Introduction
Meningioma is the commonest primary central nervous system tumor accounting for about 37.6% of them; and approximately 50% of all benign brain tumors.[1][2][3] Meningioma originates from the meningeal layers of either the brain or the spinal cord.[4] These tumors are classified into three grades, according to the World Health Organization (WHO).[4] The majority of meningiomas are benign and considered grade 1.[1][2] About 1 to 3% of meningiomas can be transformed into malignant tumors with a 5-year survival rate of 32 to 64%.[2] Several predisposing factors increase the risk of occurrence, including genetic disorders such as neurofibromatosis type 2, exposure to radiation, hormonal therapy, and family history.[3]
The clinical manifestation is dependent on the location and the size of the meningioma.[4] Therefore, some patients can be asymptomatic, while others might experience neurological deficits. Brain magnetic resonance imaging (MRI) is the gold standard radiological investigation for diagnosing meningioma.[4] Asymptomatic and slow-growing meningiomas are usually managed with observation along with routine imaging.[4] However, for fast-growing tumors, large tumors, or symptomatic patients, surgery remains the best management option. This review will primarily discuss intracranial meningiomas.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Most meningiomas are sporadic in origin, but some have been associated with certain conditions and risk factors. Environmental factors such as obesity, alcoholism, exposure to ionizing radiation, radiotherapy, hormonal factors such as exposure to exogenous hormones, hormonal replacement therapy, use of oral contraceptive pills, and breast cancer can increase the risk of incidence of meningiomas.[3][4][5]
Meningiomas express progesterone, estrogen, and androgen receptors on their membranes.[6] Progesterone receptors can be found in up to 72% of tumors.[7] Studies have shown that they exhibit changes in size during pregnancy and the luteal phase of the menstrual cycle. The higher incidence among females is due to hormonal factors. The incidence is also highly associated with meningioma-affected first-degree relatives, genetic syndromes such as neurofibromatosis type 2, von Hippel Lindau disease, multiple endocrine neoplasia type 1, Li-Fraumeni, Cowden disease, and Gorlin syndrome.[3][4]
Epidemiology
The worldwide incidence of primary brain tumors in 2015 was estimated to be 10.82 per 100,000 people per year.[8] Between the years 2010 and 2014, the incidence was 8.3 per 100,000 persons; therefore, it has been increasing.[4] The mean age of presentation is 66 years, with a female-to-male ratio of 2.3:1.[1][7][9] There is a higher incidence in African Americans, with a female-to-male ratio of 2.27 to 1.[1][4]
Histologically confirmed meningiomas accounted for 37.6% of all primary central nervous system tumors and 50% of all benign primary central nervous system tumors. It affects approximately 1.8 to 13 per 100,000 individuals per year.[10] The prevalence in the United States is 97.5/100,000 individuals, with more than 170,000 people diagnosed with meningioma.[3]
Meningioma is more common in adults than in children, with an incidence of 37.75 per 100,000 in the 75 to 84 age group. Whereas 0.14 per 100,000 in children from 0 to 19 years of age. Neurofibromatosis type 2 is associated with approximately 1% of meningiomas.[4]
According to the WHO grades for meningioma, 80-81% are considered typical or grade 1. While 17 to 18% of them are atypical or grade 2, and 1.7% are anaplastic or grade 3 meningiomas.[1][4] The recurrence rate of meningioma at ten years can reach 20%.[11] High recurrence rates have been reported in higher-grade meningiomas. In grade 3, the recurrence rate is about 50 to 94%. Whereas in grades 1 and 2, the recurrence rate is 7 to 25%, and 29 to 52%, respectively.[12]
Spinal meningiomas account for 30.7 to 38.8% of all primary intradural spinal tumors in the United States in those patients aged 20 years and older, with an age-adjusted incidence of 0.193 per 100,000 of the population.[1] These meningiomas occur at a frequency of 80.3% in females.[13]
Pathophysiology
Meningioma commonly originates from meningothelial arachnoid cap cells. Most meningiomas are sporadic, benign, and slowly growing. They usually exhibit one or more focal chromosomal deletions; however, malignant meningiomas often have multiple chromosomal mutations. More genetic mutations are associated with accelerated growth and increased tumor grade.[3]
A genetic mutation on chromosome 22 in neurofibromatosis type 2 is one of the most common predisposing conditions seen in sporadic meningiomas. Other chromosomal mutations reported in meningiomas are 1p, 6q, 14q, and 18q. There are also genes found in inherited meningiomas such as CREB binding protein, protein patched homolog 1, phosphatase and tensin homolog, cyclin-dependent kinase inhibitor 2A, Von Hippel–Lindau, and neurofibromatosis 1.
Histopathology
The WHO grade system is based on the histological features of meningioma.[14]
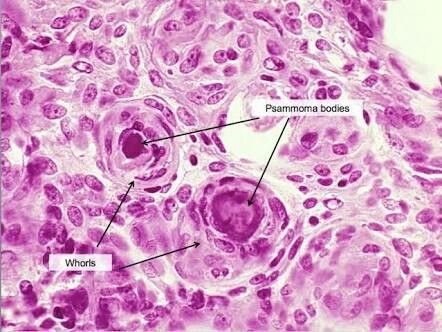
Grade 1 constitutes more than 80% of meningiomas, includes nine histological variants, and lacks anaplastic features that can be seen in other grades. These variants include meningothelial, fibroblastic, transitional or mixed, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte-rich, and metaplastic subtypes.[4][12] More than 70% of meningiomas are positive for progesterone receptors.[4][7] One of the histopathological characteristics of meningiomas is the growth of meningothelial cells that eventually mineralize to form psammoma bodies. Hyperostosis of the bone adjacent to the tumor can sometimes be identified.
Grade 2 are atypical lesions characterized by three or more of the following: necrosis, sheet-like growth, prominent nuclei, increased cellularity, or high nucleus/cytoplasm ratio. An increased mitotic activity (4-19 mitoses per 10 high-power fields) will also indicate an atypical tumor. The histological variations included in this grade include atypical, clear, and choroid cell subtypes. A meningioma with brain invasion is now considered a grade 2 tumor.[12][14]
Grade 3 are anaplastic, malignant lesions that can be similar to high-grade sarcomas, carcinoma, or melanomas with a high rate of distant metastases. A high mitotic activity (20 or more mitoses per 10 high-power fields) will also indicate a grade 3 lesion. The histological variations of this grade include papillary and rhabdoid subtypes.[12]
Cystic meningioma represents a meningioma with intratumoral or peritumoral cysts. It accounts for about 4 to 7% of meningiomas.[15] A cystic meningioma might be challenging to differentiate from an intra-axial glial or metastatic tumor. There are several hypotheses postulated on how cystic meningiomas are formed.[16] Tumor degeneration or necrosis explains that the cystic formation is due to macro-cavitation. This phenomenon results from the molecular pathogenesis of the tumor due to an intracellular regressive process. The oasis phenomenon is another explanation that occurs due to the development of an arteriolar hyalinization in the intratumoral cavity leading to ischemic necrosis. Other hypotheses include that the cystic formation might be secreted directly from the meningioma or originating from a peripheral arachnoid cyst containing CSF. Intracranial cystic meningiomas are classified into four types, according to Nauta classification.[17]
Type 1 represents a single or multiple intratumoral cysts located centrally, mainly within the tumor. Type 2 represents a single or multiple intratumoral cysts located peripherally to meningioma and surrounded by the tumor cells. Type 3 represents a single or multiple peritumoral cysts located peripherally to the meningioma lying adjacent to the parenchyma. Type 4 represents a single or multiple peritumoral cysts with walls formed by the arachnoid located between the meningioma and adjacent brain parenchyma. Another classification, el-Fiki, explain the association between the cyst contents and location categorized into four types.[18]
Type A represents a cystic meningioma containing CSF or clear fluid divided into two locations, A1 and A2. A1 represents an extratumoral cystic meningioma containing CSF and surrounded by an arachnoid membrane. A2 represents an extratumoral cystic meningioma containing clear fluid not surrounded by an arachnoid membrane. Type B represents a cystic meningioma containing a xanthochromic fluid divided into two locations, B1 and B2. B1 represents an extratumoral cystic meningioma not surrounded by a wall. B2 represents an intratumoral cystic meningioma surrounded by a thin rim of an enhancing tumor. Type C is a cystic meningioma containing a yellowish or dark brown fluid located intratumorally. Type D is a cystic meningioma containing both clear fluids of peritumoral or extratumoral cysts and small intratumoral dark brown cysts.
Meningioma en plaque (MEP) is another type of meningioma described as a carpet or sheet-like lesion. It can cause extensive angioinvasion to bones, dura, and soft tissues. These tumors grow along the dural surface and can significantly involve bony structures such as the sphenoid ridge and calvarial bones hence causing intraosseous infiltration. MEP is classified as WHO grade 1, accounting for 2 to 9% of all meningiomas, mainly occurring intracranial and rarely found in the spine.[19][20] Hyperostosis is found in 13 to 49% of MEP cases.[20][21] The histopathological evaluation of the hyperostosis in MEP usually contains whorls and syncytia of meningothelial cells. These cells can invade the medullary spaces and lead to intraosseous infiltration.[20][22] The classification by Kim et al. is based on the characteristics of the hyperostosis found in the head computed tomographic (CT) scan. The hyperostosis characteristics include the presence of homogenous, periosteal, three-layer, and diploic patterns.[23]
Radiation-induced meningioma (RIM) is a tumor that has a latent occurrence and develops as a complication from iatrogenic or environmental exposure to radiation. The incidence of RIM is not well established yet in the literature; however, these tumors have a long latency period from 2 to 63 years, with an average of approximately 38 years following radiation exposure. A high dose of radiation exposure is linked with high grades of RIM and higher tumor recurrences.[24] In contrast to spontaneous meningiomas, RIM tends to be multiple and aggressive with high recurrence rates, with some tumors containing pathological malignant features.[24][25] These tumors show a high proliferative marker MIB-1 labeling index of more than 10%, with tumor recurrence occurring within one year after surgical resection.[25] RIM can occur intracranially or in the spine, depending on the site of the radiation exposure.[26] Increased vascular endothelial growth factor levels and mRNA expression are other molecular markers reported in high-grade RIM.[24]
Ossified meningioma is an uncommon slow-growing tumor characterized by complete ossification or calcification. The ossification is different from those seen in psammomatous meningiomas.[27] Ossified meningiomas can be either located intracranially or in the spine, accounting for about 1 to 5% of all spinal meningiomas.[27] According to the WHO classification, ossified meningiomas are considered a subtype of grade 1 metaplastic meningiomas.[27] The mechanism of ossification is yet not well understood; however, it is suggested that it could be related to the metaplasia of the arachnoid and interstitial cells.[27][28]
History and Physical
The manifestations of intracranial meningioma are dependent on the affected site.[4] Most intracranial meningiomas are located supratentorial. They are commonly seen in the parasagittal, brain convexity, sphenoid ridge, anterior and posterior parafalcine areas, and olfactory groove. Other reported locations of intracranial meningiomas include suprasellar, posterior fossa (cerebellopontine), intraventricular, and intraorbital areas.[29]
Upper motor neuron signs are usually reported in intracranial meningioma, including hypertonia or clonus, hyperreflexia, positive Babinski and Hoffman signs, paresis, or paralysis. Other reported symptoms include anosmia, headaches, dizziness, visual impairments, seizures, papilledema, and behavioral changes. Patients with parasagittal and brain convexity meningiomas can present with paresis or paralysis of the affected contralateral limb. Patients with sphenoid ridge meningiomas with the involvement of the supraorbital fissure or the cavernous sinus can present with cranial nerve palsies and seizures.[4] Olfactory groove meningiomas can lead to Foster-Kennedy syndrome. This syndrome produces anosmia, contralateral papilledema, and ipsilateral optic nerve atrophy.[30][31]
Cavernous sinus meningioma can present with cranial nerve deficits of the optic, oculomotor, trochlear, trigeminal, and abducens nerves.[32] Patients with suprasellar or frontal meningiomas can present with mental, cognitive, and behavioral changes.[4] Posterior fossa and foramen magnum meningiomas can cause bulbar palsy, cerebellar symptoms, paresis, facial palsy, hearing deficit, lower cranial nerve palsies, and neck pain.[33][34] Intraventricular meningioma can lead to obstructive hydrocephalus.[35] Parasellar, cavernous sinus, and orbital meningiomas can lead to vision loss and proptosis.[36]
The most common symptoms experienced in spinal meningiomas are pain and radiculopathy, followed by neurological deficits. The neurological deficits could be either upper motor or lower motor neuron symptoms depending on the location and the compression of the nerve roots. Lower motor neuron symptoms include weakness, hypotonia, muscle fasciculations, and hyporeflexia. Local spinal tenderness is sometimes found during physical examination.[37][38]
Evaluation
The diagnosis of meningioma is based on history, physical examination, and radiological investigations. A head contrast-enhanced CT scan can be useful for patients who are not fit for MRI, to visualize hyperostosis, or in cases of calcified meningiomas.[4][39] Often meningiomas in non-contrast head CT scans appear as hyperdense or isodense dural-based lesions. While in contrast-enhanced head CT scans, most meningiomas appear as homogenous dense enhanced dural-based lesions with or without brain edema. The presence of tumor calcifications and hyperostosis, as seen in cases of ossified meningiomas and MEP, is better assessed with head CT scans.[4][21]
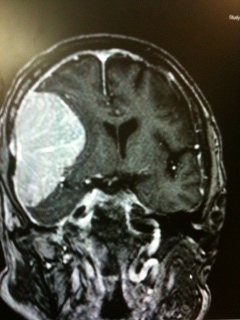
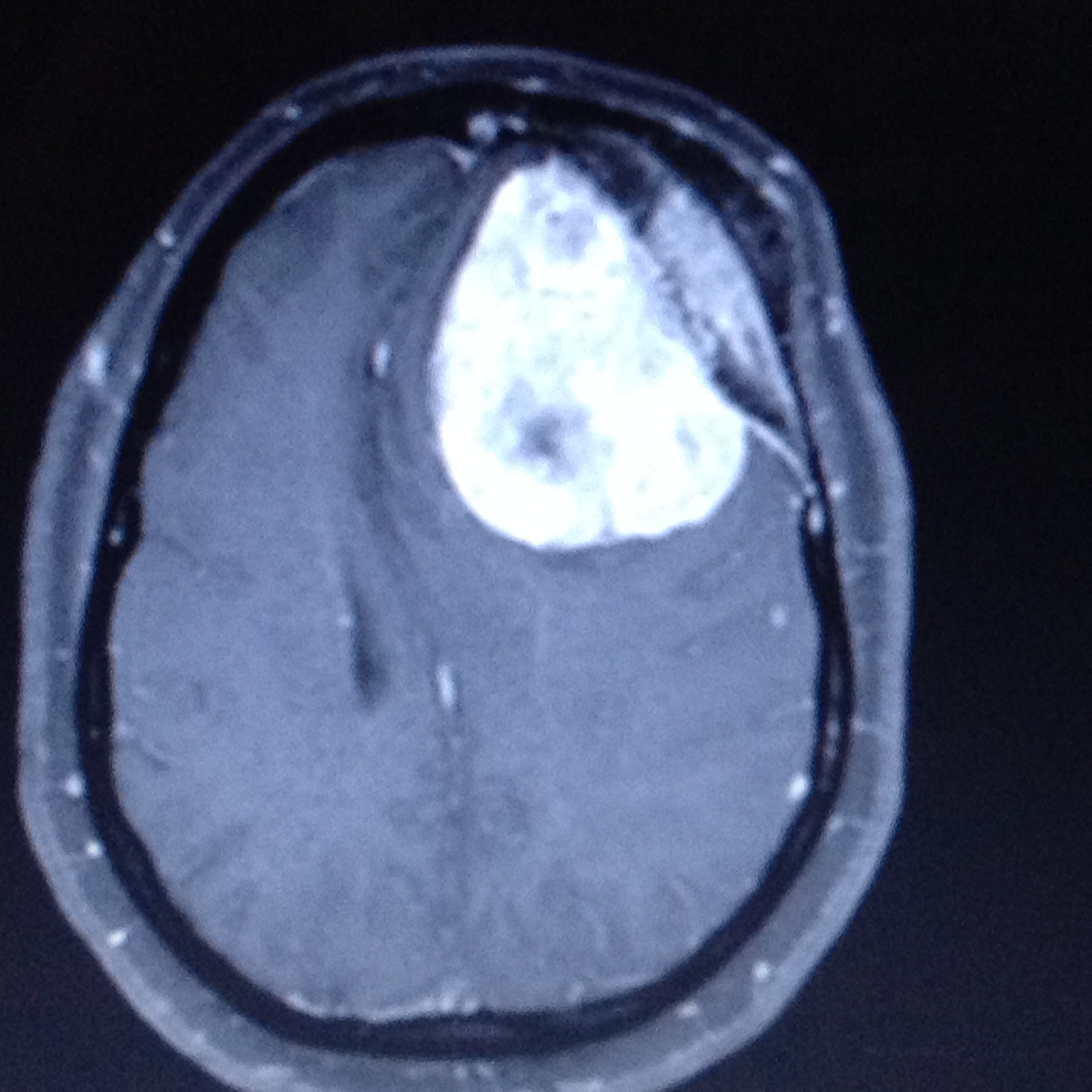
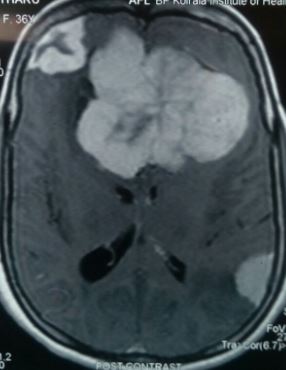
The best radiological method to diagnose meningioma is a brain MRI with contrast. It can help to distinguish extra-axial from intra-axial lesions as they have a homogenous enhancement with a feature called the dural tail. Brain MRI also helps to evaluate venous sinus involvement. Brain MRI also helps to find cystic lesions in meningiomas, which may show a sign of a mushroom-like appearance. This sign explains the tumor invagination in the brain parenchyma.[15] On brain MRI without contrast, meningiomas usually appear as a hypointense lesion on T1-weighted imaging and hyperintense on T2-weighted imaging. Some meningiomas may appear as isointense in non-contrast brain MRI on T1 and T2 weighted images. Another sign that can be seen is the CSF vascular cleft. The cleft is an entrapment of the CSF or cerebral cortical vessels found between the meningioma and the underlying cortex and is used to distinguish an extra-axial meningioma from an intra-axial lesion.[39]
Calcifications and peritumoral brain edema, which is usually of a vasogenic type, might be present. Brain edema commonly occurs due to the disruption of the blood-brain barrier. It causes the extracerebral protein-rich fluid to accumulate in the cerebral parenchyma leading to vasogenic edema.[40] Brain MRI shows edema clearly as hyperintensity in T2 weighted images. The precise causes of peritumoral edema have not been established yet in the literature. However, some features or predictive factors can influence the formation of peritumoral edema. These include the absence of an arachnoid plane on brain MRI, high levels of Ki-67 antigen labeling index, as well as the presence of irregular tumor margins.[41] Other factors include the release of vascular endothelial growth factor by the tumor cells, eventually affecting the tumor-brain barrier and causing an edematous effect.[40]
White matter buckling sign is one of the useful features to distinguish an extra-axial lesion from an intra-axial lesion. It can be seen on a head CT scan or brain MRI and is demonstrated as an inward compression or buckling of the white matter, preservation of the grey-white junction even in the presence of edema. White matter buckling sign is usually associated with extracerebral fluid collection in extra-axial lesions such as meningioma.[42]
MRI spectroscopy can be used to detect malignant meningiomas. It compares the metabolic and chemical contents of the healthy neuronal tissue with the malignant tumor. MRI spectroscopy measures the biochemical features associated with the tumor by analyzing different metabolites. Some meningiomas show increased levels of choline and decreased levels of creatinine.[39]
Digital subtraction angiography (DSA) is used to demonstrate the feeding arteries of a meningioma and to distinguish between pial and dural blood supply.[43][44] Sinus pericranii is usually found in parasagittal meningiomas. It is a vascular anomaly causing an abnormal connection between intracranial dural sinuses and extracranial veins.[43] Sunburst features, CSF-vascular cleft, and mother-in-law sign are other findings that can be found in hypervascular meningiomas.[15][45] In the mother-in-law sign, the meningioma shows an early enhancement in the arterial phase and stays well opacified during the venous phase.[15] DSA is helpful for surgery in patients with giant meningiomas. Preoperative embolization of the tumor feeders during DSA can aid the surgical resection by decreasing intraoperative bleeding and making the tumor softer.
Positron emission tomography scan is another radiological method that can be used for surgical planning and post-operative follow-up. It can help demarcate and define the vasculature and the tumor-brain interface.[46][47]
Some molecular cytogenetic abnormalities can be detected through genetic karyotyping studies. Mutations in chromosome 22 for neurofibromatosis 2, as well as the absence of copy in chromosomes 1,10, and 14, are identified in some cases of meningiomas. Other genetic alterations reported in the literature include SUFU, AKT1, PIK3CA, SMO, TRAF7, KLF4, PRKAR1A, and POLR2A.[47]
A sign that can differentiate spinal meningiomas from schwannomas is the ginkgo leaf sign. This sign is better seen with contrast-enhanced brain MRI on T1-weighted imaging. The leaf, which represents the spinal cord, is distorted and pushed to one side by the meningioma, with the streak representing the stretched denticulate ligament.[48]
Treatment / Management
Observation
Asymptomatic small-sized tumors, as well as patients with cavernous sinus meningiomas, can be followed up with close observation annually or biennially. The follow-up should include serial imaging, usually with brain MRI.
Surgery
Symptomatic lesions and those with accelerated growth are primarily treated with maximum gross total surgical resection. It can provide a 90% of 5-year progression-free survival rate. The degree of surgical resection of the tumor is a significant factor for recurrence.[49][50][51] Simpson grading system, established in 1957, is used to measure the recurrence depending on the extent of surgical resection.[52] This system has five grades; grade 1 is when there is a complete macroscopic or gross total removal with the attached dura and involved abnormal bone. Grade 2 is a complete macroscopic or gross total removal with coagulation of the dural attachment. Grade 3 is a complete macroscopic or gross total removal without resection or coagulation of the attached dura or any extradural extensions, such as the involved abnormal bone. Grade 4 is a partial resection of the tumor. Grade 5 is only a simple decompression of the tumor with or without a biopsy. For radiation-induced meningiomas, it is better and safer to perform a complete surgical resection. Prognostic factors for severe complications following surgery include surgery lasting more than 4 hours, greater than 70 years of age, and Karnofsky performance scale scores less than 70.[53] (B2)
Radiation
- External beam radiotherapy or brachytherapy after surgical resection can be used in grade II and III meningiomas. External beam radiation could improve the overall survival and progression-free survival rates in patients with meningioma.[4][54] Adjuvant radiotherapy for atypical and malignant meningiomas improves local control with longer progression-free survival and overall survival.[55][56] Adjuvant radiotherapy after gross total resection of atypical meningiomas may decrease the risk for relapse and improve local control.[55][57][58]
- Stereotactic radiosurgery (SRS) is another therapeutic method that can be used for patients who are unfit for surgery. SRS can be used for meningiomas of the skull base, recurrent or incompletely resected meningiomas. It can be given using various radio-surgical technologies. The use of SRS is not recommended in the settings of venous sinus occlusion or involvement, peritumoral edema, large-sized meningiomas, and tumors very adjacent to the brainstem or cranial nerves as they are associated with more adverse outcomes.[24] Multiple intracranial meningiomas (primary or recurrent) treated with SRS have an overall 3-year survival rate of 95% and a 5-year survival rate of 90%.[59][24] Even with the use of radiosurgery with a median marginal dose of 13.1Gy for atypical and anaplastic meningiomas, tumor control rate and overall survival remain poor.[60] More aggressive treatment using a higher marginal dose may improve outcomes. (A1)
Chemotherapy
Adjuvant therapies might be required to reduce the recurrence rate in incompletely removed meningiomas and atypical or malignant meningiomas. The use of bevacizumab, a type of chemotherapy to target molecular alterations of vascular endothelial growth factor in patients with anaplastic meningiomas after surgical resection and radiotherapy, had shown successful results of tumor regression.[61](B3)
Differential Diagnosis
Differential diagnoses of meningioma include those lesions which are dural-based, have a dural tail, and have a homogenous enhancement.[62]
Central Nervous System Solitary Fibrous Tumors
They originate from the dural mesenchyme and can occur in any organ and at any age. They consist of spindle cells, collagen bands, and vascular channels, and most cells express CD34 on their membranes. On brain MRI, they mostly appear as well-circumscribed extra-axial dural-based lesions. On the head CT scan, they mostly appear as calcified hyperdense lesions.
Intracranial Hemangiopericytoma
One of the most aggressive tumors with high rates of recurrence and metastasis. They originate from the pericytes of the meninges and can occur at a young age. They mainly consist of angular pericytes and vascular channels with high cellularity, known as staghorn vascularity. On brain MRI, they mostly appear as heterogenous, isointense lesions with a narrow or broad-based dural enhancement that could show a dural tail sign. On head CT scans, they appear as dural-based, hyperdense lesions with heterogeneous enhancement. These tumors are not commonly associated with calcifications.
Gliosarcoma
It is one of the rarest tumors, mostly composed of anaplastic astrocytes or glial cells with sarcomatous elements. These tumors can metastasize and are usually considered to be a variant of glioblastoma multiform. On brain MRI, they typically appear as well-defined cystic or inhomogeneous lesions with vasogenic edema. On the head CT scan, they appear as isodense or hyperdense lesions with a ring or heterogenous enhancement usually located at the periphery.
Leiomyosarcoma
This is one of the rarest central nervous system tumors usually seen in immunocompromised individuals, such as patients infected with the human immunodeficiency virus.
Dural Metastatic Tumors
Most common metastatic tumors arise from the breast, prostate, lung adenocarcinoma, and renal cell carcinoma. On brain MRI, they can appear as heterogeneous lesions with a dural tail sign.
Intracranial Hodgkin Lymphoma
This tumor can spread to the scalp, epidural space, and subcutaneous tissue. It can involve the skull bones but is usually seen at the late stage of the disease. It is described on a head CT scan as a hyperdense lesion. The bone window setting can view the presence of bone destruction or lytic lesions. On brain MRI with contrast, these tumors appear as homogenous dense lesions with dural tail enhancement and white matter edema.
Plasmacytoma
One of the extra-axial tumors that usually arise from the plasma cells and can be seen in patients with multiple myeloma. These tumors on head CT scan often appear as dense lesions. On contrast-enhanced brain MRI, they appear as intense homogeneous lesions with an enhanced dural tail sign.
Rosai-Dorfman Disease or Sinus Histiocytosis
A benign disease that originates from the proliferation of histocytes in the lymph nodes. Usually, the central nervous system Rosai-Dorfman disease affects the leptomeninges. On contrast-enhanced head CT scans or brain MRIs, they are described as homogenous, meningeal-based, extra-axial lesions occasionally associated with vasogenic edema.
Neurosarcoidosis
The incidence of neurosarcoidosis in patients with systemic sarcoidosis is approximately 5%. These tumors are dural-based lesions characterized by the presence of epithelioid granuloma. On contrast-enhanced brain MRI, they appear as enhanced homogenous parenchymal masses with leptomeningeal or cranial nerve involvement and vasogenic edema.
Melanocytic Tumors
They can be classified into malignant or benign lesions. They often have a leptomeningeal involvement and can be found in the posterior fossa or the spinal cord. On contrast-enhanced head CT scans, they appear as well-defined, extra-axial homogenously enhanced tumors.
Plasma Cell Granuloma
They are described as an inflammatory pseudotumor, with the cause of it still unknown. It might originate from the dura or the leptomeninges. On contrast-enhanced head CT scans, these tumors appear as markedly well-defined enhanced lesions. On brain MRI with contrast, they appear as heterogeneous enhanced lesions with or without parenchymal involvement. On T2 weighted imaging, they show low signal intensity, whereas, on T1, they show high signal intensity.
Prognosis
The 5-year progression-free survival for grade 1 is 95.7%, for grade 2 is 81.8%, and for grade 3 is 46.7%, while the 10-year progression-free survival for grade 1 is 90.4.7%, and for grade 2 is 69.4%.[51] Predictors for progression-free survival include the degree of resection, mitotic index <5%, histological grade, tumor size of 6 cm or less, tumor multiplicity, and tumor location.[51] Recurrence is associated with the histological grade; grade 1 has a 4.9%, grade 2 has 18.4%, and grade 3 has a 27.3% incidence of recurrence.[51] The 10-year survival for malignant meningiomas has been increasing due to the new therapeutic modalities available.[1][63][64] Partial tumor resection offers 85% 5-year overall survival with benign meningioma; however, it reduces to 58% with malignant meningiomas.[63]
The tumor grade in meningioma is considered a significant prognostic factor in patients undergoing radiation treatment after surgery.[65] A higher 3-year overall survival was associated with grades 1 and 2. The 5-year overall survival after the use of radiotherapy is approximately 81% associated with grade 1, whereas 53% with grade 2 and 3 meningiomas.
The recurrence rate of meningioma is associated with the extent of surgical removal. Using the original Simpson grading system, the overall recurrence rates for grades 1, 2, 3, and 4 are 9%, 19%, 29%, and 40%, respectively.[52] A low recurrence rate is correlated with complete surgical resection of the tumor, excision of the attached dura, and the involved abnormal bone. A study done in 2017 showed very similar recurrence rates for grades 1, 2, 3, and 4 meningiomas of 5%, 22%, 31%, and 35%, respectively.[50] The 5-year overall survival for patients with atypical and malignant meningioma is also affected by the extent of the resection; gross total removal confers a 91.3% for atypical meningioma and 64.5% for malignant meningioma, while subtotal removal confers a 78.2% for atypical meningioma and 41.1% for malignant meningioma.[66]
Complications
- Non-surgical: risk of recurrence, increase in size, and accelerated growth. Some meningiomas can get calcified or progress to grade 2 or 3 with a tendency of malignancy, brain invasion, and rarely metastasis.
- Surgical and medical: hematoma, infection, venous thrombosis, CSF leak, risk of injury to surrounding anatomical structures, and worsening of neurological deficits.[67] Other medical complications include aspiration pneumonia, deep vein thrombosis, pulmonary embolism, myocardial ischemia, and stroke.[68]
- Post radiosurgery: risk of cranial nerve deficits, such as optic nerve injury in intracranial meningioma. There is an increasing risk of toxicity due to radiotherapy.[69]
Deterrence and Patient Education
Meningioma is a tumor that grows from the meningeal layers, which cover and protect the brain and spinal cord. Most of these tumors are grade 1, which means that they are benign. Some of the meningiomas do not produce symptoms, and many of them are discovered incidentally during imaging studies. A head CT scan or brain MRI is used to diagnose a meningioma. The signs and symptoms depend on the location of the tumor in the brain. Symptoms such as headache, dizziness, visual impairments, weakness, convulsions, speech, or personality changes can occur. There are no specific causes of meningioma, but certain risk factors have been identified.
During pregnancy, meningiomas can grow in size because female hormones influence them. Moreover, other factors such as female hormones, exposure to radiation, use of oral contraceptive pills, hormonal replacement therapy, certain cancers such as breast cancer, some genetic diseases such as neurofibromatosis, von Hippel-Lindau disease, multiple endocrine neoplasias, Li-Fraumeni, Cowden disease, and Gorlin syndrome have been associated.[3][4] These conditions may increase the risk of having meningioma but not necessarily cause it to occur. Once someone is diagnosed with meningioma, it is necessary to have follow-up evaluations and serial imaging for observation.
Small-sized or asymptomatic meningiomas can be managed with observation and regular head CT or brain MRI scans. However, large-sized, symptomatic, or fast-growing meningiomas are usually treated by neurosurgical removal. The recurrence rate of meningioma depends on the histological grade and degree of resection.[9] Other management options include fractionated radiotherapy and stereotactic radiosurgery. As a result, radiation slows tumor growth and may reduce its size.
Unfortunately, there is no primary prevention of meningiomas has been identified yet. Although meningioma can occur in any individual, some might be at higher risk of developing meningioma. Medical history and neurological examination should be obtained to look for the commonly associated risk factors, including continuous estrogen therapy, exposure to radiation, first-degree relatives with meningioma, and patients with genetic syndromes such as neurofibromatosis type 2. In those high-risk groups of individuals, counseling should be given. Physicians should inform the patients about the chances of developing a meningioma. After the diagnosis of asymptomatic meningioma, regular neurosurgical follow-ups and radiological scans are essential to address the prognosis and outcomes.
One of the risk factors of meningioma, which can be prevented, is obesity. A large prospective cohort study showed a positive association between meningioma and a higher body mass index of greater than or equal to 30, as well as increased waist circumference, both in men and women. Accordingly, prevention can be done by maintaining an adequate weight with exercise and diet. Furthermore, obesity can alter the levels of sex steroid hormones, insulin, insulin-like growth factors 1, and inflammatory response proteins such as interleukin-6 and tumor necrosis factor. At the same time, there has been no specific association identified between meningioma and altered immunity. Nevertheless, overexpression of insulin growth factor and decreased serum level of immunoglobulin E in patients with allergies had been identified in those with meningioma. Therefore, early secondary prevention can be done by measuring the levels of serum biomarkers.[5][70][71]
Enhancing Healthcare Team Outcomes
Most meningiomas are benign and asymptomatic. Hence, many of them require close observation along with serial radiological imaging. However, complicated or symptomatic meningiomas should require immediate intervention. Accordingly, to enhance patients’ outcomes, multidisciplinary healthcare management from different specialties other than neurosurgery may be required, depending on the type and the complexity of the tumor.
Usually, multidisciplinary management is involved in complex meningiomas. The neurosurgery team often manages benign symptomatic and asymptomatic meningiomas. Atypical and malignant meningiomas necessitate comprehensive healthcare management from different specialties. With multidisciplinary management, medical treatments can be provided according to the patient’s needs and demands. Consequently, it is essential to address the medical problems that can occur in those patients to improve their prognosis. Meningiomas involving the paranasal sinuses or the temporal bone may require an otorhinolaryngologist or a craniomaxillofacial surgeon.[72] Moreover, since temporal bone meningiomas can cause hearing deficits, it is crucial to evaluate auditory function. The involvement of a comprehensive oncology team is necessary for malignant meningiomas to provide essential and amenable chemotherapies and radiation treatments.
Media
References
Ostrom QT,Cioffi G,Gittleman H,Patil N,Waite K,Kruchko C,Barnholtz-Sloan JS, CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro-oncology. 2019 Nov 1 [PubMed PMID: 31675094]
Lim YS,Kim MK,Park BJ,Kim TS,Lim YJ, Long term clinical outcomes of malignant meningiomas. Brain tumor research and treatment. 2013 Oct; [PubMed PMID: 24904897]
Level 2 (mid-level) evidenceWiemels J,Wrensch M,Claus EB, Epidemiology and etiology of meningioma. Journal of neuro-oncology. 2010 Sep; [PubMed PMID: 20821343]
Buerki RA,Horbinski CM,Kruser T,Horowitz PM,James CD,Lukas RV, An overview of meningiomas. Future oncology (London, England). 2018 Sep; [PubMed PMID: 30084265]
Level 3 (low-level) evidenceShao C,Bai LP,Qi ZY,Hui GZ,Wang Z, Overweight, obesity and meningioma risk: a meta-analysis. PloS one. 2014; [PubMed PMID: 24587258]
Level 1 (high-level) evidenceGurcay AG,Bozkurt I,Senturk S,Kazanci A,Gurcan O,Turkoglu OF,Beskonakli E, Diagnosis, Treatment, and Management Strategy of Meningioma during Pregnancy. Asian journal of neurosurgery. 2018 Jan-Mar; [PubMed PMID: 29492130]
Connolly ID,Cole T,Veeravagu A,Popat R,Ratliff J,Li G, Craniotomy for Resection of Meningioma: An Age-Stratified Analysis of the MarketScan Longitudinal Database. World neurosurgery. 2015 Dec [PubMed PMID: 26318633]
de Robles P,Fiest KM,Frolkis AD,Pringsheim T,Atta C,St Germaine-Smith C,Day L,Lam D,Jette N, The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro-oncology. 2015 Jun; [PubMed PMID: 25313193]
Level 2 (mid-level) evidenceHortobágyi T,Bencze J,Varkoly G,Kouhsari MC,Klekner Á, Meningioma recurrence. Open medicine (Warsaw, Poland). 2016; [PubMed PMID: 28352788]
Shibuya M, Pathology and molecular genetics of meningioma: recent advances. Neurologia medico-chirurgica. 2015; [PubMed PMID: 25744347]
Level 3 (low-level) evidenceVarlotto J,Flickinger J,Pavelic MT,Specht CS,Sheehan JM,Timek DT,Glantz MJ,Sogge S,Dimaio C,Moser R,Yunus S,Fitzgerald TJ,Upadhyay U,Rava P,Tangel M,Yao A,Kanekar S, Distinguishing grade I meningioma from higher grade meningiomas without biopsy. Oncotarget. 2015 Nov 10; [PubMed PMID: 26472106]
Backer-Grøndahl T,Moen BH,Torp SH, The histopathological spectrum of human meningiomas. International journal of clinical and experimental pathology. 2012; [PubMed PMID: 22558478]
Level 2 (mid-level) evidenceWestwick HJ,Shamji MF, Effects of sex on the incidence and prognosis of spinal meningiomas: a Surveillance, Epidemiology, and End Results study. Journal of neurosurgery. Spine. 2015 Sep; [PubMed PMID: 26023898]
Louis DN,Perry A,Reifenberger G,von Deimling A,Figarella-Branger D,Cavenee WK,Ohgaki H,Wiestler OD,Kleihues P,Ellison DW, The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica. 2016 Jun [PubMed PMID: 27157931]
Sotiriadis C,Vo QD,Ciarpaglini R,Hoogewoud HM, Cystic meningioma: diagnostic difficulties and utility of MRI in diagnosis and management. BMJ case reports. 2015 Mar 26; [PubMed PMID: 25814028]
Level 3 (low-level) evidenceMittal A,Layton KF,Finn SS,Snipes GJ,Opatowsky MJ, Cystic meningioma: unusual imaging appearance of a common intracranial tumor. Proceedings (Baylor University. Medical Center). 2010 Oct; [PubMed PMID: 21240328]
[New members in the health organization...]., Wisniewski W,, Pielegniarka i polozna, 1979 [PubMed PMID: 469560]
Level 3 (low-level) evidence[In the role of adviser and friend]., Krzanowska Z,, Pielegniarka i polozna, 1979 [PubMed PMID: 8869708]
Simas NM,Farias JP, Sphenoid Wing en plaque meningiomas: Surgical results and recurrence rates. Surgical neurology international. 2013; [PubMed PMID: 23956929]
Basu K,Majumdar K,Chatterjee U,Ganguli M,Chatterjee S, En plaque meningioma with angioinvasion. Indian journal of pathology [PubMed PMID: 20551544]
Level 3 (low-level) evidenceBaek JU,Cho YD,Yoo JC, An osteolytic meningioma en plaque of the sphenoid ridge. Journal of Korean Neurosurgical Society. 2008 Jan; [PubMed PMID: 19096543]
Level 3 (low-level) evidenceMatschke J,Addo J,Bernreuther C,Zustin J, Osseous changes in meningioma en plaque. Anticancer research. 2011 Feb; [PubMed PMID: 21378343]
Level 2 (mid-level) evidence[Skull and brain injuries]., Wejroch A,, Pielegniarka i polozna, 1979 [PubMed PMID: 3118666]
Mansouri A,Badhiwala J,Mansouri S,Zadeh G, The evolving role of radiosurgery in the management of radiation-induced meningiomas: a review of current advances and future directions. BioMed research international. 2014; [PubMed PMID: 25136551]
Level 3 (low-level) evidenceGoto Y,Yamada S,Yamada SM,Nakaguchi H,Hoya K,Murakami M,Yamazaki K,Ishida Y,Matsuno A, Radiation-induced meningiomas in multiple regions, showing rapid recurrence and a high MIB 1 labeling index: a case report and review of the literature. World journal of surgical oncology. 2014 Apr 26; [PubMed PMID: 24767145]
Level 3 (low-level) evidenceOikonomou A,Birbilis T,Daskalogiannakis G,Prassopoulos P, Meningioma of the conus medullaris mimicking neurofibroma--possibly radiation induced. The spine journal : official journal of the North American Spine Society. 2011 Feb; [PubMed PMID: 21193353]
Level 3 (low-level) evidenceMurakami T,Tanishima S,Takeda C,Kato S,Nagashima H, Ossified Metaplastic Spinal Meningioma Without Psammomatous Calcification: A Case Report. Yonago acta medica. 2019 Jun; [PubMed PMID: 31320828]
Level 3 (low-level) evidenceKitagawa M,Nakamura T,Aida T,Iwasaki Y,Abe H,Nagashima K, [Clinicopathologic analysis of ossification in spinal meningioma]. Noshuyo byori = Brain tumor pathology. 1994; [PubMed PMID: 8162148]
Level 3 (low-level) evidenceBhat AR,Wani MA,Kirmani AR,Ramzan AU, Histological-subtypes and anatomical location correlated in meningeal brain tumors (meningiomas). Journal of neurosciences in rural practice. 2014 Jul; [PubMed PMID: 25002762]
Marchand AA,O'Shaughnessy J, Subtle clinical signs of a meningioma in an adult: a case report. Chiropractic [PubMed PMID: 24490991]
Level 3 (low-level) evidenceRodríguez-Porcel F,Hughes I,Anderson D,Lee J,Biller J, Foster Kennedy Syndrome Due to Meningioma Growth during Pregnancy. Frontiers in neurology. 2013 [PubMed PMID: 24273529]
Hekmatpanah J, Evidence-based treatment of cavernous sinus meningioma. Surgical neurology international. 2019 [PubMed PMID: 31819821]
Gadgil N,Hansen D,Barry J,Chang R,Lam S, Posterior fossa syndrome in children following tumor resection: Knowledge update. Surgical neurology international. 2016 [PubMed PMID: 27057398]
[School work and passing examinations]., Rogula W,, Pielegniarka i polozna, 1978 [PubMed PMID: 29204199]
Elwatidy SM,Albakr AA,Al Towim AA,Malik SH, Tumors of the lateral and third ventricle: surgical management and outcome analysis in 42 cases. Neurosciences (Riyadh, Saudi Arabia). 2017 Oct; [PubMed PMID: 29057852]
Level 3 (low-level) evidenceSlentz DH,Bellur S,Taheri MR,Almira-Suarez MI,Sherman JH,Mansour TN, Orbital malignant meningioma: a unique presentation of a rare entity. Orbit (Amsterdam, Netherlands). 2018 Dec [PubMed PMID: 29485367]
Ciftdemir M,Kaya M,Selcuk E,Yalniz E, Tumors of the spine. World journal of orthopedics. 2016 Feb 18; [PubMed PMID: 26925382]
Samartzis D, Gillis CC, Shih P, O'Toole JE, Fessler RG. Intramedullary Spinal Cord Tumors: Part I-Epidemiology, Pathophysiology, and Diagnosis. Global spine journal. 2015 Oct:5(5):425-35. doi: 10.1055/s-0035-1549029. Epub 2015 Mar 31 [PubMed PMID: 26430598]
Watts J,Box G,Galvin A,Brotchie P,Trost N,Sutherland T, Magnetic resonance imaging of meningiomas: a pictorial review. Insights into imaging. 2014 Feb; [PubMed PMID: 24399610]
Hou J,Kshettry VR,Selman WR,Bambakidis NC, Peritumoral brain edema in intracranial meningiomas: the emergence of vascular endothelial growth factor-directed therapy. Neurosurgical focus. 2013 Dec; [PubMed PMID: 24289127]
Level 1 (high-level) evidenceKim BW,Kim MS,Kim SW,Chang CH,Kim OL, Peritumoral brain edema in meningiomas : correlation of radiologic and pathologic features. Journal of Korean Neurosurgical Society. 2011 Jan; [PubMed PMID: 21494359]
George AE,Russell EJ,Kricheff II, White matter buckling: CT sign of extraaxial intracranial mass. AJR. American journal of roentgenology. 1980 Nov; [PubMed PMID: 6778144]
Level 3 (low-level) evidencePapacci F,Pedicelli A,Montano N, The role of preoperative angiography in the management of giant meningiomas associated to vascular malformation. Surgical neurology international. 2015; [PubMed PMID: 26167366]
Tsuchiya K,Katase S,Yoshino A,Hachiya J, MR digital subtraction angiography in the diagnosis of meningiomas. European journal of radiology. 2003 May; [PubMed PMID: 12714229]
[Industrial Health Service]., Krasucki P,, Pielegniarka i polozna, 1978 [PubMed PMID: 1257433]
Galldiks N,Albert NL,Sommerauer M,Grosu AL,Ganswindt U,Law I,Preusser M,Le Rhun E,Vogelbaum MA,Zadeh G,Dhermain F,Weller M,Langen KJ,Tonn JC, PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro-oncology. 2017 Nov 29; [PubMed PMID: 28605532]
Nowosielski M,Galldiks N,Iglseder S,Kickingereder P,von Deimling A,Bendszus M,Wick W,Sahm F, Diagnostic challenges in meningioma. Neuro-oncology. 2017 Nov 29; [PubMed PMID: 28531331]
Yamaguchi S,Takeda M,Takahashi T,Yamahata H,Mitsuhara T,Niiro T,Hanakita J,Hida K,Arita K,Kurisu K, Ginkgo leaf sign: a highly predictive imaging feature of spinal meningioma. Journal of neurosurgery. Spine. 2015 Nov; [PubMed PMID: 26230423]
Quddusi A,Shamim MS, Simpson grading as predictor of meningioma recurrence. JPMA. The Journal of the Pakistan Medical Association. 2018 May; [PubMed PMID: 29885194]
Nanda A,Bir SC,Maiti TK,Konar SK,Missios S,Guthikonda B, Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. Journal of neurosurgery. 2017 Jan; [PubMed PMID: 27058201]
Gousias K,Schramm J,Simon M, The Simpson grading revisited: aggressive surgery and its place in modern meningioma management. Journal of neurosurgery. 2016 Sep [PubMed PMID: 26824369]
[Health and management]., Kosińska D,, Pielegniarka i polozna, 1978 [PubMed PMID: 13406590]
Bartek J Jr,Sjåvik K,Förander P,Solheim O,Gulati S,Weber C,Ingebrigtsen T,Jakola AS, Predictors of severe complications in intracranial meningioma surgery: a population-based multicenter study. World neurosurgery. 2015 May [PubMed PMID: 25655686]
Level 2 (mid-level) evidenceWalcott BP,Nahed BV,Brastianos PK,Loeffler JS, Radiation Treatment for WHO Grade II and III Meningiomas. Frontiers in oncology. 2013 Sep 2; [PubMed PMID: 24032107]
[Treatment and prevention of EPH-gestosis]., Daraz B,Piela A,, Pielegniarka i polozna, 1978 [PubMed PMID: 24696499]
Level 1 (high-level) evidencePisćević I,Villa A,Milićević M,Ilić R,Nikitović M,Cavallo LM,Grujičić D, The Influence of Adjuvant Radiotherapy in Atypical and Anaplastic Meningiomas: A Series of 88 Patients in a Single Institution. World neurosurgery. 2015 Jun [PubMed PMID: 25769488]
Level 2 (mid-level) evidenceAltering patients' responses to surgery: an extension and replication., Johnson JE,Fuller SS,Endress MP,Rice VH,, Research in nursing & health, 1978 Oct [PubMed PMID: 25535067]
Level 1 (high-level) evidenceAizer AA,Arvold ND,Catalano P,Claus EB,Golby AJ,Johnson MD,Al-Mefty O,Wen PY,Reardon DA,Lee EQ,Nayak L,Rinne ML,Beroukhim R,Weiss SE,Ramkissoon SH,Abedalthagafi M,Santagata S,Dunn IF,Alexander BM, Adjuvant radiation therapy, local recurrence, and the need for salvage therapy in atypical meningioma. Neuro-oncology. 2014 Nov [PubMed PMID: 24891451]
Level 2 (mid-level) evidenceSamblas J,Luis Lopez Guerra J,Bustos J,Angel Gutierrez-Diaz J,Wolski M,Peraza C,Marsiglia H,Sallabanda K, Stereotactic radiosurgery in patients with multiple intracranial meningiomas. Journal of B.U.ON. : official journal of the Balkan Union of Oncology. 2014 Jan-Mar; [PubMed PMID: 24659672]
Wang WH,Lee CC,Yang HC,Liu KD,Wu HM,Shiau CY,Guo WY,Pan DH,Chung WY,Chen MT, Gamma Knife Radiosurgery for Atypical and Anaplastic Meningiomas. World neurosurgery. 2016 Mar [PubMed PMID: 26485417]
Puchner MJ,Hans VH,Harati A,Lohmann F,Glas M,Herrlinger U, Bevacizumab-induced regression of anaplastic meningioma. Annals of oncology : official journal of the European Society for Medical Oncology. 2010 Dec; [PubMed PMID: 21041375]
Level 3 (low-level) evidenceChourmouzi D,Potsi S,Moumtzouoglou A,Papadopoulou E,Drevelegas K,Zaraboukas T,Drevelegas A, Dural lesions mimicking meningiomas: A pictorial essay. World journal of radiology. 2012 Mar 28; [PubMed PMID: 22468187]
Disengagement and reinforcement in the elderly., Henthorn BS,, Research in nursing & health, 1979 Mar [PubMed PMID: 27760993]
Ostrom QT,Gittleman H,Truitt G,Boscia A,Kruchko C,Barnholtz-Sloan JS, CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro-oncology. 2018 Oct 1 [PubMed PMID: 30445539]
Development of an instrument to measure exercise of self-care agency., Kearney BY,Fleischer BJ,, Research in nursing & health, 1979 Mar [PubMed PMID: 27274498]
Aizer AA,Bi WL,Kandola MS,Lee EQ,Nayak L,Rinne ML,Norden AD,Beroukhim R,Reardon DA,Wen PY,Al-Mefty O,Arvold ND,Dunn IF,Alexander BM, Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer. 2015 Dec 15 [PubMed PMID: 26308667]
Postpartum perceptions of touch received during labor., Penny KS,, Research in nursing & health, 1979 Mar [PubMed PMID: 31082552]
Sughrue ME,Rutkowski MJ,Shangari G,Chang HQ,Parsa AT,Berger MS,McDermott MW, Risk factors for the development of serious medical complications after resection of meningiomas. Clinical article. Journal of neurosurgery. 2011 Mar; [PubMed PMID: 20653395]
Shaikh N,Dixit K,Raizer J, Recent advances in managing/understanding meningioma. F1000Research. 2018; [PubMed PMID: 29770198]
Level 3 (low-level) evidenceRajaraman P, Hunting for the causes of meningioma--obesity is a suspect. Cancer prevention research (Philadelphia, Pa.). 2011 Sep; [PubMed PMID: 21893497]
Michaud DS,Bové G,Gallo V,Schlehofer B,Tjønneland A,Olsen A,Overvad K,Dahm CC,Teucher B,Boeing H,Steffen A,Trichopoulou A,Bamia C,Kyrozis A,Sacerdote C,Agnoli C,Palli D,Tumino R,Mattiello A,Bueno-de-Mesquita HB,Peeters PH,May AM,Barricarte A,Chirlaque MD,Dorronsoro M,José Sánchez M,Rodríguez L,Duell EJ,Hallmans G,Melin BS,Manjer J,Borgquist S,Khaw KT,Wareham N,Allen NE,Travis RC,Romieu I,Vineis P,Riboli E, Anthropometric measures, physical activity, and risk of glioma and meningioma in a large prospective cohort study. Cancer prevention research (Philadelphia, Pa.). 2011 Sep; [PubMed PMID: 21685234]
Level 2 (mid-level) evidenceSilva DP,Carvalho SD,Marçal N,Dias L, Giant meningioma in paranasal sinuses: an atypical nasal occupation. BMJ case reports. 2018 Jan 4; [PubMed PMID: 29301819]
Level 3 (low-level) evidence