Introduction
The mammillary bodies are brainstem nuclei on the posteroinferior aspect of the hypothalamus. There are 2 mammillary bodies on either side of the midline. Early anatomists named the structures after mammary tissue because they resembled small breasts (Figure 1A).[1]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The mammillary bodies are one of the primary nuclei within the Papez Circuit (Figure 1B). The circuit assists with spatial and episodic memory consolidation and storage.[2][3] Therefore, damage to the fornix or mammillary bodies results in amnesia.[4][5] The mammillary bodies also function to assist with emotion and reward behaviors and goal-directed behaviors.[6][7]
The primary function associated with the mammillary bodies is recollective memory. Memory information begins within the hippocampus. Theta waves activate CA3 neurons in the hippocampus. Information about memory transmits through the fornix to the mammillary bodies (orange line, Figure 1C). Mammillary bodies project to the anterior thalamic nuclei through the mammillothalamic tract. The anterior thalamic nuclei projects to the cingulate cortex, and the cingulate cortex to the entorhinal cortex. There is then a bi-directional projection between the hippocampus and the entorhinal cortex that completes the circuit; the Papez circuit (Figure 1B).
Beyond the transmission of memory information, the mammillary bodies help facilitate the creation of appropriate behavioral reactions through reciprocal connections with the tegmentum (blue line, Figure 1C). The medial mammillary nucleus has bi-directional projections to the ventral tegmental area. The lateral mammillary nucleus has bi-directional connections to the dorsal tegmental nucleus. The ventral tegmentum is rich with dopamine neurons, and thus functions as a reward (pleasure) center of the brain. Memory information from the hippocampus combines with information about reward or aversive attributes about a specific stimulus or set of stimuli in the environment, which allows the processing of the salience of environmental stimuli.[8] The mammillary bodies send information about reward or aversion through the anterior thalamic nucleus to the cingulate cortex where it assists with the creation of emotional perception.[9]
The dorsal tegmental connections to the lateral mammillary nucleus function to process goal-directed movement. The location of the dorsal tegmental nucleus is between the trochlear nuclei within the midbrain.[9] Neurons in the dorsal tegmental nucleus respond specifically to movement velocity, turning behaviors, and learned goal-directed behaviors.[10] It also has direct connections with the ventral tegmental nucleus. These connections help guide processing in the reward circuit to facilitate improved goal-directed behaviors.[10] The mammillary bodies connect this stream of information to the cerebellum.[6] Researchers hypothesize that this connection assists the cerebellum with its role in visuospatial orientation, movement learning, and motor memory.[6] If the hypothesis is correct, disruption of information flow between the cerebellum and mammillary bodies may contribute to navigational impairments during the early stages of neurodegenerative disorders.[11]
The mammillary bodies do not have interneurons. The processing of information within the mammillary bodies occurs from projection neurons of other regions of the brain. It receives both dopaminergic and acetylcholine reciprocal projections from the tegmentum (blue line, Figure 1C) that help to regulate what information the mammillary bodies send forward to the anterior thalamic nucleus (Papez circuit) and the cerebellum. Because tegmental connections are reciprocal (bi-directional), the mammillary activity also influences the tegmentum and other connections of the tegmentum (e.g., amygdala, prefrontal cortex, hippocampus, and nucleus accumbens). Activation of the acetylcholine and dopamine circuits activate norepinephrine and serotonin systems downstream.[7] Each of these neurotransmitter systems helps to mediate memory storage within the cortex. They also help to facilitate other limbic functions (e.g., attention, learning, memory, motor systems, decision, planning, and emotion).[8][10]
Embryology
In development, the mammillary bodies form from the ectoderm layer. The ectoderm layer forms the neural tube that closes at six weeks of gestation. The neural tube differentiates into the forebrain, midbrain, hindbrain, and spinal cord. The forebrain section develops into the telencephalon and diencephalon. The hypothalamus, mammillary bodies, and posterior pituitary form from the diencephalon.[7][12]
Blood Supply and Lymphatics
Mammillary bodies receive blood supply from the circle of Willis, which runs on the inferior surface of the hypothalamus. Branches off the posteromedial artery directly supply the mammillary bodies. These arteries originate from the posterior cerebral artery but can originate off of the posterior communicating artery. The posteromedial artery has three branches: rostral, deep penetrating, and caudal. The caudal branches supply the mammillary bodies.[7]
Capillary plexus around the region carry blood away from the mammillary bodies through the hypothalamo-neurohypophysial portal system.[7] Blood moves from this portal system into the cavernous sinus. From the cavernous sinus, it travels through either the superior or inferior petrosal sinuses to enter the jugular vein.
Lymphatics within the brain differ from other regions of the body. Within the brain, the blood-brain barrier regulates the movement/transport of nutrients, small molecules, metabolites, and/or cells and toxins in and out of the brain. The blood-brain barrier is a functional and structural boundary between vasculature and the brain that forms from brain vasculature endothelial cells, adjacent astrocytic end-feet, and pericytes. Tight junctions connect brain endothelial cells and create a physical barrier. This barrier forces most molecules to travel through selective transcellular routes – forming a transport barrier. In addition, there is also a synergistic relationship between brain endothelial cells and astrocytes. Perivascular astrocytic end-feet surround brain endothelial cells, but a basal lamina separates them. The barrier formed from these astrocytic end-feet and endothelial cells protect the brain from toxins and assist with transport of nutrients, wastes, glucose, lipids, and neurotransmitters between capillaries and neurons in the brain. [7]
Nerves
Mammillary bodies have three large direct connection: hippocampus, thalamus, and tegmental nuclei (Figure 1C). The hippocampus projects to the mammillary bodies through afferent fibers in the fornix (orange line, Figure 1C). The mammillary bodies then process information about memory and then relay it up to the thalamus for additional processing through the mammillothalamic tract. This tract terminates within the anterior thalamic nuclei. (red line, Figure 1C). Both the hippocampal projections to the mammillary bodies as well as the projections from the mammillary bodies to the thalamus are uni-directional. These circuits are a part of the Papez circuit (Figure 1B).[7]
Mammillary bodies also have bi-directional connections with the tegmental nuclei through the mammillotegmental tract (blue line, Figure 1C). Because the tegmentum has numerous connections to several limbic structures, mammillary body input to this region can influence a variety of limbic functions (see above).[7]
Muscles
Although the mammillary bodies do not directly contact muscle, they have projections to the cerebellum, a secondary motor brain region. Mammillary inputs from the tegmentum carry information about movement velocity, turning behaviors, and learned goal-directed behaviors.[10][6] The mammillary bodies also receive information about memory from the hippocampus and stimulus salience from the tegmentum. Therefore, the mammillary bodies may also assist the cerebellum in its motor learning functions.
Physiologic Variants
The mammillary bodies are a part of the hypothalamus. Physiology of this region of the brain is necessary for survival. Therefore, few developmental/physiological variations allow for the survival of a fetus.
Throughout life, the mammillary bodies adapt to environmental and physiological changes of an individual; this enables the individual to alter behavior based on learned salience of a stimulus or set of stimuli. Although it is ideal for the mammillary bodies to change with variations in environmental stimuli, the neurons, and connections with the mammillary bodies are susceptible to damage based on environmental factors (e.g., alcohol, thiamine deficiency).[9][13] These environmental factors, in addition to aging-related neurodegeneration, can dramatically influence the function of mammillary circuits.[14]
Surgical Considerations
The location of the mammillary bodies is one factor that surgeons use as a guide in patients with craniopharyngiomas that involve the hypothalamus. In these patients, the location of the mammillary bodies move. Surgeons can measure the angle between the base of the mammillary bodies and the plane of the floor of the fourth ventricle in a midsagittal MRI as an indicator of the location of these types of tumors. Angles less than 60 degrees indicate a tuberal-interventricular tumor. Angles greater than 90 degrees show tumor invagination of the third ventricle. Thus mammillary body position is a quick reference for the location of these types of tumors. By understanding the location of the tumors, surgeons have more information on how to treat and manage these tumors.[15]
Clinical Significance
Damage to the mammillary bodies can occur through trauma, stroke, tumors, or environmental factors (e.g., thiamine deficiency or chronic alcoholism). Thiamine deficiency commonly causes Korsakoff's syndrome. This syndrome can occur with Wernicke encephalopathy, caused by chronic alcoholism because alcoholism can induce a thiamine deficiency.[9][13] In both disorders, these environmental factors cause bilateral degeneration of the neurons or projections of the mammillary bodies. Bilateral mammillary damage causes anterograde amnesia in patients. The memory loss can occur independently or can accompany a variety of other symptoms (e.g., reckless behavior, reduced motivation, confabulation).[16][9]
Other Issues
The mammillary bodies and circuits associated with the mammillary bodies (e.g., limbic structures) influence many higher-order functions. Researchers have hypothesized that stimulation of the Papez circuit may have beneficial effects on memory and attention as well as decreasing the incidence of depression in individuals with dementia. In animal models, deep brain stimulation of the hippocampus shows increased memory function with no signs of anxiety. Researchers attributed these results to increased activation of the cingulate gyrus through the hippocampus-mammillary tract, mammillothalamic tract, and thalamic projections to the cingulate gyrus (the brain location where emotion is perceived).[17]
Deep brain stimulation has been a treatment for Alzheimer's disease, Parkinson's disease, and dementia.[18][17][19][20][21] The location of the stimulation probe has shown optimal results when placed near the fornix to stimulate the hippocampal fibers projecting to the mammillary bodies.[20] These types of treatments have altered the rate of brain atrophy in Alzheimer patients.[22] How and why atrophy slows down by activation of the Papez circuit is unknown. However, these experiments show that deep brain stimulation of the Papez circuit may be a viable clinical treatment for individuals with a variety of psychiatric or dementia disorders.
Media
(Click Image to Enlarge)
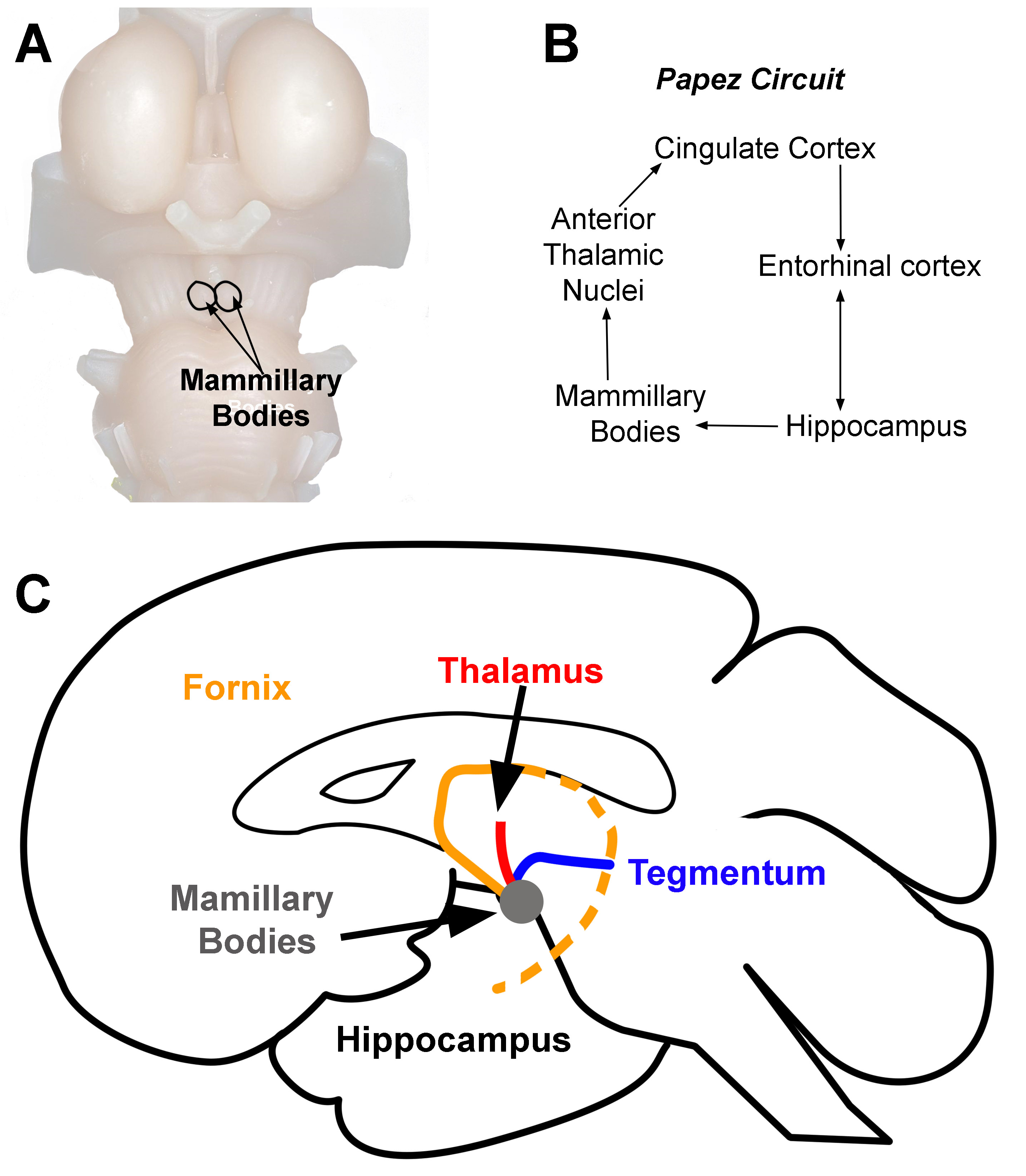
Mammillary Bodies. Location of mammillary bodies on the brainstem (A). Papez circuit (B). Arrows indicate the direction of information flow (C). Connections of the mammillary bodies (grey area). The three primary connections of the mammillary bodies are from (1) the hippocampus to the mammillary bodies (orange line). This pathway is the fornix. (2) The mammillary bodies to the thalamus (red line), and (3) the mammillary bodies to and from the tegmentum (blue line).
Contributed by D Peterson, PhD
References
Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nature reviews. Neuroscience. 2004 Jan:5(1):35-44 [PubMed PMID: 14708002]
Level 3 (low-level) evidenceVertes RP, Albo Z, Viana Di Prisco G. Theta-rhythmically firing neurons in the anterior thalamus: implications for mnemonic functions of Papez's circuit. Neuroscience. 2001:104(3):619-25 [PubMed PMID: 11440795]
Level 3 (low-level) evidenceAggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. The Behavioral and brain sciences. 1999 Jun:22(3):425-44; discussion 444-89 [PubMed PMID: 11301518]
Thomas AG, Koumellis P, Dineen RA. The fornix in health and disease: an imaging review. Radiographics : a review publication of the Radiological Society of North America, Inc. 2011 Jul-Aug:31(4):1107-21. doi: 10.1148/rg.314105729. Epub [PubMed PMID: 21768242]
Copenhaver BR, Rabin LA, Saykin AJ, Roth RM, Wishart HA, Flashman LA, Santulli RB, McHugh TL, Mamourian AC. The fornix and mammillary bodies in older adults with Alzheimer's disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry research. 2006 Oct 30:147(2-3):93-103 [PubMed PMID: 16920336]
Cacciola A, Milardi D, Calamuneri A, Bonanno L, Marino S, Ciolli P, Russo M, Bruschetta D, Duca A, Trimarchi F, Quartarone A, Anastasi G. Constrained Spherical Deconvolution Tractography Reveals Cerebello-Mammillary Connections in Humans. Cerebellum (London, England). 2017 Apr:16(2):483-495. doi: 10.1007/s12311-016-0830-9. Epub [PubMed PMID: 27774574]
Bear MH, Reddy V, Bollu PC. Neuroanatomy, Hypothalamus. StatPearls. 2023 Jan:(): [PubMed PMID: 30252249]
Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014 Jan:76 Pt B(0 0):351-9. doi: 10.1016/j.neuropharm.2013.03.019. Epub 2013 Apr 8 [PubMed PMID: 23578393]
Level 3 (low-level) evidenceBalak N, Balkuv E, Karadag A, Basaran R, Biceroglu H, Erkan B, Tanriover N. Mammillothalamic and Mammillotegmental Tracts as New Targets for Dementia and Epilepsy Treatment. World neurosurgery. 2018 Feb:110():133-144. doi: 10.1016/j.wneu.2017.10.168. Epub 2017 Nov 10 [PubMed PMID: 29129763]
Redila V,Kinzel C,Jo YS,Puryear CB,Mizumori SJ, A role for the lateral dorsal tegmentum in memory and decision neural circuitry. Neurobiology of learning and memory. 2015 Jan; [PubMed PMID: 24910282]
Level 3 (low-level) evidenceLevisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain : a journal of neurology. 2000 May:123 ( Pt 5)():1041-50 [PubMed PMID: 10775548]
Level 3 (low-level) evidenceQin C, Li J, Tang K. The Paraventricular Nucleus of the Hypothalamus: Development, Function, and Human Diseases. Endocrinology. 2018 Sep 1:159(9):3458-3472. doi: 10.1210/en.2018-00453. Epub [PubMed PMID: 30052854]
Level 2 (mid-level) evidenceKril JJ, Harper CG. Neuroanatomy and neuropathology associated with Korsakoff's syndrome. Neuropsychology review. 2012 Jun:22(2):72-80. doi: 10.1007/s11065-012-9195-0. Epub 2012 Apr 14 [PubMed PMID: 22528862]
Level 3 (low-level) evidenceRoberts DE,Killiany RJ,Rosene DL, Neuron numbers in the hypothalamus of the normal aging rhesus monkey: stability across the adult lifespan and between the sexes. The Journal of comparative neurology. 2012 Apr 15; [PubMed PMID: 21935936]
Level 3 (low-level) evidencePascual JM, Prieto R, Carrasco R, Barrios L. Displacement of mammillary bodies by craniopharyngiomas involving the third ventricle: surgical-MRI correlation and use in topographical diagnosis. Journal of neurosurgery. 2013 Aug:119(2):381-405. doi: 10.3171/2013.1.JNS111722. Epub 2013 Mar 29 [PubMed PMID: 23540270]
Level 2 (mid-level) evidenceDusoir H, Kapur N, Byrnes DP, McKinstry S, Hoare RD. The role of diencephalic pathology in human memory disorder. Evidence from a penetrating paranasal brain injury. Brain : a journal of neurology. 1990 Dec:113 ( Pt 6)():1695-706 [PubMed PMID: 2276041]
Level 3 (low-level) evidenceHescham S, Jahanshahi A, Meriaux C, Lim LW, Blokland A, Temel Y. Behavioral effects of deep brain stimulation of different areas of the Papez circuit on memory- and anxiety-related functions. Behavioural brain research. 2015 Oct 1:292():353-60. doi: 10.1016/j.bbr.2015.06.032. Epub 2015 Jun 25 [PubMed PMID: 26119240]
Hescham S, Lim LW, Jahanshahi A, Blokland A, Temel Y. Deep brain stimulation in dementia-related disorders. Neuroscience and biobehavioral reviews. 2013 Dec:37(10 Pt 2):2666-75. doi: 10.1016/j.neubiorev.2013.09.002. Epub 2013 Sep 20 [PubMed PMID: 24060532]
Level 3 (low-level) evidenceGratwicke J, Kahan J, Zrinzo L, Hariz M, Limousin P, Foltynie T, Jahanshahi M. The nucleus basalis of Meynert: a new target for deep brain stimulation in dementia? Neuroscience and biobehavioral reviews. 2013 Dec:37(10 Pt 2):2676-88. doi: 10.1016/j.neubiorev.2013.09.003. Epub 2013 Sep 11 [PubMed PMID: 24035740]
Stypulkowski PH, Stanslaski SR, Giftakis JE. Modulation of hippocampal activity with fornix Deep Brain Stimulation. Brain stimulation. 2017 Nov-Dec:10(6):1125-1132. doi: 10.1016/j.brs.2017.09.002. Epub 2017 Sep 6 [PubMed PMID: 28927833]
Zhang C, Hu WH, Wu DL, Zhang K, Zhang JG. Behavioral effects of deep brain stimulation of the anterior nucleus of thalamus, entorhinal cortex and fornix in a rat model of Alzheimer's disease. Chinese medical journal. 2015 May 5:128(9):1190-5. doi: 10.4103/0366-6999.156114. Epub [PubMed PMID: 25947402]
Sankar T,Chakravarty MM,Bescos A,Lara M,Obuchi T,Laxton AW,McAndrews MP,Tang-Wai DF,Workman CI,Smith GS,Lozano AM, Deep Brain Stimulation Influences Brain Structure in Alzheimer's Disease. Brain stimulation. 2015 May-Jun; [PubMed PMID: 25814404]