Indications
Minoxidil, also known as 2,4-pyrimidinediamine,6-(1-piperidinyl)-, 3-oxide, was initially developed as a potent peripheral vasodilator agent for treating severe refractory hypertension. However, owing to the significant adverse effects of oral minoxidil use, this drug is currently used only for patients with resistant hypertension who do not adequately respond to the maximum doses of 3 different antihypertensive medications.
Furthermore, approximately one-fifth of patients undergoing oral minoxidil treatment developed hypertrichosis. In 1987, a topical formulation of minoxidil was developed to treat androgenic alopecia, initially targeting males and later expanding its use to include females. This topic focuses on the clinical application of topical minoxidil therapy for androgenic alopecia and oral minoxidil therapy for resistant hypertension.[1][2]
Topical minoxidil is available in both liquid solution and foam formulations. The liquid form of topical minoxidil contains alcohol and propylene glycol, the 2 crucial molecules that help dissolve the drug in a patient's body, facilitating tissue absorption. Formulations containing 2% and 5% minoxidil commonly treat scalp alopecia in individuals aged 18 and above. Long-term use of minoxidil is necessary to uphold the achieved clinical outcomes of the medication, as these effects diminish when the drug is discontinued.[3][4]
Topical Minoxidil
Androgenic alopecia is the only indication approved by the U.S. Food and Drug Administration (FDA) for topical minoxidil.
Off-label uses of topical minoxidil are as follows:
- Alopecia areata: Minoxidil has demonstrated the ability to elicit a favorable clinical response when used as a standalone treatment for alopecia areata or in conjunction with other medications, such as corticosteroids.
- Chemotherapy-induced alopecia: In this case, minoxidil has exhibited the capacity to reduce hair loss and expedite the process of hair regrowth.
- Hair transplant: Hair loss, also known as telogen effluvium, is a common observation after a hair transplant. Minoxidil can be used before and after hair transplantation to reduce hair loss in patients. However, the treatment should be temporarily suspended for 3 days before the hair transplant to prevent excessive bleeding.
- Scarring alopecia: Minoxidil has displayed evidence of having an antifibrotic effect on this condition. Consequently, topical minoxidil treatment could be a viable therapeutic option during the initial stages of dermatoses, leading to scarring alopecia, such as those stemming from scalp burns.
- Monilethrix: Minoxidil induces the synchronization of hair follicles entering the anagen phase in patients experiencing this condition.
- Hereditary alopecia or hypotrichosis: Minoxidil has demonstrated its beneficial effects by promoting hair shaft thickening in hypotrichosis.
Oral Minoxidil
According to the American College of Cardiology and the American Heart Association (AHA), resistant hypertension is characterized by elevated blood pressure levels exceeding the target range, despite the simultaneous utilization of 3 different classes of antihypertensive medications. These classes typically include a combination of a calcium channel blocker, a renin-angiotensin-aldosterone system blocker (angiotensin-converting enzyme inhibitor or angiotensin receptor blocker), and a diuretic. These drugs are administered at the highest daily doses a patient can tolerate. The FDA approves minoxidil for maintaining blood pressure levels in patients with resistant hypertension.[5]
Many forms of alopecia are off-label indications for oral or sublingual minoxidil. Recently, multiple studies have explored using low-dose oral minoxidil (<5 mg daily) for treating many forms of alopecia, including male-patterned and female-patterned hair loss, to treat hair loss without adverse reactions.[6] The low adverse-effect profile of low-dose oral minoxidil aids in long-term adherence to treatment and positive clinical response.[7][8] More studies are needed to test the efficacy of oral and sublingual minoxidil when treating various alopecia to improve patient outcomes and provide an alternative for those with difficulty with topical formulations.[6]
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
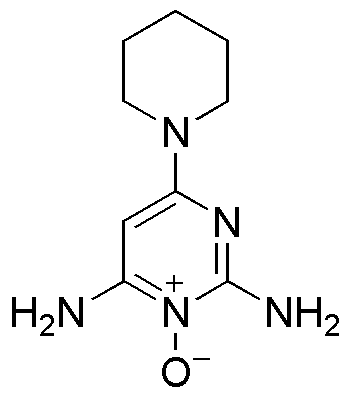
Topical minoxidil, with the empirical formula C9H15N5O, is a stimulant for hair growth (see Image. Skeletal Formula of Minoxidil). The precise mechanism of action for the drug remains inadequately understood. The sulfotransferase enzyme in the human scalp converts minoxidil into minoxidil sulfate, which is the active form of the molecule. Differences in sulfotransferase activity among individuals can affect minoxidil's effectiveness, leading to inconsistencies in the therapy.[9]
Minoxidil shortens the telogen phase to prompt the dormant hair follicles for premature transition into the anagen phase. The shortening of the telogen phase might lead to telogen effluvium following minoxidil therapy. Furthermore, minoxidil extends the anagen phase, resulting in increased hair length and thickness, thereby representing the observable outcomes of minoxidil therapy.[10]
The initial outcomes of minoxidil become apparent after approximately 8 weeks of initiating the treatment, with the maximum effects manifesting around 4 months. Minoxidil affects the potassium channels present in vascular smooth muscles and hair follicles.[10] This potassium channel activity may induce the following effects:
- Stimulation of the microcirculation around the hair follicles induces arteriolar vasodilation, thereby encouraging conditions conducive to hair growth.
- Induction of the vascular endothelial growth factor expression leads to heightened vascularization around the hair follicles, thereby promoting hair growth.
- Activation of the prostaglandin-endoperoxide synthase-1 enzyme leads to the enhancement of hair growth.
- Inhibition of androgen-related effects on androgen-sensitive hair follicles.
- Direct stimulation of the hair follicles as the drug acts as an epidermal growth factor on the matrix cells, slowing their aging process and extending their anagen phase. This process is achieved through the activation of the beta-catenin pathway.
- Display of antifibrotic characteristics due to its impact on collagen synthesis.
Hypertension
Minoxidil exerts its antihypertensive effect by opening adenosine triphosphate (ATP)-sensitive potassium channels. As a result, when the smooth muscle vasculature is relaxed, it reduces peripheral resistance, which ultimately helps to lower blood pressure levels. Consequently, this process increases plasma renin activity, which, in turn, triggers salt and water retention in a patient's body. Additionally, minoxidil triggers the activation of the sympathetic nervous system, resulting in tachycardia and heightened cardiac output. Therefore, clinicians prescribe beta-blockers and diuretics alongside minoxidil to mitigate potential adverse effects.[5]
Pharmacokinetics
Absorption: Minoxidil is absorbed from the gastrointestinal tract at a rate of 95%, and the drug achieves peak levels within the initial hour. Conversely, only 1.4% of topical minoxidil is absorbed through the skin.[7]
Distribution: Minoxidil shows no binding affinity to plasma proteins. This drug experiences broad distribution, with a distribution volume ranging from 2.8 to 3.3 L/kg.
Metabolism: Minoxidil undergoes metabolism through conjugation, sulfation, and hydroxylation processes. The resulting metabolites generally exhibit lower pharmacological activity than the parent drug. Notably, as previously mentioned, topical minoxidil is metabolized within the hair follicles by the sulfotransferase enzyme, leading to the formation of minoxidil sulfate.[11]
Elimination: Although the elimination half-life of minoxidil is around 3 to 4 hours, the hypotensive impact of the medication may persist for up to 72 hours. Notably, the excretion of minoxidil and its metabolites predominantly occurs through the kidneys.[12]
Administration
Minoxidil is available as an over-the-counter topical agent in the United States. Furthermore, the medication is marketed in an oral tablet formulation and as a topical agent in foam and solution formulations for both male and female patients.
Adult Dosage
Topical minoxidil:
- Patients can apply a 5% aerosol formulation of topical minoxidil directly onto their scalp at a one-half capful dose. The medication should be used twice daily by male patients and once daily by female patients.
- Women should apply 1 mL of the 2% topical minoxidil solution to their scalp twice daily.
- Men should apply 1 mL of 2% or 5% topical minoxidil solution to their scalp twice daily.
Scalp massage is not required following the application of the medication. Minoxidil uptake reaches approximately 50% within an hour and increases to 75% after 4 hours. Although certain practitioners use microneedling with topical minoxidil to potentially enhance the drug's efficacy, further studies are necessary to evaluate the potential significance of this practice.[13][14][15]
A 2% topical minoxidil solution is effective for male patients experiencing androgenic alopecia in their frontotemporal and vertex regions. However, the 5% topical minoxidil solution provides a more significant clinical benefit than the 2% solution. The clinical response to minoxidil is notably more pronounced when the onset of alopecia occurs within 5 years, primarily among young adults, and when the hair follicles are not extensively miniaturized.[16]
Oral minoxidil:
This formulation is supplied in tablet formulations, with options of 2.5 mg and 10 mg doses. Although the FDA does not approve the oral formulation of minoxidil for treating hair loss in individuals, clinical trials have demonstrated its effectiveness at dosages ranging from 0.25 to 2.5 mg daily for maintaining blood pressure levels in patients with resistant hypertension.[8]
The recommended initial dosage of minoxidil for hypertensive therapy is 5 mg, administered once daily to patients younger than 12, which can be gradually escalated up to a maximum of 40 mg per day. Higher doses should be equally divided and administered to individuals 2 or 3 times daily to prevent excessive hypotension. The maximum recommended dosage of oral minoxidil is 100 mg per day.[12] As per the AHA's guidelines for managing resistant hypertension, minoxidil should be prescribed alongside a loop diuretic and a beta-blocker to mitigate potential adverse effects in patients.[2]
The recommended off-label dosing of oral minoxidil for treating alopecia is 0.25 to 2.5 mg, administered once or twice daily in 1 or 2 equally divided doses.
Specific Patient Population
Patient with hepatic impairment: The product labeling for topical or oral minoxidil does not offer specific dosage adjustment information. However, caution is advised when considering its use in such cases.
Patient with renal impairment: When dosing oral minoxidil, it is advisable to consider dose reduction for individuals with renal impairment. However, specific dosage adjustment information is not supplied for topical minoxidil.
Pregnancy considerations: As animal reproductive studies have indicated specific adverse effects of minoxidil in pregnant women, it is not advisable to use this drug. Minoxidil holds an FDA pregnancy category C classification.[17]
Breastfeeding considerations: As minoxidil is excreted in breast milk, its use is not recommended in breastfeeding women.[10]
Pediatric patients: The recommended initial dosage of oral minoxidil in patients younger than 12 is 0.2 mg/kg daily. Typically, the maintenance dosage of minoxidil ranges from 0.25 to 1 mg/kg per day, and the maximum recommended dosage is 50 mg per day.[18] Clinicians may suggest topical minoxidil in children; however, it is considered an off-label drug indication.[19]
Older patients: Oral minoxidil should be initiated at a lower dose in older patients as clinicians recommend, considering comorbidities, polypharmacy, and increased risk of falls in this population.
Adverse Effects
Although minoxidil is generally well tolerated, topical minoxidil is associated with the following adverse effects, as listed below.[10][20]
- Minoxidil-induced telogen effluvium: The shortening of the telogen phase caused by minoxidil can result in excessive hair shedding in individuals.
- Skin irritation: This condition can lead to erythema, discomfort, and a burning sensation on the scalp.
- Scaly changes of the scalp: This condition can entail irritation or a potential worsening of seborrheic dermatitis.
- Isolated pruritus: appears in the area of application.
- Allergic contact dermatitis: This can lead to symptoms such as erythema, eczematous skin reactions, and pruritus. Minoxidil and propylene glycol are the primary allergens implicated in cases of allergic contact dermatitis. Patch testing can aid in identifying the underlying causative agent. If allergic contact dermatitis arises due to propylene glycol, topical minoxidil as a foam formulation can be an alternative option, as it does not contain propylene glycol.
- Localized or generalized hypertrichosis: Both oral and topical minoxidil usage can lead to hypertrichosis. However, this effect is more frequently observed with the oral formulation and when the 5% topical minoxidil solution is used compared to the 2% solution. Research indicates that hypertrichosis is linked to minoxidil extending the anagen phase. In addition, cases of hypertrichosis have been documented in infants due to unintentional direct skin contact.[21]
Oral minoxidil is also associated with significant adverse effects, as listed below.
- Rare but severe reactions include pericarditis, pericardial effusion, cardiac tamponade, exacerbating congestive heart failure, and worsening angina.
- Oral minoxidil administration can lead to significant hypotension and potential complications such as thrombocytopenia and leukopenia in individuals taking the medication.[22]
- Breast tenderness and gynecomastia have also been reported as adverse effects of the medication.[23]
- Hypertrichosis, edema, tachycardia, and weight gain are also caused by oral minoxidil.
Drug-Drug Interactions
- Systemic cyclosporine can exacerbate adverse effects such as hypertrichosis when combined with topical minoxidil. Notably, hypertrichosis symptoms significantly improved after discontinuing topical minoxidil for 2 months.[24]
- Coadministration of low-dose aspirin and minoxidil may diminish the effectiveness of topical minoxidil. This decrease in effectiveness is attributed to the inhibitory effect of low-dose aspirin on sulfotransferase enzymes in human hair.[25]
- Concurrent use of guanethidine with minoxidil can cause severe hypotension.[26]
Contraindications
Contraindications include the following:
- Minoxidil should not be used in patients with a known history of hypersensitivity to the drug or its constituents, including propylene glycol.
- The utilization of minoxidil is not recommended for pregnant and breastfeeding women. Although minoxidil is not recognized as a teratogenic agent, there are occasional reports of congenital anomalies.[10]
- The product intended for use in males is not recommended for use in females.
- This product is also contraindicated in patients younger than 18 and those who experience sudden, uncertain, or patchy hair loss, hair loss after childbirth, scalp infections or inflammation, or use another scalp medication.
Box Warning
Oral minoxidil can cause serious adverse effects, including pericardial effusion, that can advance to cardiac tamponade. Reports of angina pectoris worsening have also been reported in patients using the medication. As a result, careful oversight is imperative when administering minoxidil to patients.[27]
Patients receiving guanethidine should be admitted to a hospital for monitoring when minoxidil is administered due to the substantial risk of severe orthostatic hypotension.[26] Coadministration of beta-blockers with minoxidil is advised to help manage the elevated myocardial workload and tachycardia.[12] Oral minoxidil should be used with a loop diuretic to prevent severe fluid accumulation in a patient's body.[28]
Monitoring
Patients using topical minoxidil should undergo regular monitoring for any alterations in the scalp and the occurrence of localized or generalized hypertrichosis.[29] Hypotension is a rare occurrence in patients using topical minoxidil. Nonetheless, some clinicians advise consistent monitoring of blood pressure, heart rate, and electrocardiographic changes for patients undergoing topical minoxidil therapy. Clinicians should conduct thorough assessments to eliminate secondary causes of hypertension, such as renal artery stenosis, primary aldosteronism, pheochromocytoma, and Cushing syndrome, warranting minoxidil treatment. Regular monitoring of fundoscopic examination, renal function, echocardiogram, and ankle-brachial index is recommended to assess potential target organ damage for patients taking the medication.[2]
Toxicity
Percutaneous toxicity is a rare occurrence following standard minoxidil application. In cases of significant oral ingestion, there is currently no recognized antidote for minoxidil toxicity. Unintentional oral consumption of minoxidil may lead to vomiting and, in rare instances, necessitate hospitalization. Instances of hypotension, tachycardia, and changes in the electrocardiogram have been reported following accidental ingestion. Intravenous fluids and pharmaceutical vasopressors can address malignant hypotension. In substantial inadvertent minoxidil ingestion, gastric lavage technique and activated charcoal administration might be essential to avert systemic toxicity in patients.[30]
A case was reported where a young girl unintentionally ingested a topical solution of minoxidil prescribed to her father. After ingesting the medication, the girl encountered adverse effects such as hypotension and prolonged tachycardia.[31] Intravenous fluids are essential in cases of oral minoxidil overdose, causing hypotension in individuals. Vasopressors should be administered to patients for hypotension that does not respond to standard treatment.[32][33]
Enhancing Healthcare Team Outcomes
Topical minoxidil has been a trusted medication in the medical field for more than 30 years and is commonly prescribed by dermatologists, primary care physicians, nurse practitioners, and physician assistants. Minoxidil is also prescribed as a treatment option for resistant hypertension in patients. According to AHA, it is recommended to consult hypertension specialists for cases of resistant hypertension. A retrospective study demonstrated enhanced blood pressure control in patients with resistant hypertension under the care of hypertension specialists.[5][34][35]
Secondary causes of hypertension require consultation with nephrologists and endocrinologists. Pharmacists are critical in ensuring no potential drug-drug interactions are of concern in patients with hypertension. Although minoxidil is regarded as a primary treatment option to promote hair growth, the efficacy of the medication can vary inconsistently depending on various cases. Some patients experience substantial improvements, whereas others observe only minimal changes. Healthcare professionals should inform patients that individual responses to minoxidil can vary and constant adherence to medication is crucial for optimal hair growth.
Emergency medicine and critical care physicians should focus on stabilizing the patient in case of a minoxidil overdose. To ensure the best outcomes for patients receiving minoxidil therapy for alopecia and hypertension, physicians, advanced practice practitioners, specialists, pharmacists, and nursing staff must work collaboratively, utilizing an interprofessional approach and open communication to facilitate effective teamwork to achieve optimal outcomes with the fewest adverse events.
Media
References
Jimenez-Cauhe J, Saceda-Corralo D, Rodrigues-Barata R, Hermosa-Gelbard A, Moreno-Arrones OM, Fernandez-Nieto D, Vaño-Galvan S. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. Journal of the American Academy of Dermatology. 2019 Aug:81(2):648-649. doi: 10.1016/j.jaad.2019.04.054. Epub 2019 May 2 [PubMed PMID: 31054970]
Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB, American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension (Dallas, Tex. : 1979). 2018 Nov:72(5):e53-e90. doi: 10.1161/HYP.0000000000000084. Epub [PubMed PMID: 30354828]
Level 2 (mid-level) evidenceVerma K, Tegta GR, Verma G, Gupta M, Negi A, Sharma R. A Study to Compare the Efficacy of Platelet-rich Plasma and Minoxidil Therapy for the Treatment of Androgenetic Alopecia. International journal of trichology. 2019 Mar-Apr:11(2):68-79. doi: 10.4103/ijt.ijt_64_18. Epub [PubMed PMID: 31007475]
Gajjar PC, Mehta HH, Barvaliya M, Sonagra B. Comparative Study between Mesotherapy and Topical 5% Minoxidil by Dermoscopic Evaluation for Androgenic Alopecia in Male: A Randomized Controlled Trial. International journal of trichology. 2019 Mar-Apr:11(2):58-67. doi: 10.4103/ijt.ijt_89_18. Epub [PubMed PMID: 31007474]
Level 2 (mid-level) evidenceMundt HM, Matenaer M, Lammert A, Göttmann U, Krämer BK, Birck R, Benck U. Minoxidil for Treatment of Resistant Hypertension in Chronic Kidney Disease--A Retrospective Cohort Analysis. Journal of clinical hypertension (Greenwich, Conn.). 2016 Nov:18(11):1162-1167. doi: 10.1111/jch.12847. Epub 2016 Jun 1 [PubMed PMID: 27246772]
Level 2 (mid-level) evidenceRamírez-Marín HA, Tosti A. Role of Oral Minoxidil in Patterned Hair Loss. Indian dermatology online journal. 2022 Nov-Dec:13(6):729-733. doi: 10.4103/idoj.idoj_246_22. Epub 2022 Oct 12 [PubMed PMID: 36386734]
Gupta AK, Talukder M, Venkataraman M, Bamimore MA. Minoxidil: a comprehensive review. The Journal of dermatological treatment. 2022 Jun:33(4):1896-1906. doi: 10.1080/09546634.2021.1945527. Epub 2021 Jul 20 [PubMed PMID: 34159872]
Randolph M, Tosti A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. Journal of the American Academy of Dermatology. 2021 Mar:84(3):737-746. doi: 10.1016/j.jaad.2020.06.1009. Epub 2020 Jul 2 [PubMed PMID: 32622136]
Freire PCB, Riera R, Martimbianco ALC, Petri V, Atallah AN. Minoxidil for patchy alopecia areata: systematic review and meta-analysis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2019 Sep:33(9):1792-1799. doi: 10.1111/jdv.15545. Epub 2019 Jun 28 [PubMed PMID: 30835901]
Level 1 (high-level) evidenceSuchonwanit P, Thammarucha S, Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug design, development and therapy. 2019:13():2777-2786. doi: 10.2147/DDDT.S214907. Epub 2019 Aug 9 [PubMed PMID: 31496654]
Ramos PM, Goren A, Sinclair R, Miot HA. Oral minoxidil bio-activation by hair follicle outer root sheath cell sulfotransferase enzymes predicts clinical efficacy in female pattern hair loss. Journal of the European Academy of Dermatology and Venereology : JEADV. 2020 Jan:34(1):e40-e41. doi: 10.1111/jdv.15891. Epub 2019 Sep 11 [PubMed PMID: 31420889]
Sica DA. Minoxidil: an underused vasodilator for resistant or severe hypertension. Journal of clinical hypertension (Greenwich, Conn.). 2004 May:6(5):283-7 [PubMed PMID: 15133413]
Sharma A, Goren A, Dhurat R, Agrawal S, Sinclair R, Trüeb RM, Vañó-Galván S, Chen G, Tan Y, Kovacevic M, Situm M, McCoy J. Tretinoin enhances minoxidil response in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. Dermatologic therapy. 2019 May:32(3):e12915. doi: 10.1111/dth.12915. Epub 2019 Apr 23 [PubMed PMID: 30974011]
Sung CT, Juhasz ML, Choi FD, Mesinkovska NA. The Efficacy of Topical Minoxidil for Non-Scarring Alopecia: A Systematic Review. Journal of drugs in dermatology : JDD. 2019 Feb 1:18(2):155-160 [PubMed PMID: 30794366]
Level 1 (high-level) evidenceFabbrocini G, Cantelli M, Masarà A, Annunziata MC, Marasca C, Cacciapuoti S. Female pattern hair loss: A clinical, pathophysiologic, and therapeutic review. International journal of women's dermatology. 2018 Dec:4(4):203-211. doi: 10.1016/j.ijwd.2018.05.001. Epub 2018 Jun 19 [PubMed PMID: 30627618]
Kanti V, Messenger A, Dobos G, Reygagne P, Finner A, Blumeyer A, Trakatelli M, Tosti A, Del Marmol V, Piraccini BM, Nast A, Blume-Peytavi U. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. Journal of the European Academy of Dermatology and Venereology : JEADV. 2018 Jan:32(1):11-22. doi: 10.1111/jdv.14624. Epub 2017 Nov 27 [PubMed PMID: 29178529]
Smorlesi C, Caldarella A, Caramelli L, Di Lollo S, Moroni F. Topically applied minoxidil may cause fetal malformation: a case report. Birth defects research. Part A, Clinical and molecular teratology. 2003 Dec:67(12):997-1001 [PubMed PMID: 14745922]
Level 3 (low-level) evidenceMeyers RS, Siu A. Pharmacotherapy review of chronic pediatric hypertension. Clinical therapeutics. 2011 Oct:33(10):1331-56. doi: 10.1016/j.clinthera.2011.09.003. Epub 2011 Oct 7 [PubMed PMID: 21982385]
Chu JJ, Chen XJ, Shen SS, Zhang XF, Chen LY, Zhang JM, He J, Zhao JF. A poor performance in comprehensive geriatric assessment is associated with increased fall risk in elders with hypertension: a cross-sectional study. Journal of geriatric cardiology : JGC. 2015 Mar:12(2):113-8. doi: 10.11909/j.issn.1671-5411.2015.02.006. Epub [PubMed PMID: 25870613]
Level 2 (mid-level) evidenceRossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent patents on inflammation & allergy drug discovery. 2012 May:6(2):130-6 [PubMed PMID: 22409453]
Level 3 (low-level) evidenceRica Echevarría I, García Del Monte J, Delgado Rubio A, Arcangeli F, Lotti T. Severe hypertrichosis in infants due to transdermic exposure to 5% and 7% topical minoxidil. Dermatologic therapy. 2020 Nov:33(6):e14230. doi: 10.1111/dth.14230. Epub 2020 Sep 21 [PubMed PMID: 32844481]
Sánchez-Díaz M, López-Delgado D, Montero-Vílchez T, Salvador-Rodríguez L, Molina-Leyva A, Tercedor-Sánchez J, Arias-Santiago S. Systemic Minoxidil Accidental Exposure in a Paediatric Population: A Case Series Study of Cutaneous and Systemic Side Effects. Journal of clinical medicine. 2021 Sep 20:10(18):. doi: 10.3390/jcm10184257. Epub 2021 Sep 20 [PubMed PMID: 34575367]
Level 2 (mid-level) evidence. Minoxidil. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012:(): [PubMed PMID: 31643713]
Sever MS, Sonmez YE, Kocak N. Limited use of minoxidil in renal transplant recipients because of the additive side-effects of cyclosporine on hypertrichosis. Transplantation. 1990 Sep:50(3):536 [PubMed PMID: 2402807]
Level 3 (low-level) evidenceGoren A, Sharma A, Dhurat R, Shapiro J, Sinclair R, Situm M, Kovacevic M, Lukinovic Skudar V, Goldust M, Lotti T, McCoy J. Low-dose daily aspirin reduces topical minoxidil efficacy in androgenetic alopecia patients. Dermatologic therapy. 2018 Nov:31(6):e12741. doi: 10.1111/dth.12741. Epub 2018 Oct 8 [PubMed PMID: 30226287]
Mutterperl RE, Diamond FB, Lowenthal DT. Long-term effects of minoxidil in the treatment of malignant hypertension in chronic renal failure. Journal of clinical pharmacology. 1976 Oct:16(10 Pt 1):498-509 [PubMed PMID: 789412]
Oye M, Oye M, Ali A. Signs of early cardiac tamponade induced by Minoxidil. The American journal of emergency medicine. 2021 Feb:40():226.e1-226.e2. doi: 10.1016/j.ajem.2020.07.050. Epub 2020 Jul 24 [PubMed PMID: 32778436]
Gbadamosi WA, Melvin J, Lopez M. Atypical Case of Minoxidil-Induced Generalized Anasarca and Pleuropericardial Effusion. Cureus. 2021 Jun:13(6):e15424. doi: 10.7759/cureus.15424. Epub 2021 Jun 3 [PubMed PMID: 34249570]
Level 3 (low-level) evidenceGargallo V, Gutierrez C, Vanaclocha F, Guerra-Tapia A. Generalized Hypertrichosis Due to Topical Minoxidil. Actas dermo-sifiliograficas. 2015 Sep:106(7):599-600. doi: 10.1016/j.ad.2014.12.016. Epub 2015 Feb 14 [PubMed PMID: 25688008]
Level 3 (low-level) evidenceMacMillan AR, Warshawski FJ, Steinberg RA. Minoxidil overdose. Chest. 1993 Apr:103(4):1290-1 [PubMed PMID: 8131492]
Level 3 (low-level) evidence. Topical minoxidil: accidental poisoning in children. Prescrire international. 2015 Apr:24(159):97 [PubMed PMID: 25941701]
Garrard A, Wood A, Sollee D, Aaronson P. Refractory hypotension due to Rogaine® (minoxidil) ingestion managed with midodrine. Clinical toxicology (Philadelphia, Pa.). 2011 Dec:49(10):907-9. doi: 10.3109/15563650.2011.624988. Epub 2011 Nov 11 [PubMed PMID: 22077158]
Level 3 (low-level) evidenceChakar B, Salter M, Roberts DM. Minoxidil overdose with hypotension effectively managed with norepinephrine, rather than dopamine. Clinical toxicology (Philadelphia, Pa.). 2023 Feb:61(2):133-134. doi: 10.1080/15563650.2022.2159831. Epub 2023 Feb 13 [PubMed PMID: 36779868]
Denker MG, Haddad DB, Townsend RR, Cohen DL. Blood pressure control 1 year after referral to a hypertension specialist. Journal of clinical hypertension (Greenwich, Conn.). 2013 Sep:15(9):624-9. doi: 10.1111/jch.12146. Epub 2013 Jun 17 [PubMed PMID: 24034654]
Level 2 (mid-level) evidenceGarg JP, Elliott WJ, Folker A, Izhar M, Black HR, RUSH University Hypertension Service. Resistant hypertension revisited: a comparison of two university-based cohorts. American journal of hypertension. 2005 May:18(5 Pt 1):619-26 [PubMed PMID: 15882544]
Level 2 (mid-level) evidence