Introduction
Mantle cell lymphoma (MCL) is a rare subtype of B-cell non-Hodgkin lymphomas (NHLs) characterized by an (11,14) translocation resulting in overexpression of the cyclin D1 (CCND1) gene. The variety of morphologic variants may make this a challenging diagnosis, although some cases are uncomplicated. It typically follows an aggressive clinical course (aggressive MCL), although an indolent leukemia variant (indolent MCL) has been described.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Mantle cell lymphoma is typically sporadic, but it may have a higher incidence in some families.
Epidemiology
Mantle cell lymphoma (MCL) is a rare subtype of B-cell non-Hodgkin lymphoma (NHL) with an annual incidence of one case per 200 000 people. MCL comprises around 5% of all non-Hodgkins lymphomas. MCL is more common in men (3 to 1), and the median age at diagnosis ranges from 60 to 70 years old.
Pathophysiology
MCL is characterized by reciprocal chromosomal translocation t(11;14)(q13:q32), resulting in the juxtapositioning of cyclin D1 locus to immunoglobulin heavy chain gene locus.[1] This leads to constitutive expression of cyclin D1 (CCND1), which plays a significant role in tumor cell proliferation via cell cycle dysregulation, chromosomal instability, and epigenetic regulation. Rare cases without this translocation may have a CCND2 or 3 translocations.[2] Hypothetical models of molecular subtypes have been proposed based on the cell of origin that correlates to clinical phenotypes. Arising from naive B cells that have no or limited iGVH mutations and express SOX 11 are the classical MCL or aggressive MCL. SOX 11 is a neural transcription factor reported to block terminal B cell differentiation.[3] Arising from antigen-experienced B cells that have undergone IGVH somatic hypermutations and typically SOX 11 negative and genetically stable B cells are the indolent variant of MCL.[4]
Histopathology
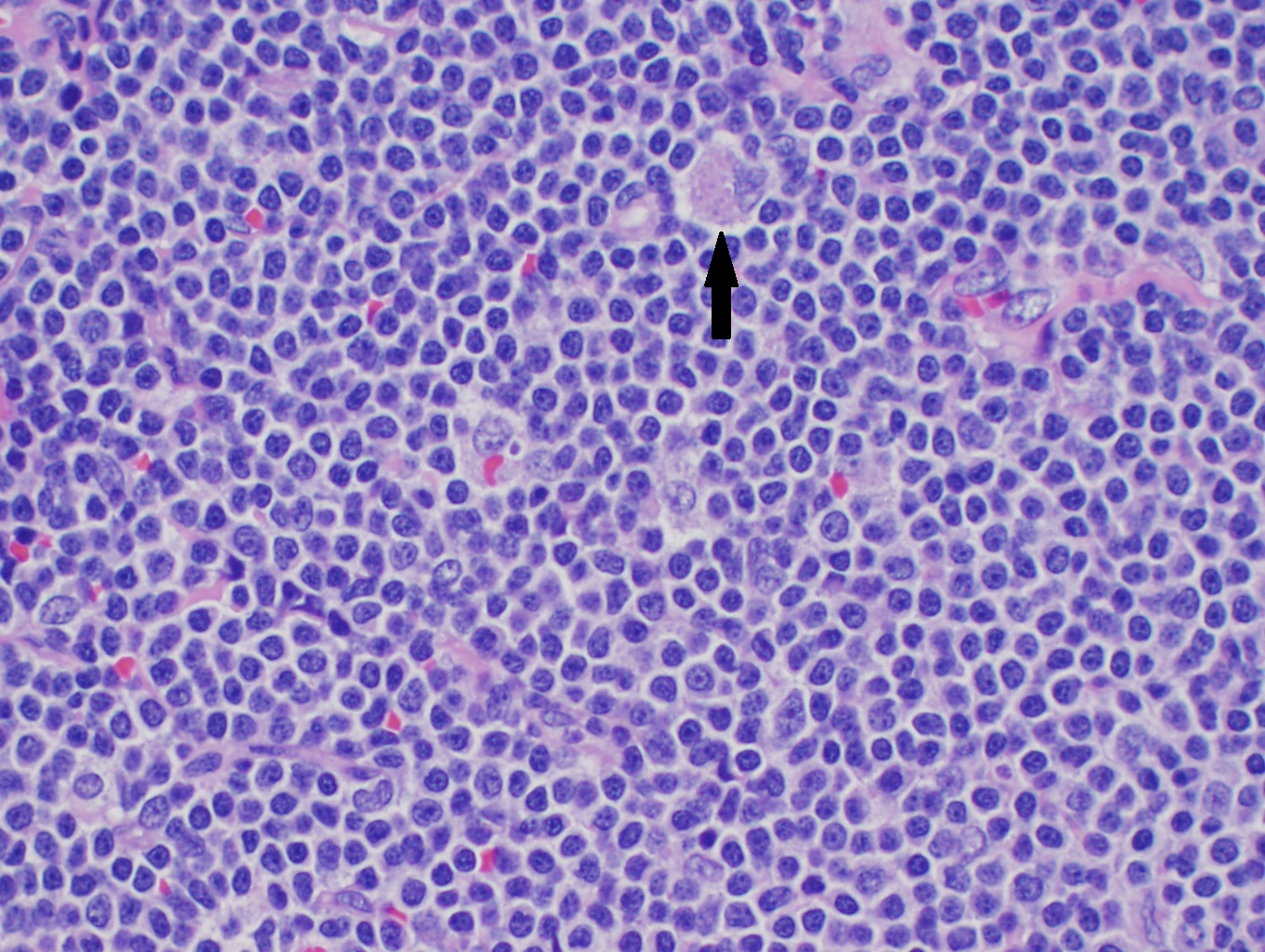
Although only a single translocation defines MCL, there are several morphologic variations that one must know. In lymph nodes, there is often diffuse or nodular effacement of the lymph node. MCL may also show expansion of the mantle zones with a monocytoid appearance surrounding reactive germinal centers. Often pink histiocytes may be mixed with lymphoma cells (see image). Pathologic exam with samples from tissue biopsy is essential for the diagnosis of MCL, which typically shows small lymphocytes with cells characterized by notched nuclei in the small cell or blastoid variant type.
The cytology of the cells is highly variable as well. In tissue, cells may appear small and mature with irregular nuclei, but in the blastoid variant, they may appear immature with fine chromatin mimicking acute leukemia. The pleomorphic variant shows a marked variation in nuclear size and shape. The proliferation index and mitotic are also highly variable. In blood and bone marrow aspirate smears, lymphoma cells may have discrete vacuoles, which may be a clue in subtle cases.[5]
History and Physical
The disease is typically widespread at diagnosis. The most common manifestations of MCL include extensive lymphadenopathy, fevers, night sweats, weight loss symptoms, splenomegaly-related discomfort, and blood count abnormalities due to bone marrow involvement and symptoms related to extranodal organ involvement.[6] Extranodal involvement, especially of the gastrointestinal tract, spleen, and bone marrow, is fairly common in MCL.[7] MCL has shared features of both indolent (incurable nature) and aggressive (aggressive clinical phenotype).
Evaluation
Excisional biopsy of a lymph node or an involved extranodal site is essential in the diagnosis of MCL. Laboratory exams, including CBC with differential, LDH, and beta-2 microglobulin, bone marrow biopsy, and imaging (CT or FDG-PET/CT) are also recommended as the standard diagnostic workup. Further, the Ki-67 index and mutation status of p53, ATM, and CCND1 are also important in selecting the optimal treatment. A CSF study is also necessary to rule out CNS involvement and guide appropriate management in MCL patients with a high Ki-67 index (greater than 30%), blastoid variant, or neurologic symptoms.[8] Though GI tract involvement is common in MCL, the routine endoscopic evaluation is unnecessary for all cases since it does not affect the management. But GI workup is recommended in the suspected stage I and non-bulky stage II for appropriately staging early-stage MCL.
Immunohistochemistry on pathology specimens plays an essential role in differentiating MCL from other NHL, including follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL). MCL is positive for B-cell markers (CD19, CD20, CD22, CD79a). It is distinguished from other B-cell lymphomas by diffuse positivity for cyclin D1 and SOX11. SOX 11 is usually negative in indolent MCL. Rare cases are negative for cyclin D1 but can still be positive for SOX11. MCL is typically negative for BCL6, CD10, and CD23 (while CLL is CD23 positive). Rare cases of MCL are CD10 positive.[9] Peripheral blood flow cytometry can have CD 200 expression characteristic of MCL. Testing for P53 mutation is recommended to help in the prognostication of MCL.
MIPI ( Mantle cell lymphoma international prognostic index) is a scoring system is used to stratify the risk of patients with MCL with pneumonic APLE to remember for readers.[10]
- Age
- Performance status
- LDH
- Elevated white blood count
Using these parameters, scoring calculators are available for free (online) that can help educate patients about their prognosis and expected survival based on these calculations of their survival. However, the MIPI scoring system falls short in predicting prognosis in all MCL patients. Therefore the addition of KI 67, cytogenetics, and TP 53 mutations can help improve the risk stratification of MCL.
High-risk MIPI score patients have median Overall survival (OS ) of 2.8 yrs, while low-risk MIPI had survival longer than 5 years.[11][12]
Typically based on symptoms and presentation, patients can be divided into indolent MCL or aggressive MCL.
| Indolent MCL | Aggressive MCL |
| Asymptomatic | Symptomatic (B symptoms) |
| Extranodal/leukemic | Usually nodal involvement |
| KI 67 less than 30% | KI 67 greater than 30% |
| Low MIPI score | High MIPI score |
| Small cells on morphology | Can have blastoid cells |
| Usually, SOX 11 negative | SOX 11 positive |
| Absence of 179/p53 mutation | P 53 mutation/complex karyotype |
Treatment / Management
MCL remains a largely incurable disease with a median overall survival (OS) ranging from 1.8 to 9.4 years, depending on whether it is aggressive or indolent MCL. The choice of optimal treatment usually has its basis in the aggressiveness of the disease, performance status, age, and mantle cell international prognostic index (MIPI) score since there is no curative standard treatment established for MCL.
Indolent forms of MCL with low MIPI score, hypermutated IGH genes, SOX11 negativity, and non-complex karyotype may be monitored without immediate treatment. As such, watch and wait is appropriate in patients who have 1) Ki-67 = 30%, 2) maximum tumor diameter less than 3 cm, normal serum LDH level, 4) normal beta-2 microglobulin level, 5) no B symptoms, and 6) non-blastoid histology.[13]
| Indolent/Asymptomatic MCL | Classic/Aggressive/symptomatic MCL |
| Wait and close monitoring | Chemoimmunotherapy |
It is rare to find early stage I or non-bulky stage II that can be treated by involved field radiation alone or with nonaggressive regimens used for transplant-ineligible patients.
Symptomatic patients with classic MCL (aggressive MCL) that need treatments (Stage II bulky or stage III or stage IV can be classified as stem cell transplant eligible or nontransplant eligible based on performance status and comorbidities.
| Stem cell transplant-eligible patients | Not eligible for Bone marrow transplant |
| Chemo regimens that can be used: R CHOP, BR, Lenalidomide-Rituximab |
Aggressive chemo regimens used: RCHOP alternating with RDHAP; or NORDIC regimen ( Maxi RCHOP alternating with Rituximab and high dose Cytarabine or Hyper-CVAD that includes a CHOP-like regimen alternating with high-dose methotrexate and cytarabine Once in CR or very good PR, autologous stem cell transplant is recommended |
| Usually, maintenance with rituximab is performed after R CHOP chemo; however, no benefit exists for maintenance after the BR regimen | Post-transplant rituximab maintenance every 8 weeks for 3 years is recommended for those in CR |
R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone), BR (bendamustine, rituximab), R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin), hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high dose methotrexate and high dose cytarabine)
Patients with relapsed or refractory disease require more aggressive treatment, which is typically a salvage regimen followed by HDC and autologous or allogeneic SCT. Other second-line targeted agents with fewer side effects that are now FDA-approved include
(A1)| Second-line agents | Main action | How do they work | Reference Study that led to approval and survival data |
| Ibrutinib, znubritinib, acalubritinib | BTK -inhibitors |
Inhibit constitutively activated B cell receptor (BCR) signaling through inhibiting BTK downstream from BCR in mantle cell and also immunomodulatory effects on T cells Acalubritinib and zanubrutinib bind to cysteine 481 of BTK and irreversibly inhibit target BTK; they have less action on off-targets [14] |
Ibrutinib: ORR 66%, PFS 12.3 months, OS 25 months [15][16][17] Acalubritinb PFS 20 months OS not reached at 26 month follow-up [18] Acalubritinib and zanubrutinib are not effective for BTK C481 S mutation |
| Venetoclax | BCL 2 inhibitors | BCL 2 is overexpressed in MCL, inhibition of BCL2 increases apoptosis of MCL cells |
Venetoclax approval based on Phase I ORR 75%, CR 21% in patients not previously on BTK inhibitors [21] Approved in combination with rituximab or ibrutinib |
| Bortezomib | Proteosome Inhibitor | Decreases NF KB signaling, inhibiting p 27 degradation and thus cell cycle arrest |
In relapsed MCL not exposed to BTK inhibitors, single-agent bortezomib OS 23.5 months, the median time to progression was 6.7 months [22][23] |
|
Lenalidomide |
Immunomodulatory agent | Direct cytotoxicity on MCL cells, enhancement of dendritic cells, NK, and T cell activation, and suppressing angiogenesis | Lenalidomide single-agent ORR 28%, duration of response 16.6 months months [24][25] |
| Rituximab | Monoclonal antibodies | Antibodies engineered for a specific receptor on B cells | Rituximab in combination regimens is approved [26][27] |
|
Polatuzumab, inotuzumab, pinatuzumab, loncastuximab and naratuximab |
Antibody-drug conjugates |
Anti CD79, Anti CD 22, Anti CD 19, Anti CD 37 antibody binders that bind to targets and delivers cytotoxic drugs | In clinical trials |
|
Brexucabtagene autoleucel |
CAR T cell (Chimeric antigen receptor T cell) therapy | Autologous T cells genetically modified transduced with CD 19 receptor to fight against lymphoma cells | ZUMA-2 ORR 87%, CR of 62% in relapsed MCL [28] |
|
Blinatumomab Mosunetuzumab |
Bite-antibodies |
An antibody that cross-links B cells and T cells by ligating CD3 and CD 19 /CD20 | In clinical trials |
|
Venetoclax + ibrutinib |
Combination BTK and BCL2 inhibitor |
Inhibiting 2 pathways, BTK and BCL2, and having a synergistic effect | AIM study CR 42-62%, OS 32 months [26] |
Ibrutinib, acalabrutinib, zanubrutinib, venetoclax +/- rituximab, bortezomib +/- rituximab, lenalidomide plus rituximab, ibrutinib and lenalidomide + rituximab and chimeric antigen receptor T-cells (CAR-T) received FDA approval and several other agents including BiTE antibodies, antibody-drug conjugates and are under clinical trials.
BCR- B cell receptor, BTK - Bruton's tyrosine kinase
Differential Diagnosis
The differential diagnosis primarily includes SLL/CLL and DLBCL. Both SLL/CLL and mantle cell lymphoma are CD5-positive, mature B-cell lymphomas and may be difficult to distinguish by flow cytometry alone. When there is positivity for CD200 and CD23, the diagnosis is most likely SLL/CLL. Mantle cell lymphoma is typically negative for CD23; it is positive for FMC7. Confirmation of MCL is typically performed by either FISH for t(11;14) or immunohistochemistry for cyclin D1 and/or SOX11. In tissue, uniform positive immunohistochemical expression of B-cells by Cyclin D1 and SOX 11 supports MCL. Focal positivity for Cyclin D1 may be seen in the proliferation centers of SLL/CLL and should not prompt a diagnosis of MCL.[29] Diffuse large B-cell lymphoma is entertained when large cell proliferation is negative for Cyclin D1 and SOX11.
When the architecture of a lymph node is intact and only shows focal involvement by atypical cyclin D1 positive cells, mantle cell neoplasia in situ may be a diagnostic consideration.
Surgical Oncology
Excisional Biopsies are recommended for diagnosis. The role of surgery as treatment is not generally recommended, considering the systemic nature of this illness.
Radiation Oncology
Involved site radiation therapy (ISRT) to 24-36 Gy for stages IA and stage II A MCL is recommended with or without combination chemoimmunotherapy. This recommendation is based on retrospective and anecdotal data with PFS at 5 years of 68%.[30] Considering the rarity of finding such early-stage MCL patients and the nonavailability of clinical trial data, ISRT is included in guidelines based on retrospective data.
Medical Oncology
Chemoimmunotherapies as frontline treatment, and targeted drugs as second-line treatments.
Ongoing clinical trials are evaluating combination treatment strategies and new drugs. While it is beyond the scope of this discussion to list out all the ongoing clinical trials, we felt it is important to elucidate the concept underlying these trials as below for the readers.
| Concept | Combination strategy/Investigational Agent/ Maintenance | transplant eligible | Transplant ineligible | Front line | Relapsed/refractory |
|
Ibrutinib + Venetoclax |
Combination Regimens |
yes Phase III SYMPATICO trial |
yes | ||
|
Obintuzumab + Lenalidomide |
Combination Regimens |
yes | To sensitize MCL resistance to rituximab | ||
|
R-BAC after ibrutinib failure (Rituximab, Bendamustine, cytarabine) , |
Combination Regimens |
yes | To bridge to an allo transplant | ||
| CDK4/6 Inhibitor | Investigational Agents | yes | yes | To get deeper responses. | |
| Lenalidomide | Maintenance | yes | To increase the time to relapse | ||
| Bortezomib combination to transplant | Maintenance | yes | yes | yes | To get deeper responses and to reduce relapse |
| Chemotherapy-free options | Combination of ibrutinib with obinutuzumab and venetoclax | yes | To reduce chemo toxicity in elderly patients | ||
| Risk adapted approaches | Measuring Ct DNA and MRD status by ASO-PCR and evaluating maintenance treatments based on that | yes | yes | To reduce toxicity and have personalized treatments |
MRD: Minimal residual disease; ASO-PCR: allele-specific oligonucleotide PCR; ctDNA: circulating tumor DNA.
Staging
Initial staging requires comprehensive scanning, including a contrast CT chest/abdomen/pelvis and/or whole-body PET/CT scan, along with a bone marrow biopsy with aspirate to assess the burden of disease.
The staging system that is used for non-Hodgkin's lymphomas is used for the staging of MCL.
| Stage I | Involvement of one lymph node or group of adjacent lymph nodes or one extra lymphatic site |
| Stage II | Involvement of two or more nodal groups on the same side of the diaphragm or stage I or II with contiguous extranodal involvement |
| Stage III | Involvement of lymph nodes on either side of the diaphragm |
| Stage IV | involvement of bone marrow or noncontiguous extranodal involvement |
Prognosis
Although International Prognostic Index (IPI) failed to show prognostic power in MCL, MCL IPI (MIPI) that incorporates age, performance status, normalized LDH level, and white blood cell (WBC) count, was shown to discriminate MCL patients into three risk groups; low, intermediate, and high with a five-year overall survival (OS) of 60%, 35%, and 20%, respectively. Two other prognostic indexes were developed recently to accommodate the prognostic impact of Ki-67 (MIPIb) and simplify calculation (sMIPI), although they require further clinical validation.[31] Further, CDKN2A and TP53 deletions showed inferior OS independent to Ki-67 in patients treated with autologous stem cell transplant (SCT), and TP53 overexpression was shown to correlate with a worse prognosis.[32] Patients who present with involvement of the peripheral blood and bone marrow (leukemic non-nodal MCL) may follow an indolent course.[33]
Complications
Complications of the MCL due to the disease process itself based on the site of MCL involvement include splenic rupture due to splenomegaly, GI bleeding or obstruction or perforation due to MCL in the gastrointestinal tract, and tumor lysis in the blastoid variant of MCL are all the considerations.[34] Due to chemotherapy and stem cell transplants, complications of life-threatening infections, neutropenic sepsis, cytopenias needing transfusions, nausea, vomiting, and hair loss are possible. With targeted drug approvals in MCL with agents like ibrutinib, aBTK inhibitor, hypertension, arrhythmias, diarrhea, skin rashes, and bleeding risk are reported. These side effects are felt to be more related to off-target actions on EGFR, ITK, and PI3K. Second-generation BTK inhibitors like acalabrutinib and zanubrutinib are more specific to BTK and have fewer off-target side effects in terms of hypertension, arrhythmias, or rashes.[14] BCL-2 inhibitor venetoclax is now approved for MCL venetoclax drug tumor lysis syndrome, while lenalidomide edema, cytopenias, risk of blood clots, and diarrhea are expected complications and or side effects to be aware of. Brexucabtagene (CAR-T cell therapy ) is associated with encephalopathy, cytopenias, and pyrexia from cytokine release syndrome, which must be addressed during the treatments.
ITK - Interleukin-2-inducible T-cell kinase, EGFR-epidermal growth factor inhibitor, PI3K-phosphoinositide 3-kinase.
Consultations
Consultations with a medical oncologist, bone marrow transplant team, and clinical trial teams are an integral part of the management of aggressive MCL.
Pearls and Other Issues
- Mantle cell lymphoma is a rare and conventionally aggressive B-cell lymphoma with a heterogeneous disease profile.
- Cases are typically diagnosed with diffuse cyclin D1 positivity by immunohistochemistry or FISH for t(11;14).
- Rare cases that are negative for cyclin D1 for t(11;14) are positive for SOX11 by immunohistochemistry.
- High Ki67 proliferation index and p53 mutations are adverse prognostic findings and likely imply the need for more prompt treatment and clinical trial enrollment if no good response.
- Chemoimmunotherapy is conventionally the choice as front-line therapy among patients necessitating treatment with a potential role of targeted therapies in the salvage setting.
- The role of autologous and allogeneic bone marrow transplant and stratifying patients as transplant eligible and ineligible in choosing the treatment choices are elucidated.
- The role of second-generation drugs in improving outcomes and the side effects of these drugs are discussed.
- Investigational new drugs alone and in combination are described. Risk-adapted approaches in the pipeline for improving the care of mantle cell lymphoma patients are discussed.
Enhancing Healthcare Team Outcomes
Mantle cell lymphoma is typically an uncomplicated diagnosis, but morphologic variants may mimic other lymphoid neoplasms. Having a low threshold for ordering additional testing for cyclin D 1, SOX 11 immunohistochemistry, or confirmatory FISH studies will prevent misdiagnosis. The disorder is best managed by an interprofessional team that includes a hematologist-medical oncologist, internist, radiologist, radiation-oncologist, bone marrow transplant team, experienced nursing staff, and clinical trial teams. It is important to recognize the indolent form of MCL that might require monitoring with aggressive forms that require immediate systemic treatments. With the availability now of multiple second-line treatments in relapsed settings, including CAR-T cell treatments recognizing the complications and side effects related to these treatments can help guide the management of MCL patients effectively.
Media
(Click Image to Enlarge)
References
Li S, Xu J, You MJ. The pathologic diagnosis of mantle cell lymphoma. Histology and histopathology. 2021 Oct:36(10):1037-1051. doi: 10.14670/HH-18-351. Epub 2021 Jun 11 [PubMed PMID: 34114641]
Fu K, Weisenburger DD, Greiner TC, Dave S, Wright G, Rosenwald A, Chiorazzi M, Iqbal J, Gesk S, Siebert R, De Jong D, Jaffe ES, Wilson WH, Delabie J, Ott G, Dave BJ, Sanger WG, Smith LM, Rimsza L, Braziel RM, Müller-Hermelink HK, Campo E, Gascoyne RD, Staudt LM, Chan WC, Lymphoma/Leukemia Molecular Profiling Project. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood. 2005 Dec 15:106(13):4315-21 [PubMed PMID: 16123218]
Vegliante MC, Palomero J, Pérez-Galán P, Roué G, Castellano G, Navarro A, Clot G, Moros A, Suárez-Cisneros H, Beà S, Hernández L, Enjuanes A, Jares P, Villamor N, Colomer D, Martín-Subero JI, Campo E, Amador V. SOX11 regulates PAX5 expression and blocks terminal B-cell differentiation in aggressive mantle cell lymphoma. Blood. 2013 Mar 21:121(12):2175-85. doi: 10.1182/blood-2012-06-438937. Epub 2013 Jan 15 [PubMed PMID: 23321250]
Level 3 (low-level) evidenceNavarro A, Beà S, Jares P, Campo E. Molecular Pathogenesis of Mantle Cell Lymphoma. Hematology/oncology clinics of North America. 2020 Oct:34(5):795-807. doi: 10.1016/j.hoc.2020.05.002. Epub 2020 Jul 22 [PubMed PMID: 32861278]
Lynch DT, Foucar K. Discrete vacuoles in lymphocytes as a subtle clue to mantle cell lymphoma. Blood. 2016 Jun 23:127(25):3292. doi: 10.1182/blood-2016-03-706101. Epub [PubMed PMID: 28092871]
Argatoff LH, Connors JM, Klasa RJ, Horsman DE, Gascoyne RD. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997 Mar 15:89(6):2067-78 [PubMed PMID: 9058729]
Level 3 (low-level) evidenceRomaguera JE, Medeiros LJ, Hagemeister FB, Fayad LE, Rodriguez MA, Pro B, Younes A, McLaughlin P, Goy A, Sarris AH, Dang NH, Samaniego F, Brown HM, Gagneja HK, Cabanillas F. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer. 2003 Feb 1:97(3):586-91 [PubMed PMID: 12548600]
Hoster E, Rosenwald A, Berger F, Bernd HW, Hartmann S, Loddenkemper C, Barth TF, Brousse N, Pileri S, Rymkiewicz G, Kodet R, Stilgenbauer S, Forstpointner R, Thieblemont C, Hallek M, Coiffier B, Vehling-Kaiser U, Bouabdallah R, Kanz L, Pfreundschuh M, Schmidt C, Ribrag V, Hiddemann W, Unterhalt M, Kluin-Nelemans JC, Hermine O, Dreyling MH, Klapper W. Prognostic Value of Ki-67 Index, Cytology, and Growth Pattern in Mantle-Cell Lymphoma: Results From Randomized Trials of the European Mantle Cell Lymphoma Network. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016 Apr 20:34(12):1386-94. doi: 10.1200/JCO.2015.63.8387. Epub 2016 Feb 29 [PubMed PMID: 26926679]
Level 1 (high-level) evidenceXu J, Medeiros LJ, Saksena A, Wang M, Zhou J, Li J, Yin CC, Tang G, Wang L, Lin P, Li S. CD10-positive mantle cell lymphoma: clinicopathologic and prognostic study of 30 cases. Oncotarget. 2018 Feb 20:9(14):11441-11450. doi: 10.18632/oncotarget.23571. Epub 2017 Dec 15 [PubMed PMID: 29545910]
Level 3 (low-level) evidenceGeisler CH, Kolstad A, Laurell A, Räty R, Jerkeman M, Eriksson M, Nordström M, Kimby E, Boesen AM, Nilsson-Ehle H, Kuittinen O, Lauritzsen GF, Ralfkiaer E, Ehinger M, Sundström C, Delabie J, Karjalainen-Lindsberg ML, Brown P, Elonen E, Nordic Lymphoma Group. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT). Blood. 2010 Feb 25:115(8):1530-3. doi: 10.1182/blood-2009-08-236570. Epub 2009 Dec 23 [PubMed PMID: 20032504]
Hoster E. Prognostic relevance of clinical risk factors in mantle cell lymphoma. Seminars in hematology. 2011 Jul:48(3):185-8. doi: 10.1053/j.seminhematol.2011.06.001. Epub [PubMed PMID: 21782060]
Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, Pfreundschuh M, Reiser M, Metzner B, Einsele H, Peter N, Jung W, Wörmann B, Ludwig WD, Dührsen U, Eimermacher H, Wandt H, Hasford J, Hiddemann W, Unterhalt M, German Low Grade Lymphoma Study Group (GLSG), European Mantle Cell Lymphoma Network. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008 Jan 15:111(2):558-65 [PubMed PMID: 17962512]
Lee C, Martin P. Watch and Wait in Mantle Cell Lymphoma. Hematology/oncology clinics of North America. 2020 Oct:34(5):837-847. doi: 10.1016/j.hoc.2020.06.002. Epub 2020 Jul 29 [PubMed PMID: 32861281]
Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, van Hoek M, de Zwart E, Mittag D, Demont D, Verkaik S, Krantz F, Pearson PG, Ulrich R, Kaptein A. Acalabrutinib (ACP-196): A Covalent Bruton Tyrosine Kinase Inhibitor with a Differentiated Selectivity and In Vivo Potency Profile. The Journal of pharmacology and experimental therapeutics. 2017 Nov:363(2):240-252. doi: 10.1124/jpet.117.242909. Epub 2017 Sep 7 [PubMed PMID: 28882879]
Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, Barrientos JC, Chmielowska E, Radford J, Stilgenbauer S, Dreyling M, Jedrzejczak WW, Johnson P, Spurgeon SE, Zhang L, Baher L, Cheng M, Lee D, Beaupre DM, Rule S. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015 Aug 6:126(6):739-45. doi: 10.1182/blood-2015-03-635326. Epub 2015 Jun 9 [PubMed PMID: 26059948]
Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, Offner F, Caballero D, Joao C, Witzens-Harig M, Hess G, Bence-Bruckler I, Cho SG, Bothos J, Goldberg JD, Enny C, Traina S, Balasubramanian S, Bandyopadhyay N, Sun S, Vermeulen J, Rizo A, Rule S. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet (London, England). 2016 Feb 20:387(10020):770-8. doi: 10.1016/S0140-6736(15)00667-4. Epub 2015 Dec 7 [PubMed PMID: 26673811]
Level 1 (high-level) evidenceRule S, Dreyling M, Goy A, Hess G, Auer R, Kahl B, Cavazos N, Liu B, Yang S, Clow F, Goldberg JD, Beaupre D, Vermeulen J, Wildgust M, Wang M. Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: a pooled analysis from three open-label studies. British journal of haematology. 2017 Nov:179(3):430-438. doi: 10.1111/bjh.14870. Epub 2017 Aug 18 [PubMed PMID: 28832957]
Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, Damaj G, Doorduijn J, Lamy T, Morschhauser F, Panizo C, Shah B, Davies A, Eek R, Dupuis J, Jacobsen E, Kater AP, Le Gouill S, Oberic L, Robak T, Covey T, Dua R, Hamdy A, Huang X, Izumi R, Patel P, Rothbaum W, Slatter JG, Jurczak W. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet (London, England). 2018 Feb 17:391(10121):659-667. doi: 10.1016/S0140-6736(17)33108-2. Epub 2017 Dec 11 [PubMed PMID: 29241979]
Song Y, Zhou K, Zou D, Zhou J, Hu J, Yang H, Zhang H, Ji J, Xu W, Jin J, Lv F, Feng R, Gao S, Guo H, Zhou L, Elstrom R, Huang J, Novotny W, Wei R, Zhu J. Treatment of Patients with Relapsed or Refractory Mantle-Cell Lymphoma with Zanubrutinib, a Selective Inhibitor of Bruton's Tyrosine Kinase. Clinical cancer research : an official journal of the American Association for Cancer Research. 2020 Aug 15:26(16):4216-4224. doi: 10.1158/1078-0432.CCR-19-3703. Epub 2020 May 27 [PubMed PMID: 32461234]
Tam CS, Opat S, Simpson D, Cull G, Munoz J, Phillips TJ, Kim WS, Rule S, Atwal SK, Wei R, Novotny W, Huang J, Wang M, Trotman J. Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood advances. 2021 Jun 22:5(12):2577-2585. doi: 10.1182/bloodadvances.2020004074. Epub [PubMed PMID: 34152395]
Level 3 (low-level) evidenceDavids MS, Roberts AW, Kenkre VP, Wierda WG, Kumar A, Kipps TJ, Boyer M, Salem AH, Pesko JC, Arzt JA, Mantas M, Kim SY, Seymour JF. Long-term Follow-up of Patients with Relapsed or Refractory Non-Hodgkin Lymphoma Treated with Venetoclax in a Phase I, First-in-Human Study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2021 Sep 1:27(17):4690-4695. doi: 10.1158/1078-0432.CCR-20-4842. Epub 2021 Jun 3 [PubMed PMID: 34083230]
Goy A, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, Epner E, Krishnan A, Leonard JP, Lonial S, Nasta S, O'Connor OA, Shi H, Boral AL, Fisher RI. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Annals of oncology : official journal of the European Society for Medical Oncology. 2009 Mar:20(3):520-5. doi: 10.1093/annonc/mdn656. Epub 2008 Dec 12 [PubMed PMID: 19074748]
Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, Epner E, Krishnan A, Leonard JP, Lonial S, Stadtmauer EA, O'Connor OA, Shi H, Boral AL, Goy A. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Oct 20:24(30):4867-74 [PubMed PMID: 17001068]
Goy A, Sinha R, Williams ME, Kalayoglu Besisik S, Drach J, Ramchandren R, Zhang L, Cicero S, Fu T, Witzig TE. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 Oct 10:31(29):3688-95. doi: 10.1200/JCO.2013.49.2835. Epub 2013 Sep 3 [PubMed PMID: 24002500]
Zinzani PL, Vose JM, Czuczman MS, Reeder CB, Haioun C, Polikoff J, Tilly H, Zhang L, Prandi K, Li J, Witzig TE. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Annals of oncology : official journal of the European Society for Medical Oncology. 2013 Nov:24(11):2892-7. doi: 10.1093/annonc/mdt366. Epub 2013 Sep 12 [PubMed PMID: 24030098]
Wang ML, Lee H, Chuang H, Wagner-Bartak N, Hagemeister F, Westin J, Fayad L, Samaniego F, Turturro F, Oki Y, Chen W, Badillo M, Nomie K, DeLa Rosa M, Zhao D, Lam L, Addison A, Zhang H, Young KH, Li S, Santos D, Medeiros LJ, Champlin R, Romaguera J, Zhang L. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. The Lancet. Oncology. 2016 Jan:17(1):48-56. doi: 10.1016/S1470-2045(15)00438-6. Epub 2015 Nov 28 [PubMed PMID: 26640039]
Lamm W, Kaufmann H, Raderer M, Hoffmann M, Chott A, Zielinski C, Drach J. Bortezomib combined with rituximab and dexamethasone is an active regimen for patients with relapsed and chemotherapy-refractory mantle cell lymphoma. Haematologica. 2011 Jul:96(7):1008-14. doi: 10.3324/haematol.2011.041392. Epub 2011 Apr 12 [PubMed PMID: 21486866]
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, McSweeney PA, Miklos DB, Pagel JM, Kersten MJ, Milpied N, Fung H, Topp MS, Houot R, Beitinjaneh A, Peng W, Zheng L, Rossi JM, Jain RK, Rao AV, Reagan PM. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. The New England journal of medicine. 2020 Apr 2:382(14):1331-1342. doi: 10.1056/NEJMoa1914347. Epub [PubMed PMID: 32242358]
Teixeira Mendes LS, Peters N, Attygalle AD, Wotherspoon A. Cyclin D1 overexpression in proliferation centres of small lymphocytic lymphoma/chronic lymphocytic leukaemia. Journal of clinical pathology. 2017 Oct:70(10):899-902. doi: 10.1136/jclinpath-2017-204364. Epub 2017 Apr 13 [PubMed PMID: 28408436]
Leitch HA, Gascoyne RD, Chhanabhai M, Voss NJ, Klasa R, Connors JM. Limited-stage mantle-cell lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology. 2003 Oct:14(10):1555-61 [PubMed PMID: 14504058]
Level 2 (mid-level) evidenceDreyling M, Ferrero S, Vogt N, Klapper W, European Mantle Cell Lymphoma Network. New paradigms in mantle cell lymphoma: is it time to risk-stratify treatment based on the proliferative signature? Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 Oct 15:20(20):5194-206. doi: 10.1158/1078-0432.CCR-14-0836. Epub [PubMed PMID: 25320369]
Eskelund CW, Dahl C, Hansen JW, Westman M, Kolstad A, Pedersen LB, Montano-Almendras CP, Husby S, Freiburghaus C, Ek S, Pedersen A, Niemann C, Räty R, Brown P, Geisler CH, Andersen MK, Guldberg P, Jerkeman M, Grønbæk K. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017 Oct 26:130(17):1903-1910. doi: 10.1182/blood-2017-04-779736. Epub 2017 Aug 17 [PubMed PMID: 28819011]
Maddocks K. Update on mantle cell lymphoma. Blood. 2018 Oct 18:132(16):1647-1656. doi: 10.1182/blood-2018-03-791392. Epub 2018 Aug 28 [PubMed PMID: 30154113]
Sapkota S, Shaikh H. Non-Hodgkin Lymphoma. StatPearls. 2024 Jan:(): [PubMed PMID: 32644754]