Introduction
The constellation of motor pathways within the human central and peripheral nervous system involves two entities that guide voluntary movement: upper motor neurons (UMN) and lower motor neurons (LMN). Although these entities share familiar nomenclature, they each serve distinct functions in steering spinal mechanics. The collaborative effect of the UMN with the LMN is crucial in facilitating voluntary movement.
Upper motor neurons relay information from the brain to the spinal cord and brainstem, where they activate lower motor neurons, which directly stimulate muscles to contract. Upper motor neurons are first-order neurons regulated by the neurotransmitter glutamate, are found in the primary motor cortex (precentral gyrus), and terminate in the spinal cord or brainstem, unable to leave the central nervous system (CNS). Contrarily, lower motor neurons directly innervate skeletal muscle and have cell bodies in the anterior horn of the spinal cord (ventral horn) and at cranial nerve nuclei. Because lower motor neurons are cholinergic and directly innervate skeletal muscle, they can exist in both the central and peripheral nervous system (PNS). Damage both to upper and lower motor neurons results in distinctly identifiable deficits that can localize the cause of the deficit.[1]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Lower motor neurons transmit impulses via spinal peripheral nerves or cranial nerves to skeletal muscles. Three distinct types of motor neurons are categorized based on the target they innervate: branchial, visceral, and somatic motor neurons.[2]
Branchial Motor Neurons
These motor neurons are located in the brainstem and are responsible for forming the LMNs of the cranial nerve nuclei. They are called "branchial" because they innervate the muscles formed by the branchial (pharyngeal) arch, which includes muscles innervated by cranial nerves V, VII, IX, and X.[3] The V3 portion of the trigeminal nerve (CN V) is derived from the 1st branchial arch and innervates the muscles of mastication (temporalis, masseter, medial pterygoid, and lateral pterygoid) as well as smaller muscles supplied by CN V. An abnormality in the development of the 1st branchial arch may result in Pierre Robin sequence or Treacher Collins syndrome. The facial nerve (CN VII) innervates muscles derived from the 2nd branchial arch, which includes the muscles of facial expression, allowing one to smile. The glossopharyngeal nerve (CNIX) innervates muscles derived from the 3rd branchial arch, including the stylopharyngeus muscle, which assists in swallowing. Lastly, the vagus nerve (CN X) innervates muscles derived from the 4th and 6th branchial arches, which permit swallowing and speaking. Lesions at any region along the nerve from the cranial nerve nuclei in the brainstem to these muscles would result in their respective LMN deficits.
Visceral Motor Neurons
These motor neurons are a component of the autonomic nervous system (ANS) and regulate smooth muscles and glands. These nerves can be further broken down into the sympathetic and parasympathetic nervous system. Motor neurons of the sympathetic nervous system are present from T1 to L2 segments of the spinal cord. These segments regulate the "fight or flight" response, which can dictate body metabolism, awareness, and energy storage.[3] Conversely, the parasympathetic nervous system contributes to CN III, VII, IX, and X, as well as the S2 through S4 segments of the sacrum. These segments regulate the "rest and digest" response, which has a significant role in GI motility and sexual drive. Because the LMNs don't directly innervate these visceral targets, visceral motor neurons have been an exception to the role of traditional lower motor neurons.
Somatic Motor Neurons
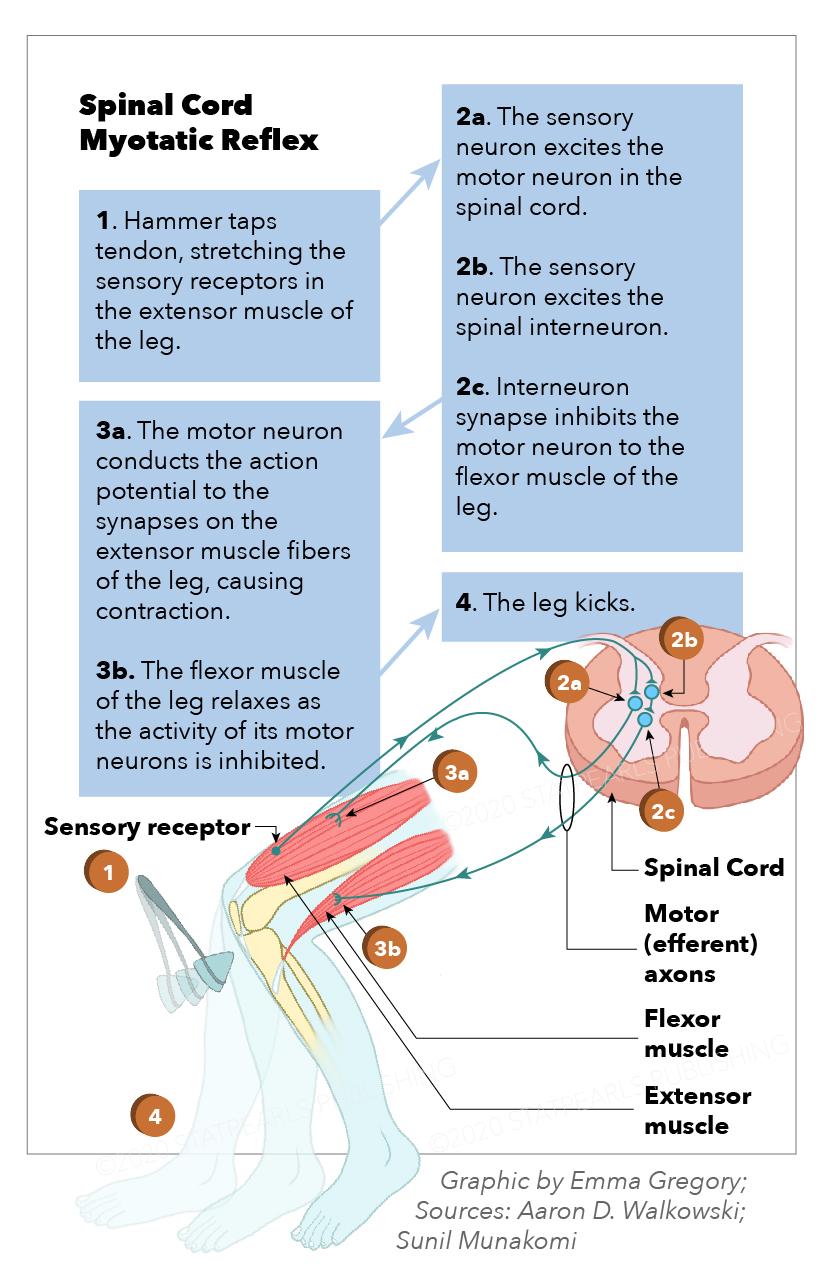
These motor neurons are the traditional motor neurons located in the ventral horn of the spinal cord and directly innervate skeletal muscle. They receive stimuli from the UMNs from the primary motor cortex and relay that information from the spinal cord to their target. Somatic motor neurons can be further divided into alpha, beta, and gamma, depending on the type of muscle fiber they innervate.[3] Alpha motor neurons innervate extrafusal fibers and are responsible for muscle contraction. Beta motor neurons innervate intrafusal fibers, which act like proprioceptors and detect the change in muscle length. Gamma muscle fibers also innervate intrafusal muscle fibers. Abnormalities in somatic motor neurons classically present in spinal muscular atrophy and poliomyelitis.
Embryology
Motor development begins early in embryonic development and continues throughout childhood into adulthood. All spinal motor neurons develop in the ventral region of the ventral-dorsal axis during the 4th week of development via spinal progenitor motor neurons. Various transcription factors must be present for both initial development and the further specification of all motor neurons.[4]
Surgical Considerations
Extreme care should be taken during the spinal cord surgeries not to damage the anterior horn cells.
Clinical Significance
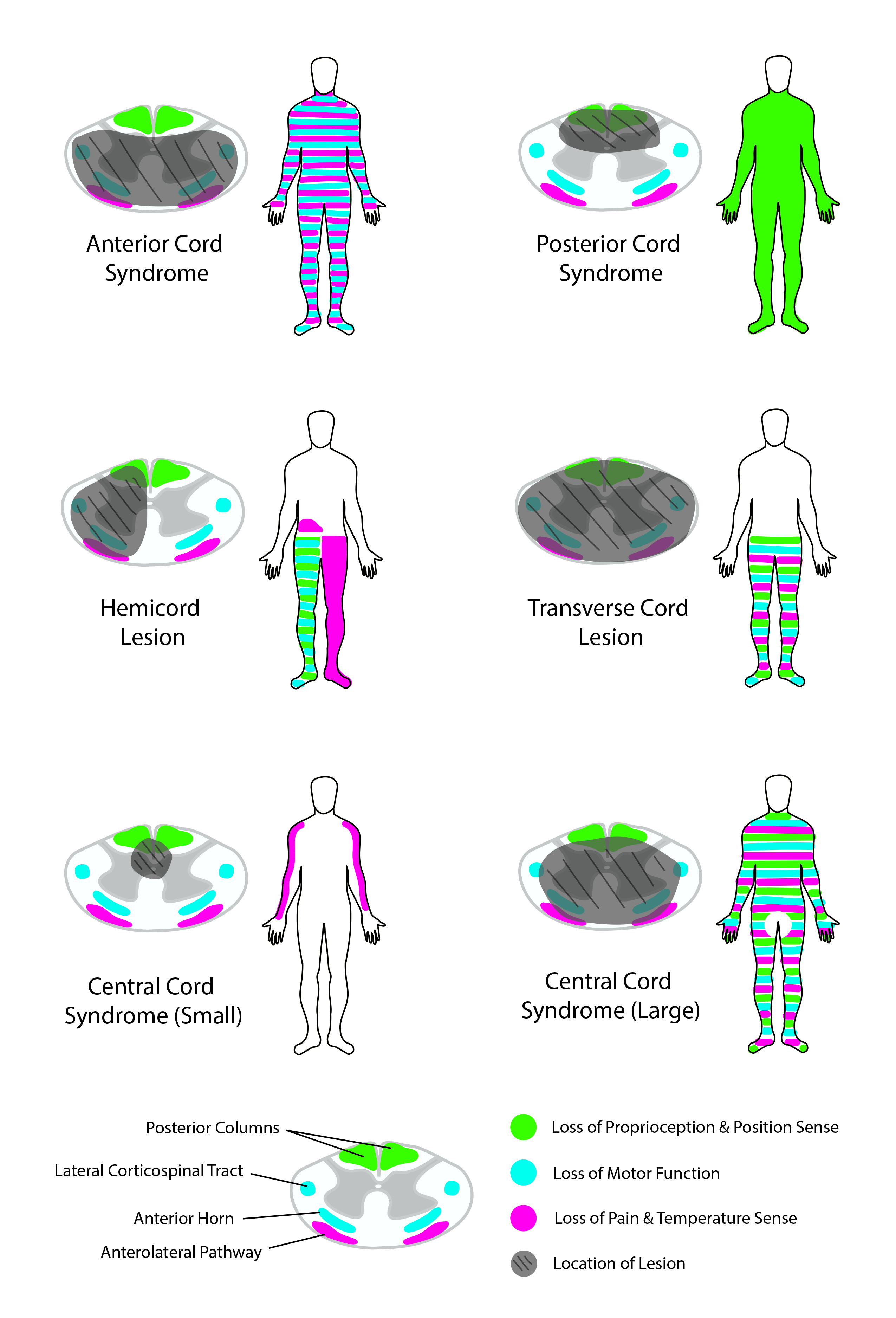
Although both upper and motor neuron lesions result in muscle weakness, they are clinically distinct due to various other manifestations. Unlike UMNs, LMN lesions present with muscle atrophy, fasciculations (muscle twitching), decreased reflexes, decreased tone, negative Babinsky sign, and flaccid paralysis. These findings are crucial when differentiating UMN vs. LMN lesions and must be distinguished from UMN characteristics to formulate a proper differential diagnosis. Although various diseases involve lower motor neurons, poliomyelitis and spinal muscular atrophy are two classic examples of isolated LMN disease.
Poliomyelitis
A classic example of solely LMN paralysis, poliomyelitis, has a fecal-oral transmission and is caused by a type of picornavirus: poliovirus. Once infected, the virus replicated in the oropharynx and small intestine before spreading via the bloodstream to the CNS. While replicating in the Peyer's patches of the small intestine, 95% of patients are asymptomatic, and it can only be found in the stool or via an oral swab.[5] In the CNS, the virus destroys the anterior (ventral) horn of the spinal cord, resulting in LMN paralysis. Because LMNs originate in the anterior horn of the spinal cord, this results in LMN signs such as asymmetric weakness, flaccid paralysis, fasciculations, hyporeflexia, and muscle atrophy. The infection could also result in respiratory involvement leading to respiratory paralysis. Other systemic signs of infection include fever, headache, nausea, and malaise. The cerebrospinal fluid would demonstrate increased WBC's and a slight elevation of protein, which is consistent with the viral infection. Once a prominent cause of paralysis, poliomyelitis has almost been eradicated due to widespread vaccination.[6][7]
Spinal Muscular Atrophy
This disease is due to congenital degeneration of the anterior horn of the spinal cord. Unlike polio, this results in symmetric weakness, flaccid paralysis, fasciculations, hyporeflexia, and muscle atrophy. Because it is congenital, it has classically had associations with a "floppy baby" with marked hypotonia and tongue fasciculations. This disease carries an autosomal recessive inheritance and is due to a mutation in the SMN1 gene. Spinal muscular atrophy type I is also known as Werdnig-Hoffmann disease, which is the most severe form of the disease and usually results in childhood death due to respiratory failure.[8] Spinal muscular atrophy type II and III are less severe and often result in a lifelong inability to ambulate.
Bell Palsy
Bell palsy is the most common etiology of peripheral facial nerve palsy. Although it is not always a lower motor neuron deficit, it is a perfect example to demonstrate LMN signs. It usually develops after herpes virus reactivation, but it can also result from Lyme disease, herpes zoster (Ramsay-Hunt syndrome), sarcoidosis, tumors of the parotid gland, and diabetes mellitus.
If any part of the corticobulbar tract from the motor cortex to the facial nerve nucleus is damaged, it will result in UMN deficits; this will result in contralateral facial paralysis involving the lower muscles of facial expression. Because there is bilateral UMN innervation to the muscles of the forehead, there is sparing of the forehead.
However, if the lesion occurs anywhere from the facial nucleus along CN VII, it will result in LMN deficits, affecting the ipsilateral side of the face and involve both the upper and lower muscles of facial expressions. This condition presents as incomplete eye closure (orbicularis oculi), dry eyes, corneal ulceration, hyperacusis, and taste sensation loss to the anterior tongue. Because the forehead is involved, the affected individual will be unable to wrinkle their forehead (lift their eyebrows).[9][10]
Media
(Click Image to Enlarge)
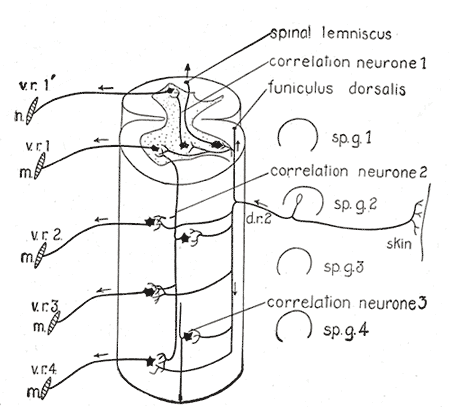
Central Connections of the Spinal Cord. This illustration portrays a diagram of the spinal cord reflex apparatus, spinal lemniscus, correlation neurone, and funiculus dorsalis.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Tindle J, Tadi P. Neuroanatomy, Parasympathetic Nervous System. StatPearls. 2023 Jan:(): [PubMed PMID: 31985934]
Zayia LC, Tadi P. Neuroanatomy, Motor Neuron. StatPearls. 2023 Jan:(): [PubMed PMID: 32119503]
Stifani N. Motor neurons and the generation of spinal motor neuron diversity. Frontiers in cellular neuroscience. 2014:8():293. doi: 10.3389/fncel.2014.00293. Epub 2014 Oct 9 [PubMed PMID: 25346659]
Elshazzly M, Lopez MJ, Reddy V, Caban O. Embryology, Central Nervous System. StatPearls. 2023 Jan:(): [PubMed PMID: 30252280]
Mehndiratta MM, Mehndiratta P, Pande R. Poliomyelitis: historical facts, epidemiology, and current challenges in eradication. The Neurohospitalist. 2014 Oct:4(4):223-9. doi: 10.1177/1941874414533352. Epub [PubMed PMID: 25360208]
Van Wittenberghe IC, Peterson DC. Corticospinal Tract Lesion. StatPearls. 2023 Jan:(): [PubMed PMID: 31194358]
Lohia A, McKenzie J. Neuroanatomy, Pyramidal Tract Lesions. StatPearls. 2023 Jan:(): [PubMed PMID: 31082020]
Arnold WD, Kassar D, Kissel JT. Spinal muscular atrophy: diagnosis and management in a new therapeutic era. Muscle & nerve. 2015 Feb:51(2):157-67. doi: 10.1002/mus.24497. Epub 2014 Dec 16 [PubMed PMID: 25346245]
Warner MJ, Hutchison J, Varacallo M. Bell Palsy. StatPearls. 2023 Jan:(): [PubMed PMID: 29493915]
Crouch AE, Hohman MH, Moody MP, Andaloro C. Ramsay Hunt Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 32491341]