Subacute Cutaneous Lupus Erythematosus
Subacute Cutaneous Lupus Erythematosus
Introduction
Lupus erythematosus (LE) is an inflammatory connective tissue disorder characterized by pathogenic autoantibodies, immune complex formation, deposition, and attributed to the loss of immune tolerance. Dermatological manifestations of lupus constitute one of the diagnostic criteria for systemic lupus erythematosus (SLE). Cutaneous findings in lupus encompass a broad spectrum of findings and may exist with systemic involvement as well as independent of SLE.[1] They may be described as LE specific and LE nonspecific.[2] LE specific manifestations include various subtypes of cutaneous lupus erythematosus (CLE) and are subdivided into three different categories described by Gillian et al., namely: acute cutaneous lupus, subacute cutaneous lupus, and chronic cutaneous lupus erythematosus.[3]
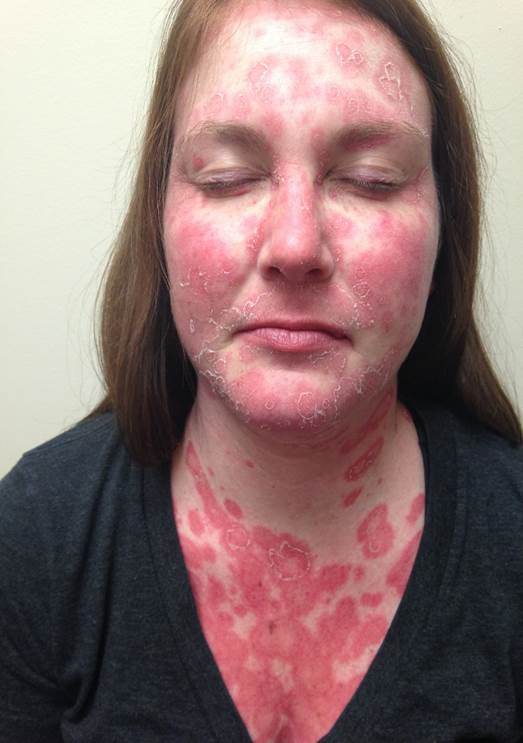
This activity will discuss subacute cutaneous lupus erythematosus (SCLE). SCLE is a subtype of CLE presenting as symmetric, non-scarring photosensitive erythematous rash usually over syn-exposed areas like face, neck, arms, upper back, and shoulders.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
There is no well-defined etiology for systemic lupus erythematosus. The classical precipitating factor is sunlight exposure in a patient with the abnormal milieu of genetic predisposition and immune dysregulation. Drug-induced SCLE has been reported as well. Commonly used drugs that have been associated with SCLE are angiotensin-converting enzyme inhibitors, anticonvulsants, beta-blockers, and immune modulators, including TNF alpha inhibitors. There have been case reports of the development of SCLE in malignancies.
Epidemiology
Systemic lupus erythematosus primarily occurs in young to middle-aged females, with females 3 to 4 times more likely to develop the lesions compared to males.[1] It usually presents in the third or fourth decade of life. The mean age of onset of SCLE described by Bizar et al. ranged from 50 to 52 years. SCLE is highly photosensitive, with 48% to 90% of patients meeting the ACR definition of increased photosensitivity.[2] Drug-induced forms may be seen in either sex and at older ages of onset.
Pathophysiology
The pathogenesis of systemic lupus erythematosus is multifactorial. SCLE is thought to develop due to genetics, environmental triggers, and or immunologic factors. Recent data suggests some potential candidate genes involved with SCLE.[3] HLA1, B8, DR3, HLA1, B8, DR3, DQ2, DRw52, DR3, and C4 null ancestral haplotype have all been associated with SCLE.[4][5] Moreover, deficiencies of C2 and C4 components of complement have been associated with SCLE.[6] Amongst the environmental factors, UV light and drugs have been found to play a role in the development of SCLE. Studies have shown the role of both innate and cell-mediated immunity, abnormal expression of T helper (Th) cells Th1, Th2, and Th17, several cytokines, and adhesion molecules implicated in the development of SCLE.[7][8] Anti-Ro/SS-A antibodies, apoptotic keratinocytes, including UVB-irradiated keratinocytes, have been implicated in pathogenesis in SCLE, as studies have shown deposition of, autoantibodies, immunoglobulin, and complement at the dermo-epidermal junction. Antibody-dependent cell-mediated cytotoxicity (ADCC), CD8+ cytotoxicity, complement-mediated cytolysis are all involved in the destruction of keratinocytes.[9][10][11]
Histopathology
Histologically, LE specific cutaneous findings share many features, but systemic lupus erythematosus has some distinct findings. Most characteristic findings are moderate hyperkeratosis with focal disorientation, interface dermatitis, and peri-appendageal mononuclear cell infiltrate confined to the superficial dermis. Basement membrane thickening is usually not seen in SCLE, dermal edema may be noted. The inflammatory infiltrate of the interface dermatitis consists mainly of activated T cells and macrophages.[12] Immunofluorescence studies show the deposition of immunoglobulin G (IgG) and/or complement components in a granular pattern at the dermal-epidermal junction, in about 60% of patients with SCLE. Anti-SS-A/Ro and anti-SS-B/La autoantibodies have been strongly associated with SCLE.[13]
History and Physical
The systemic lupus erythematosus lesions have a characteristic distribution with either papulosquamous lesions or annular lesions with central clearing. These two forms can occur concurrently, and the lesions typically heal without atrophy or scarring. The patient may have hypopigmentation or telangiectasia, but in most patients, the skin returns to normal. Patients with SCLE frequently have a mild illness with musculoskeletal complaints and serologic abnormalities.[12] SCLE has a predilection for sun-exposed areas like neck, shoulders, chest, and extensor surfaces of the arms and usually spares the face.
Evaluation
Systemic lupus erythematosus, along with other forms of CLE, is mainly a clinical diagnosis supported by clinical features. Confirmatory histopathologic examination with skin biopsy is indicated when there is diagnostic uncertainty. Laboratory studies like anti-SSA/Ro and anti-SSB/La are indicated as well. Direct immunofluorescence can be used to help support the diagnosis if histopathology is uncertain. Immunofluorescence shows a granular pattern of deposition of immunoglobulin at the dermal-epidermal junction.
Treatment / Management
Management of systemic lupus erythematosus includes lifestyle management and pharmacological therapy. It is important to address physical protection like broad-brimmed hats and sun-protective clothing, along with the proper use of sunscreen. Educating patients about their disease and avoiding potential triggers like minimizing sun exposure and strict sunscreen adherence with chemical and/or physical blocking agents is essential. The application of broad-spectrum sunscreen with a sun protection factor (SPF) of at least 50 should be applied in sufficient amounts about 20 to 30 minutes before expected exposure.[14]
Tobacco is phototoxic, enhances toll-like receptor 9 responsiveness, and IFN type 1 production in plasmacytoid dendritic cells and up-regulates expression of metalloproteinases 1 through 8.[15] Hence, smoking cessation education is very important.
For the pharmacological approach, the use of topical corticosteroids (CS) and calcineurin inhibitors (tacrolimus 0.1% and pimecrolimus 0.3%) are usually the first-line treatment. Intralesional CS could be used in localized areas. When a patient with SCLE does not respond appropriately to topical therapy and/or the cutaneous disease is widespread, the use of systemic therapy can be considered.
It is recommended that all patients be started on antimalarials (hydroxychloroquine, chloroquine, or quinacrine) due to their photoprotective and anti-inflammatory properties. Dosage recommendations are as follows: hydroxychloroquine, 6.0 to 6.5 mg/kg of ideal body weight; chloroquine, 3.5 to 4 mg/kg of ideal body weight; quinacrine, 100 mg/day.
In patients not responding to the initial treatment, further immunosuppressive agents may be added. Immunosuppressants that have been used methotrexate, dapsone, mycophenolate (MMF), azathioprine, and thalidomide.
Methotrexate was introduced in 1965 and is considered a second line of therapy. A significant reduction in autoantibodies in patients with lupus compared with the control group was found with the use of methotrexate.[16] The dosage ranges from 7.5 mg to 25 mg once per week orally, intravenously, or subcutaneously. MMF is used in lesions refractory to antimalarials and other immunomodulatory agents. MMF acts on both T and B cells by inducing apoptosis of T cells and preventing the B cells from producing antibodies. The dosing for MMF is 1.0 to 3.0 g/day and also needs renal dosage adjustment. Dosing for thalidomide is 400 mg daily, and for lenalidomide 5 to 10 mg/day, with the mechanism of action for this being inhibition of synthesis of tumor necrosis factor-α.(A1)
Third-line treatments include belimumab and dapsone. Belimumab is an IgG1 monoclonal antibody that binds to B lymphocyte stimulator, found to be efficacious in SCLE. Cutaneous symptoms are among the treatment areas in which belimumab, in addition to standard therapy in these patients, is most efficacious.[17] Dapsone is an antibiotic that blocks the myeloperoxidase enzyme and has an anti-inflammatory and immunomodulatory effect. Dapsone is started at 50 mg daily, with a maximum dosage of 200 mg daily.
Furthermore, there have been case reports of using intravenous immunoglobulin (IVIG) and rituximab. Rituximab is a chimeric anti-CD20 monoclonal antibody; it induces B-cell lysis through antibody-dependent cellular toxicity. It is the first choice for patients with severe autoimmune diseases, resistant to conventional treatment, including severe cases of subacute lupus.[14][18] IVIG has shown some promising results in refractory cases of SCLE as well.[19](B3)
Differential Diagnosis
- Psoriasis
- Tinea corporis
- Nummular eczema
- Dermatomyositis
- Pityriasis rubra pilaris
- Sarcoidosis
- Cutaneous T cell lymphoma
- Drug eruptions [20]
Prognosis
Since systemic lupus erythematosus is a photosensitive rash, around 50% of patients with SCLE would meet the criteria for classification as SLE. However, systemic disease is mild, arthralgia, myalgia, oral ulcers, a positive antinuclear antibody (ANA), positive anti-dsDNA, and low complement levels being the most common findings.[21][22] Severe systemic disease is not as common. Central nervous system involvement, vasculitis, or nephritis is seen in around 10% of patients.[23] Renal disease has been associated with papulosquamous variants of SCLE.[24]
Complications
Due to sun protection, patients can develop vitamin D deficiency. Systemic lupus erythematosus rarely can involve large body surface areas to cause excessive discomfort and may affect the quality of life. Systemic manifestations of SCLE may lead to vital organ involvement and result in complications.
Consultations
Consultations with dermatology and rheumatology are recommended. Moreover, depending on organ involvement, other specialties may need to get involved, included but not limited to nephrology, pulmonary, and neurology.
Deterrence and Patient Education
Patients need to be counseled and educated about sun protection, smoking cessation, and vitamin D replacement. Patients should also be advised to avoid any drugs that can trigger or exacerbate SCLE.
Pearls and Other Issues
Smoking cessation and correction of vitamin D deficiency are other aspects of management.
Enhancing Healthcare Team Outcomes
Early recognition of systemic lupus erythematosus can help initiation of treatment and thus help avoid complications. Communication of primary care providers and specialists is key to limit morbidity patients with diseases like SCLE or SLE. Primary care providers can help reinforce lifestyle changes, including sun protection and smoking cessation, which can improve long term outcomes. Dermatology nurses assist with patient education and arrange for follow up appointments and labs. Pharmacists review prescriptions, check for drug interactions, and inform patients about the importance of compliance and potential side effects. They can also consult with the clinician regarding optimal agent selection and dosing. Both nurses and pharmacists need to alert the clinician if they encounter any issues of concern. These examples of interprofessional coordination can help drive better patient outcomes for systemic lupus erythematosus. [Level 5]
Media
(Click Image to Enlarge)
References
Parodi A, Caproni M, Cardinali C, Bernacchi E, Fuligni A, De Panfilis G, Zane C, Papini M, Veller FC, Vaccaro M, Fabbri P. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. A review by the Italian group of immunodermatology. Dermatology (Basel, Switzerland). 2000:200(1):6-10 [PubMed PMID: 10681606]
Level 2 (mid-level) evidenceBiazar C, Sigges J, Patsinakidis N, Ruland V, Amler S, Bonsmann G, Kuhn A, EUSCLE co-authors. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmunity reviews. 2013 Jan:12(3):444-54. doi: 10.1016/j.autrev.2012.08.019. Epub 2012 Sep 18 [PubMed PMID: 23000206]
Level 2 (mid-level) evidenceMillard TP, Kondeatis E, Cox A, Wilson AG, Grabczynska SA, Carey BS, Lewis CM, Khamashta MA, Duff GW, Hughes GR, Hawk JL, Vaughan RW, McGregor JM. A candidate gene analysis of three related photosensitivity disorders: cutaneous lupus erythematosus, polymorphic light eruption and actinic prurigo. The British journal of dermatology. 2001 Aug:145(2):229-36 [PubMed PMID: 11531784]
Level 2 (mid-level) evidenceSontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. The Journal of clinical investigation. 1981 Jan:67(1):312-6 [PubMed PMID: 7451656]
Watson RM, Talwar P, Alexander E, Bias WB, Provost TT. Subacute cutaneous lupus erythematosus-immunogenetic associations. Journal of autoimmunity. 1991 Feb:4(1):73-85 [PubMed PMID: 2031665]
Callen JP, Hodge SJ, Kulick KB, Stelzer G, Buchino JJ. Subacute cutaneous lupus erythematosus in multiple members of a family with C2 deficiency. Archives of dermatology. 1987 Jan:123(1):66-70 [PubMed PMID: 3467658]
Level 3 (low-level) evidenceBennion SD, Norris DA. Ultraviolet light modulation of autoantigens, epidermal cytokines and adhesion molecules as contributing factors of the pathogenesis of cutaneous LE. Lupus. 1997:6(2):181-92 [PubMed PMID: 9061667]
Robinson ES, Werth VP. The role of cytokines in the pathogenesis of cutaneous lupus erythematosus. Cytokine. 2015 Jun:73(2):326-34. doi: 10.1016/j.cyto.2015.01.031. Epub 2015 Mar 9 [PubMed PMID: 25767072]
Norris DA, Lee LA. Pathogenesis of cutaneous lupus erythematosus. Clinics in dermatology. 1985 Jul-Sep:3(3):20-35 [PubMed PMID: 2463862]
Norris DA, Lee LA. Antibody-dependent cellular cytotoxicity and skin disease. The Journal of investigative dermatology. 1985 Jul:85(1 Suppl):165s-175s [PubMed PMID: 3874244]
Level 3 (low-level) evidenceFurukawa F, Kashihara-Sawami M, Lyons MB, Norris DA. Binding of antibodies to the extractable nuclear antigens SS-A/Ro and SS-B/La is induced on the surface of human keratinocytes by ultraviolet light (UVL): implications for the pathogenesis of photosensitive cutaneous lupus. The Journal of investigative dermatology. 1990 Jan:94(1):77-85 [PubMed PMID: 2132545]
Bangert JL, Freeman RG, Sontheimer RD, Gilliam JN. Subacute cutaneous lupus erythematosus and discoid lupus erythematosus. Comparative histopathologic findings. Archives of dermatology. 1984 Mar:120(3):332-7 [PubMed PMID: 6703733]
Level 2 (mid-level) evidenceLee LA, Roberts CM, Frank MB, McCubbin VR, Reichlin M. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Archives of dermatology. 1994 Oct:130(10):1262-8 [PubMed PMID: 7944507]
Nutan F, Ortega-Loayza AG. Cutaneous Lupus: A Brief Review of Old and New Medical Therapeutic Options. The journal of investigative dermatology. Symposium proceedings. 2017 Oct:18(2):S64-S68. doi: 10.1016/j.jisp.2017.02.001. Epub [PubMed PMID: 28941497]
Ortiz A, Grando SA. Smoking and the skin. International journal of dermatology. 2012 Mar:51(3):250-62. doi: 10.1111/j.1365-4632.2011.05205.x. Epub [PubMed PMID: 22348557]
Miyawaki S, Nishiyama S, Aita T, Yoshinaga Y. The effect of methotrexate on improving serological abnormalities of patients with systemic lupus erythematosus. Modern rheumatology. 2013 Jul:23(4):659-66. doi: 10.1007/s10165-012-0707-9. Epub 2012 Jul 19 [PubMed PMID: 23011357]
Level 1 (high-level) evidenceManzi S, Sánchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, Ginzler EM, D'Cruz DP, Doria A, Cooper S, Zhong ZJ, Hough D, Freimuth W, Petri MA, BLISS-52 and BLISS-76 Study Groups. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Annals of the rheumatic diseases. 2012 Nov:71(11):1833-8. doi: 10.1136/annrheumdis-2011-200831. Epub 2012 May 1 [PubMed PMID: 22550315]
Kieu V, O'Brien T, Yap LM, Baker C, Foley P, Mason G, Prince HM, McCormack C. Refractory subacute cutaneous lupus erythematosus successfully treated with rituximab. The Australasian journal of dermatology. 2009 Aug:50(3):202-6. doi: 10.1111/j.1440-0960.2009.00539.x. Epub [PubMed PMID: 19659984]
Level 3 (low-level) evidenceGoodfield M, Davison K, Bowden K. Intravenous immunoglobulin (IVIg) for therapy-resistant cutaneous lupus erythematosus (LE). The Journal of dermatological treatment. 2004 Jan:15(1):46-50 [PubMed PMID: 14754650]
Ziemer M, Milkova L, Kunz M. Lupus erythematosus. Part II: clinical picture, diagnosis and treatment. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2014 Apr:12(4):285-301; quiz 302. doi: 10.1111/ddg.12254. Epub 2014 Jan 15 [PubMed PMID: 24423191]
Cohen MR, Crosby D. Systemic disease in subacute cutaneous lupus erythematosus: a controlled comparison with systemic lupus erythematosus. The Journal of rheumatology. 1994 Sep:21(9):1665-9 [PubMed PMID: 7799346]
Level 2 (mid-level) evidenceTiao J, Feng R, Carr K, Okawa J, Werth VP. Using the American College of Rheumatology (ACR) and Systemic Lupus International Collaborating Clinics (SLICC) criteria to determine the diagnosis of systemic lupus erythematosus (SLE) in patients with subacute cutaneous lupus erythematosus (SCLE). Journal of the American Academy of Dermatology. 2016 May:74(5):862-9. doi: 10.1016/j.jaad.2015.12.029. Epub 2016 Feb 18 [PubMed PMID: 26897388]
Sontheimer RD. Subacute cutaneous lupus erythematosus. Clinics in dermatology. 1985 Jul-Sep:3(3):58-68 [PubMed PMID: 3880024]
Callen JP, Kulick KB, Stelzer G, Fowler JF. Subacute cutaneous lupus erythematosus. Clinical, serologic, and immunogenetic studies of forty-nine patients seen in a nonreferral setting. Journal of the American Academy of Dermatology. 1986 Dec:15(6):1227-37 [PubMed PMID: 3543071]