancers is catching up with laryngeal cancer in the NORDIC countries through a half century. Cancer medicine. 2024 Jan 2:13(1):. doi: 10.1002/cam4.6867. Epub 2024 Jan 2 [PubMed PMID: 38164108]
Virtaniemi JA, Hirvikoski PP, Kumpulainen EJ, Johansson RT, Pukkala E, Kosma VM. Is the subsite distribution of laryngeal cancer related to smoking habits? Acta oncologica (Stockholm, Sweden). 2000:39(1):77-9 [PubMed PMID: 10752658]
Misono S, Marmor S, Yueh B, Virnig BA. Treatment and survival in 10,429 patients with localized laryngeal cancer: a population-based analysis. Cancer. 2014 Jun 15:120(12):1810-7. doi: 10.1002/cncr.28608. Epub 2014 Mar 17 [PubMed PMID: 24639148]
Amanian A, Anderson DW, Durham JS, Prisman E, Ng T, Hu A. Treatment of Laryngeal Verrucous Carcinoma: 28-Year Retrospective Cohort Study and Literature Review. OTO open. 2023 Apr-Jun:7(2):e50. doi: 10.1002/oto2.50. Epub 2023 Jun 1 [PubMed PMID: 37275458]
Level 2 (mid-level) evidenceOztürkcan S, Katilmiş H, Ozdemir I, Tuna B, Güvenç IA, Dündar R. Occult contralateral nodal metastases in supraglottic laryngeal cancer crossing the midline. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2009 Jan:266(1):117-20. doi: 10.1007/s00405-008-0721-x. Epub 2008 Jun 10 [PubMed PMID: 18542980]
Khan U, MacKay C, Rigby M, Trites J, Corsten M, Taylor SM. Management of positive resection margins following transoral laser microsurgery for glottic cancer. Laryngoscope investigative otolaryngology. 2023 Dec:8(6):1579-1583. doi: 10.1002/lio2.1184. Epub 2023 Nov 23 [PubMed PMID: 38130264]
O'Sullivan B, Warde P, Keane T, Irish J, Cummings B, Payne D. Outcome following radiotherapy in verrucous carcinoma of the larynx. International journal of radiation oncology, biology, physics. 1995 Jun 15:32(3):611-7 [PubMed PMID: 7790246]
Level 2 (mid-level) evidenceBallo MT, Garden AS, El-Naggar AK, Gillenwater AM, Morrison WH, Goepfert H, Ang KK. Radiation therapy for early stage (T1-T2) sarcomatoid carcinoma of true vocal cords: outcomes and patterns of failure. The Laryngoscope. 1998 May:108(5):760-3 [PubMed PMID: 9591559]
Level 2 (mid-level) evidenceHaddad G, Sataloff RT, Hamdan AL. Laryngeal Metastatic Lesions: A Literature Review. Journal of voice : official journal of the Voice Foundation. 2022 Jul 30:():. pii: S0892-1997(22)00170-9. doi: 10.1016/j.jvoice.2022.06.016. Epub 2022 Jul 30 [PubMed PMID: 35918235]
Cîrstea AI, Berteșteanu ȘVG, Scăunașu RV, Popescu B, Bejenaru PL, Simion-Antonie CB, Berteșteanu GS, Diaconu TE, Taher PB, Rujan SA, Oașă ID, Grigore R. Management of Locally Advanced Laryngeal Cancer-From Risk Factors to Treatment, the Experience of a Tertiary Hospital from Eastern Europe. International journal of environmental research and public health. 2023 Mar 8:20(6):. doi: 10.3390/ijerph20064737. Epub 2023 Mar 8 [PubMed PMID: 36981644]
Knief J, Herber K, Muenscher A, Thorns C, Moeckelmann N. Tumor-stroma ratio in preoperative biopsies and matched surgical specimens in oral squamous cell carcinoma: Concordance and impact on recurrence-free and overall survival. Pathology, research and practice. 2024 Mar:255():155211. doi: 10.1016/j.prp.2024.155211. Epub 2024 Feb 16 [PubMed PMID: 38368663]
Tran CL, Han M, Kim B, Park EY, Kim YI, Oh JK. Gastroesophageal reflux disease and risk of cancer: Findings from the Korean National Health Screening Cohort. Cancer medicine. 2023 Sep:12(18):19163-19173. doi: 10.1002/cam4.6500. Epub 2023 Sep 7 [PubMed PMID: 37676071]
Jahshan F, Marshak T, Qarawany J, Markel B, Sberro A, Lahav Y, Layous E, Eisenbach N, Shochat I, Sela E, Ronen O. Incidental Laryngeal Findings in Routine Laryngopharyngeal Reflux Diagnosis. The Israel Medical Association journal : IMAJ. 2024 Jan:26(1):40-44 [PubMed PMID: 38420641]
Wang W, Wang W, Zhang D, Zeng P, Wang Y, Lei M, Hong Y, Cai C. Creation of a machine learning-based prognostic prediction model for various subtypes of laryngeal cancer. Scientific reports. 2024 Mar 18:14(1):6484. doi: 10.1038/s41598-024-56687-x. Epub 2024 Mar 18 [PubMed PMID: 38499632]
Osazuwa-Peters N, Adjei Boakye E, Hussaini AS, Sujijantarat N, Ganesh RN, Snider M, Thompson D, Varvares MA. Characteristics and predictors of oral cancer knowledge in a predominantly African American community. PloS one. 2017:12(5):e0177787. doi: 10.1371/journal.pone.0177787. Epub 2017 May 17 [PubMed PMID: 28545057]
Noordman BJ, Comans E, Lips P, Rinkel RN. False-positive uptake of 124I in a laryngeal cyst mimicking thyroid remnant after thyroidectomy and 131i therapy for follicular thyroid carcinoma. Clinical nuclear medicine. 2014 Oct:39(10):898-9. doi: 10.1097/RLU.0000000000000360. Epub [PubMed PMID: 24561682]
Al-Ibraheem A, Abdlkadir AS, Shagera QA, Saraireh O, Al-Adhami D, Al-Rashdan R, Anwar F, Moghrabi S, Mohamad I, Muylle K, Estrada E, Paez D, Mansour A, Lopci E. The Diagnostic and Predictive Value of (18)F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Laryngeal Squamous Cell Carcinoma. Cancers. 2023 Nov 17:15(22):. doi: 10.3390/cancers15225461. Epub 2023 Nov 17 [PubMed PMID: 38001720]
Pakkanen P, Irjala H, Ilmarinen T, Halme E, Lindholm P, Mäkitie A, Wigren T, Aaltonen LM. Survival and Larynx Preservation in Early Glottic Cancer: A Randomized Trial Comparing Laser Surgery and Radiation Therapy. International journal of radiation oncology, biology, physics. 2022 May 1:113(1):96-100. doi: 10.1016/j.ijrobp.2022.01.010. Epub 2022 Feb 11 [PubMed PMID: 35164976]
Level 1 (high-level) evidencePrasad A, Carey RM, Panara K, Rajasekaran K, Cannady SB, Newman JG, Brant JA, Brody RM. Nodal metastasis in surgically treated laryngeal squamous cell carcinoma. Head & neck. 2023 Sep:45(9):2303-2312. doi: 10.1002/hed.27437. Epub 2023 Jul 5 [PubMed PMID: 37403903]
Forastiere AA, Ismaila N, Lewin JS, Nathan CA, Adelstein DJ, Eisbruch A, Fass G, Fisher SG, Laurie SA, Le QT, O'Malley B, Mendenhall WM, Patel S, Pfister DG, Provenzano AF, Weber R, Weinstein GS, Wolf GT. Use of Larynx-Preservation Strategies in the Treatment of Laryngeal Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018 Apr 10:36(11):1143-1169. doi: 10.1200/JCO.2017.75.7385. Epub 2017 Nov 27 [PubMed PMID: 29172863]
Level 1 (high-level) evidenceRosenthal DI, Mohamed AS, Weber RS, Garden AS, Sevak PR, Kies MS, Morrison WH, Lewin JS, El-Naggar AK, Ginsberg LE, Kocak-Uzel E, Ang KK, Fuller CD. Long-term outcomes after surgical or nonsurgical initial therapy for patients with T4 squamous cell carcinoma of the larynx: A 3-decade survey. Cancer. 2015 May 15:121(10):1608-19. doi: 10.1002/cncr.29241. Epub 2015 Jan 13 [PubMed PMID: 25586197]
Level 3 (low-level) evidenceRuytenberg T, Verbist BM, Vonk-Van Oosten J, Astreinidou E, Sjögren EV, Webb AG. Improvements in High Resolution Laryngeal Magnetic Resonance Imaging for Preoperative Transoral Laser Microsurgery and Radiotherapy Considerations in Early Lesions. Frontiers in oncology. 2018:8():216. doi: 10.3389/fonc.2018.00216. Epub 2018 Jun 6 [PubMed PMID: 29928638]
Zhang MJ, Mu JW, Chen XR, Zhang X, Feng C. Effect of voice rehabilitation training on the patients with laryngeal cancer after radiotherapy. Medicine. 2018 Jun:97(26):e11268. doi: 10.1097/MD.0000000000011268. Epub [PubMed PMID: 29953001]
Dziegielewski PT, Reschly WJ, Morris CG, DeJesus RD, Silver N, Boyce BJ, Santiago I 3rd, Amdur RJ, Mendenhall WM. Tumor volume as a predictor of survival in T3 glottic carcinoma: A novel approach to patient selection. Oral oncology. 2018 Apr:79():47-54. doi: 10.1016/j.oraloncology.2018.02.015. Epub 2018 Feb 22 [PubMed PMID: 29598950]
Yamazaki H, Suzuki G, Nakamura S, Yoshida K, Konishi K, Teshima T, Ogawa K. Radiotherapy for laryngeal cancer-technical aspects and alternate fractionation. Journal of radiation research. 2017 Jul 1:58(4):495-508. doi: 10.1093/jrr/rrx023. Epub [PubMed PMID: 28898958]
Dixon LM, Douglas CM, Shaukat SI, Garcez K, Lee LW, Sykes AJ, Thomson D, Slevin NJ. Conventional fractionation should not be the standard of care for T2 glottic cancer. Radiation oncology (London, England). 2017 Nov 14:12(1):178. doi: 10.1186/s13014-017-0915-8. Epub 2017 Nov 14 [PubMed PMID: 29137654]
Bloomer CH, Gavrila E, Burcher KM, Kalada JM, Chang MJ, Gebeyehu RR, Asare E, Khoury LM, Kinney R, Frizzell B, Sullivan CA, Bunch PM, Porosnicu M. Exceptional response to cetuximab monotherapy after failure of immunotherapy with a checkpoint inhibitor in a patient with metastatic head and neck squamous cell cancer: case report and review of the literature. Therapeutic advances in medical oncology. 2023:15():17588359231193722. doi: 10.1177/17588359231193722. Epub 2023 Aug 31 [PubMed PMID: 37667781]
Level 3 (low-level) evidenceSchlachtenberger G, Doerr F, Menghesha H, Lauinger P, Wolber P, Sabashnikov A, Popov AF, Macherey-Meyer S, Bennink G, Klussmann JP, Wahlers T, Hekmat K, Heldwein MB. Patients with Pulmonary Metastases from Head and Neck Cancer Benefit from Pulmonary Metastasectomy, A Systematic Review. Medicina (Kaunas, Lithuania). 2022 Jul 27:58(8):. doi: 10.3390/medicina58081000. Epub 2022 Jul 27 [PubMed PMID: 35893115]
Level 1 (high-level) evidenceGasne C, Atallah S, Dauzier E, Thariat J, Fakhry N, Verillaud B, Classe M, Vergez S, Moya-Plana A, Costes-Martineau V, Righini C, de Gabory L, Digue L, Dupin C, Ferrand FR, Even C, Baujat B, REFCOR members. Twelve years after: The french national network on rare head and neck tumours (REFCOR). Oral oncology. 2024 Apr:151():106762. doi: 10.1016/j.oraloncology.2024.106762. Epub 2024 Mar 20 [PubMed PMID: 38513311]
Gazda P, Baujat B, Sarini J, Gomez-Brouchet A, Philouze P, Moya-Plana A, Malard O, Fakhry N, De Mones Del Pujol E, Garrel R, Page C, Mouawad F, Vaz E, Evrard D, Bach C, Dufour X, Lelonge Y, Schultz P, Mauvais O, Brenet E, Vergez S, Atallah S. Functional or radical surgical treatment of laryngeal chondrosarcoma, analysis of survival and prognostic factors: A REFCOR and NetSarc-ResOs multicenter study of 74 cases. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2024 Feb:50(2):107315. doi: 10.1016/j.ejso.2023.107315. Epub 2023 Dec 22 [PubMed PMID: 38219696]
Level 2 (mid-level) evidenceSuppah M, Kamal A, Karle WE, Saadoun R, Lott DG. Outcomes of KTP Laser Ablation in Glottic Neoplasms: A Systematic Review and Meta-Analysis. The Laryngoscope. 2023 Aug:133(8):1806-1814. doi: 10.1002/lary.30547. Epub 2023 Jan 6 [PubMed PMID: 36606671]
Level 1 (high-level) evidenceRemacle M, Eckel HE, Antonelli A, Brasnu D, Chevalier D, Friedrich G, Olofsson J, Rudert HH, Thumfart W, de Vincentiis M, Wustrow TP. Endoscopic cordectomy. A proposal for a classification by the Working Committee, European Laryngological Society. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2000:257(4):227-31 [PubMed PMID: 10867840]
Olsen KD, Thomas JV, DeSanto LW, Suman VJ. Indications and results of cordectomy for early glottic carcinoma. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1993 Mar:108(3):277-82 [PubMed PMID: 8464642]
Remacle M, Hantzakos A, Eckel H, Evrard AS, Bradley PJ, Chevalier D, Djukic V, de Vincentiis M, Friedrich G, Olofsson J, Peretti G, Quer M, Werner J. Endoscopic supraglottic laryngectomy: a proposal for a classification by the working committee on nomenclature, European Laryngological Society. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2009 Jul:266(7):993-8. doi: 10.1007/s00405-008-0901-8. Epub 2009 Jan 8 [PubMed PMID: 19130072]
Davis RK, Kriskovich MD, Galloway EB 3rd, Buntin CS, Jepsen MC. Endoscopic supraglottic laryngectomy with postoperative irradiation. The Annals of otology, rhinology, and laryngology. 2004 Feb:113(2):132-8 [PubMed PMID: 14994769]
Carta F, Mariani C, Sambiagio GB, Chuchueva N, Lecis E, Gerosa C, Puxeddu R. CO(2) Transoral Microsurgery for Supraglottic Squamous Cell Carcinoma. Frontiers in oncology. 2018:8():321. doi: 10.3389/fonc.2018.00321. Epub 2018 Sep 4 [PubMed PMID: 30234007]
Yan H, Wu D, Mai JH, Zhao Z, Xu P, Liao L, Lin H, Zhang XR, Liu XK. Laryngeal function-preserving of frontolateral vertical partial laryngectomy (FLVPL) for selected T4a glottic cancer with thyroid cartilage invasion adherence to the anterior commissure: an innovative attempt. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2022 Dec:279(12):5735-5740. doi: 10.1007/s00405-022-07459-8. Epub 2022 Jun 9 [PubMed PMID: 35680654]
Lei WB, Jiang AY, Chai LP, Zhu XL, Wang ZF, Wen YH, Su ZZ, Wen WP. Middle frontal horizontal partial laryngectomy (MFHPL): a treatment for stage T1b squamous cell carcinoma of the glottic larynx involving anterior vocal commissure. PloS one. 2013:8(1):e52723. doi: 10.1371/journal.pone.0052723. Epub 2013 Jan 9 [PubMed PMID: 23326350]
Mourad M, Sadoughi B. Transcervical Conservation Laryngeal Surgery: An Anatomic Understanding to Enhance Functional and Oncologic Outcomes. Otolaryngologic clinics of North America. 2015 Aug:48(4):703-15. doi: 10.1016/j.otc.2015.04.014. Epub [PubMed PMID: 26233793]
Level 3 (low-level) evidenceSaturno M, Shaari AL, Yun J, Wein LE, Shaari D, Kappauf C, Laitman BM, Chai RL. Outcomes of Supracricoid Partial Laryngectomy Performed in the United States: A Systematic Review. The Laryngoscope. 2024 Jul:134(7):3003-3011. doi: 10.1002/lary.31273. Epub 2024 Jan 22 [PubMed PMID: 38251796]
Level 1 (high-level) evidenceGrasso M, Fusconi M, De Luca P, Camaioni A, Belizzi M, Flaccadoro F, Agolli G, Ruoppolo G, de Vincentiis M, Di Maria D, Ralli M, Di Stadio A, Colizza A, Greco A. Partial Horizontal Supracricoid Laryngectomy: Which Factors Impact on Post-decannulation Swallowing Outcomes? A Prospective Single-Center Experience. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2023 Sep:75(3):1917-1922. doi: 10.1007/s12070-023-03790-6. Epub 2023 Apr 20 [PubMed PMID: 37636747]
Palmer AD, Graville DJ, Bolognone RK, Gorecki J, Groth S, March J, Schindler JS. Longitudinal Voice Outcomes and Neoglottic Function After Supracricoid Partial Laryngectomy: The Development of a New Scale. The Annals of otology, rhinology, and laryngology. 2023 Oct:132(10):1206-1215. doi: 10.1177/00034894221141518. Epub 2022 Dec 21 [PubMed PMID: 36541624]
Agrawal N, Goldenberg D. Primary and salvage total laryngectomy. Otolaryngologic clinics of North America. 2008 Aug:41(4):771-80, vii. doi: 10.1016/j.otc.2008.02.001. Epub [PubMed PMID: 18570958]
Patel UA, Moore BA, Wax M, Rosenthal E, Sweeny L, Militsakh ON, Califano JA, Lin AC, Hasney CP, Butcher RB, Flohr J, Arnaoutakis D, Huddle M, Richmon JD. Impact of pharyngeal closure technique on fistula after salvage laryngectomy. JAMA otolaryngology-- head & neck surgery. 2013 Nov:139(11):1156-62. doi: 10.1001/jamaoto.2013.2761. Epub [PubMed PMID: 23576219]
Level 2 (mid-level) evidenceMendenhall WM, Werning JW, Hinerman RW, Amdur RJ, Villaret DB. Management of T1-T2 glottic carcinomas. Cancer. 2004 May 1:100(9):1786-92 [PubMed PMID: 15112257]
Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, Horiot JC, Le Maître A, Pajak TF, Poulsen MG, O'Sullivan B, Dobrowsky W, Hliniak A, Skladowski K, Hay JH, Pinto LH, Fallai C, Fu KK, Sylvester R, Pignon JP, Meta-Analysis of Radiotherapy in Carcinomas of Head and neck (MARCH) Collaborative Group. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet (London, England). 2006 Sep 2:368(9538):843-54 [PubMed PMID: 16950362]
Level 1 (high-level) evidenceDe Felice F, de Vincentiis M, Luzzi V, Magliulo G, Tombolini M, Ruoppolo G, Polimeni A. Late radiation-associated dysphagia in head and neck cancer patients: evidence, research and management. Oral oncology. 2018 Feb:77():125-130. doi: 10.1016/j.oraloncology.2017.12.021. Epub 2018 Jan 4 [PubMed PMID: 29362118]
Brook I. Early side effects of radiation treatment for head and neck cancer. Cancer radiotherapie : journal de la Societe francaise de radiotherapie oncologique. 2021 Jul:25(5):507-513. doi: 10.1016/j.canrad.2021.02.001. Epub 2021 Mar 5 [PubMed PMID: 33685809]
Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, Thorstad W, Wagner H, Ensley JF, Cooper JS. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 Mar 1:31(7):845-52. doi: 10.1200/JCO.2012.43.6097. Epub 2012 Nov 26 [PubMed PMID: 23182993]
Level 1 (high-level) evidenceMassonet H, Goeleven A, Van den Steen L, Vergauwen A, Baudelet M, Van Haesendonck G, Vanderveken O, Bollen H, van der Molen L, Duprez F, Tomassen P, Nuyts S, Van Nuffelen G. Home-based intensive treatment of chronic radiation-associated dysphagia in head and neck cancer survivors (HIT-CRAD trial). Trials. 2022 Oct 22:23(1):893. doi: 10.1186/s13063-022-06832-6. Epub 2022 Oct 22 [PubMed PMID: 36273210]
Riegel AC, Antone J, Schwartz DL. Comparative dosimetry of volumetric modulated arc therapy and limited-angle static intensity-modulated radiation therapy for early-stage larynx cancer. Medical dosimetry : official journal of the American Association of Medical Dosimetrists. 2013 Spring:38(1):66-9. doi: 10.1016/j.meddos.2012.07.002. Epub 2012 Aug 15 [PubMed PMID: 22901745]
Level 2 (mid-level) evidenceGujral DM, Long M, Roe JW, Harrington KJ, Nutting CM. Standardisation of Target Volume Delineation for Carotid-sparing Intensity-modulated Radiotherapy in Early Glottis Cancer. Clinical oncology (Royal College of Radiologists (Great Britain)). 2017 Jan:29(1):42-50. doi: 10.1016/j.clon.2016.09.017. Epub 2016 Nov 1 [PubMed PMID: 27815039]
Lacas B, Bourhis J, Overgaard J, Zhang Q, Grégoire V, Nankivell M, Zackrisson B, Szutkowski Z, Suwiński R, Poulsen M, O'Sullivan B, Corvò R, Laskar SG, Fallai C, Yamazaki H, Dobrowsky W, Cho KH, Beadle B, Langendijk JA, Viegas CMP, Hay J, Lotayef M, Parmar MKB, Aupérin A, van Herpen C, Maingon P, Trotti AM, Grau C, Pignon JP, Blanchard P, MARCH Collaborative Group. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. The Lancet. Oncology. 2017 Sep:18(9):1221-1237. doi: 10.1016/S1470-2045(17)30458-8. Epub 2017 Jul 27 [PubMed PMID: 28757375]
Level 1 (high-level) evidenceAkbaba S, Lang K, Held T, Bulut OC, Mattke M, Uhl M, Jensen A, Plinkert P, Rieken S, Herfarth K, Debus J, Adeberg S. Accelerated Hypofractionated Active Raster-Scanned Carbon Ion Radiotherapy (CIRT) for Laryngeal Malignancies: Feasibility and Safety. Cancers. 2018 Oct 18:10(10):. doi: 10.3390/cancers10100388. Epub 2018 Oct 18 [PubMed PMID: 30340397]
Level 2 (mid-level) evidenceKaanders JH, van Daal WA, Hoogenraad WJ, van der Kogel AJ. Accelerated fractionation radiotherapy for laryngeal cancer, acute, and late toxicity. International journal of radiation oncology, biology, physics. 1992:24(3):497-503 [PubMed PMID: 1399736]
Carew JF, Shah JP. Advances in multimodality therapy for laryngeal cancer. CA: a cancer journal for clinicians. 1998 Jul-Aug:48(4):211-28 [PubMed PMID: 9676535]
Level 3 (low-level) evidenceMilinis K, King R, Lancaster J, Brooker R, Zammitt R, Wilkie MD, Fleming JC, Davies K. Predictors of non-functional larynx following (chemo)radiotherapy for locally advanced laryngeal cancer. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2023 Sep:48(5):773-778. doi: 10.1111/coa.14074. Epub 2023 May 9 [PubMed PMID: 37577927]
Obid R, Redlich M, Tomeh C. The Treatment of Laryngeal Cancer. Oral and maxillofacial surgery clinics of North America. 2019 Feb:31(1):1-11. doi: 10.1016/j.coms.2018.09.001. Epub [PubMed PMID: 30449522]
Campbell G, Glazer TA, Kimple RJ, Bruce JY. Advances in Organ Preservation for Laryngeal Cancer. Current treatment options in oncology. 2022 Apr:23(4):594-608. doi: 10.1007/s11864-022-00945-5. Epub 2022 Mar 18 [PubMed PMID: 35303749]
Level 3 (low-level) evidenceShah NK, Qureshi MM, Dyer MA, Patel SA, Kim K, Everett PC, Grillone GA, Jalisi SM, Truong MT. Optimal sequencing of chemoradiotherapy for locally advanced laryngeal cancer. The Laryngoscope. 2019 Oct:129(10):2313-2320. doi: 10.1002/lary.27771. Epub 2019 Jan 9 [PubMed PMID: 30628077]
Bar-Ad V, Palmer J, Yang H, Cognetti D, Curry J, Luginbuhl A, Tuluc M, Campling B, Axelrod R. Current management of locally advanced head and neck cancer: the combination of chemotherapy with locoregional treatments. Seminars in oncology. 2014 Dec:41(6):798-806. doi: 10.1053/j.seminoncol.2014.09.018. Epub 2014 Oct 7 [PubMed PMID: 25499638]
Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. The New England journal of medicine. 2003 Nov 27:349(22):2091-8 [PubMed PMID: 14645636]
Level 1 (high-level) evidenceGao P, Gong L, Wang X. Induction chemotherapy in patients with resectable laryngeal cancer: A meta-analysis. Molecular and clinical oncology. 2018 Aug:9(2):155-162. doi: 10.3892/mco.2018.1645. Epub 2018 Jun 5 [PubMed PMID: 30101013]
Level 1 (high-level) evidenceOu X, Zhai R, Wei W, Chen J, Ou D, Liao T, Xu T, Zhu Y, Wang Y, Huang S, Shi R, Wu B, Chen T, Li Y, Yang Z, Zhou C, Liu Y, Jiang Z, Zeng M, Liu X, Ji D, Ying H, Zhang Z, Hu C, Lu X, Ji Q, He X, Wang Y. Induction Toripalimab and Chemotherapy for Organ Preservation in Locally Advanced Laryngeal and Hypopharyngeal Cancer: A Single-Arm Phase II Clinical Trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2024 Jan 17:30(2):344-355. doi: 10.1158/1078-0432.CCR-23-2398. Epub [PubMed PMID: 37955629]
Level 1 (high-level) evidenceLarizadeh MH, Mohammadi F, Shabani M, Damghani MA. Induction Chemotherapy Followed by either Chemoradiotherapy or Bioradiotherapy in Laryngeal Cancer. Asian Pacific journal of cancer prevention : APJCP. 2021 May 1:22(5):1633-1637. doi: 10.31557/APJCP.2021.22.5.1633. Epub 2021 May 1 [PubMed PMID: 34048195]
Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem J, Ang KK, Lefèbvre JL. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head & neck. 2005 Oct:27(10):843-50 [PubMed PMID: 16161069]
Level 2 (mid-level) evidenceSteuer CE, El-Deiry M, Parks JR, Higgins KA, Saba NF. An update on larynx cancer. CA: a cancer journal for clinicians. 2017 Jan:67(1):31-50. doi: 10.3322/caac.21386. Epub 2016 Nov 29 [PubMed PMID: 27898173]
Li MM, Zhao S, Eskander A, Rygalski C, Brock G, Parikh AS, Haring CT, Swendseid B, Zhan KY, Bradford CR, Teknos TN, Carrau RL, VanKoevering KK, Seim NB, Old MO, Rocco JW, Puram SV, Kang SY. Stage Migration and Survival Trends in Laryngeal Cancer. Annals of surgical oncology. 2021 Nov:28(12):7300-7309. doi: 10.1245/s10434-021-10318-1. Epub 2021 Jul 15 [PubMed PMID: 34263369]
Multidisciplinary Larynx Cancer Working Group, Mulcahy CF, Mohamed ASR, Kanwar A, Hutcheson KA, Ghosh A, Vock D, Weber RS, Lai SY, Gunn GB, Zafereo M, Morrison WH, Ferrarotto R, Garden AS, Rosenthal DI, Fuller CD. Age-adjusted comorbidity and survival in locally advanced laryngeal cancer. Head & neck. 2018 Sep:40(9):2060-2069. doi: 10.1002/hed.25200. Epub 2018 May 13 [PubMed PMID: 29756307]
Patel TR, Eggerstedt M, Toor J, Tajudeen BA, Husain I, Stenson K, Al-Khudari S. Occult Lymph Node Metastasis in Early-Stage Glottic Cancer in the National Cancer Database. The Laryngoscope. 2021 Apr:131(4):E1139-E1146. doi: 10.1002/lary.28995. Epub 2020 Aug 18 [PubMed PMID: 32809243]
Nahavandipour A, Jakobsen KK, Grønhøj C, Hebbelstrup Jensen D, Kim Schmidt Karnov K, Klitmøller Agander T, Specht L, von Buchwald C. Incidence and survival of laryngeal cancer in Denmark: a nation-wide study from 1980 to 2014. Acta oncologica (Stockholm, Sweden). 2019 Jul:58(7):977-982. doi: 10.1080/0284186X.2019.1572923. Epub 2019 Mar 1 [PubMed PMID: 30821560]
Petrakos I, Kontzoglou K, Nikolopoulos TP, Papadopoulos O, Kostakis A. Glottic and supraglottic laryngeal cancer: epidemiology, treatment patterns and survival in 164 patients. Journal of B.U.ON. : official journal of the Balkan Union of Oncology. 2012 Oct-Dec:17(4):700-5 [PubMed PMID: 23335528]
Yang F, He L, Rao Y, Feng Y, Wang J. Survival analysis of patients with subglottic squamous cell carcinoma based on the SEER database. Brazilian journal of otorhinolaryngology. 2022 Nov-Dec:88 Suppl 4(Suppl 4):S70-S80. doi: 10.1016/j.bjorl.2021.09.001. Epub 2021 Oct 19 [PubMed PMID: 34716102]
MacNeil SD, Patel K, Liu K, Shariff S, Yoo J, Nichols A, Fung K, Garg AX. Survival of patients with subglottic squamous cell carcinoma. Current oncology (Toronto, Ont.). 2018 Dec:25(6):e569-e575. doi: 10.3747/co.25.3864. Epub 2018 Dec 1 [PubMed PMID: 30607125]
Zhou J, Zhou L, Tao L, Zhang M, Wu H, Chen X, Li X, Li C, Gong H. Oncological outcomes of surgical treatment for T3 supraglottic laryngeal squamous cell carcinoma patients. Acta oto-laryngologica. 2018 Nov:138(11):1028-1034. doi: 10.1080/00016489.2018.1490031. Epub 2019 Feb 8 [PubMed PMID: 30735065]
Hay A, Simo R, Hall G, Tharavai S, Oakley R, Fry A, Cascarini L, Lei M, Guerro-Urbano T, Jeannon JP. Outcomes of salvage surgery for the oropharynx and larynx: a contemporary experience in a UK Cancer Centre. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2019 Apr:276(4):1153-1159. doi: 10.1007/s00405-019-05295-x. Epub 2019 Jan 21 [PubMed PMID: 30666441]