Indications
Montelukast is an orally dosed drug (available as a film-coated tablet, chewable tablet, or oral granules) that is FDA-approved for treating the following conditions.
- Asthma: Monteleulast is indicated for asthma prophylaxis and chronic treatment in adults and pediatric patients 12 months of age and older.[1][2]
- Exercise-Induced Bronchoconstriction (EIB): Montelukast is indicated to prevent exercise-induced bronchoconstriction (EIB) in patients six years of age and older.[3]
- Seasonal Allergic Rhinitis: Montelukast is indicated to relieve symptoms of seasonal allergic rhinitis in patients two years of age and more; and perennial allergic rhinitis in patients six months of age and older. Because the benefits of montelukast may not outweigh the risk of neuropsychiatric adverse reactions in patients with allergic rhinitis, only used for patients who have an intolerance or an inadequate response to alternative therapies.[4]
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
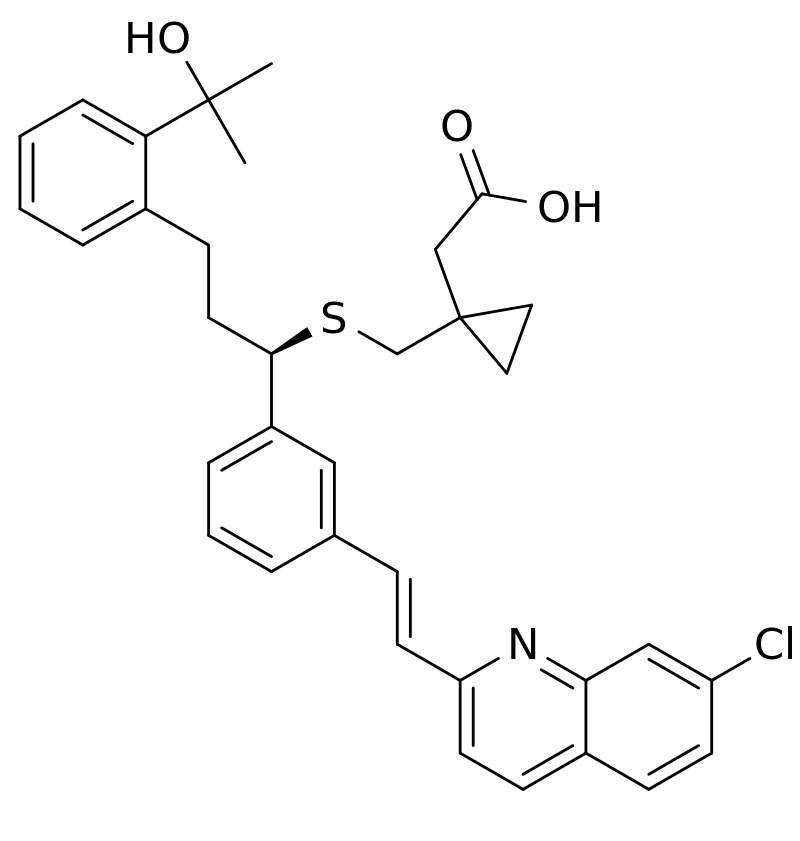
Montelukast (empirical formula C35H35ClNNaO3S) is a highly selective leukotriene receptor antagonist that binds with high affinity to the cysteinyl leukotriene receptor for leukotrienes D4 and E4. These leukotrienes are excreted by various cells, such as mast cells, and are involved in the inflammatory process that may cause asthma and allergic rhinitis signs and symptoms. Leukotriene receptors are found in airway cells, such as macrophages and smooth muscle cells. When bound to leukotriene receptors, montelukast inhibits leukotriene physiologic effects (such as airway edema, smooth muscle contraction, and impairment of normal cellular activity) without exhibiting any agonist activity. In asthmatics, low doses of montelukast (5 mg) induce a significant inhibition of bronchoconstriction caused by leukotriene D4. Furthermore, in a crossover study, montelukast induced inhibition of both early and late phase bronchoconstriction caused by a challenge with antigen in 12 asthmatic patients. Although most international asthma guidelines advise that children ≤5 years with asthma be treated with daily low- moderate dose inhaled corticosteroids (ICS) as the preferred controller and montelukast as an alternative therapy.[5]
In controlled studies, montelukast is reported to significantly reduce beta2-agonist use (p<0.001), asthma symptoms (p=0.001), blood eosinophils (p=0.009) and significantly increase morning peak expiratory flow (p=0.001). These parameters demonstrate that montelukast decreases airway eosinophilic inflammation and improves clinical symptoms. Its efficacy in the treatment of chronic asthma may be due, in part, to the effect on airway inflammation.[6]
Pharmacokinetics
- Absorption: Montelukast is quickly absorbed following oral administration. After the 10 mg film-coated tablet is administered to fasted adults, the mean peak montelukast plasma concentration (C max) is achieved in 3 to 4 hours (T max). The mean oral bioavailability is 64%. A standard meal does not influence the oral bioavailability and C max in the morning.
- Distribution: Montelukast is approximately 99% bound to plasma proteins.
- Metabolism: Montelukast is primarily metabolized by the liver. At clinically relevant concentrations, CYP2C8 appears to play a major role in the metabolism of montelukast.
- Elimination: Montelukast and metabolites are excreted almost singly via the bile. The pharmacokinetics of montelukast is linear for doses up to 50 mg.
Administration
Montelukast may be taken without regard to food or meals. Patients with phenylketonuria who receive montelukast should be aware that chewable tablets contain phenylalanine. There is no need to adjust doses when montelukast is co-administered with other systemic treatments.
- For the treatment of chronic asthma, it is best to administer the dose in the evening. Recommended doses are 10 mg for patients aged 15 years and older, 5 mg for patients aged six to 14 years, and 4 mg for patients 12 months to five years old. For patients aged 12 to 23 months, tablets are not indicated, and only oral granules are used. In the absence of clinical data on efficacy and safety in asthma patients under the age of 12 months, montelukast is not indicated for this patient population. In addition, Montelukast is not suitable for the treatment of acute asthma exacerbations, such as status asthmatics.
- For prophylaxis of exercise-induced bronchoconstriction, montelukast should be administered at least 2 hours before initiating exercise. Recommended doses are 10 mg for patients aged 15 years and older and 5 mg for patients aged six to 14 years. In the absence of clinical data on efficacy and safety in patients under the age of 6 years with exercise-induced bronchoconstriction, montelukast is not indicated for this patient population. Daily doses of montelukast should be separated by at least 24 hours. A regular intake of montelukast for the treatment of chronic asthma does not prevent exercise-induced bronchoconstriction.
- In patients with allergic rhinitis, the dose may be taken in the morning or the evening. For seasonal allergic rhinitis, recommended doses are 10 mg for patients aged 15 years and older, 5 mg for patients aged six to 14 years, and 4 mg for patients aged two to five years. In the absence of clinical data on efficacy and safety in patients younger than two years of age with seasonal allergic rhinitis, montelukast is not indicated in that patient population.
- For perennial allergic rhinitis, recommended doses are 10 mg for patients aged 15 years and older, 5 mg for patients aged six to 14 years, and 4 mg for patients aged six months to five years. For patients aged six months to 23 months, tablets are not indicated, and only oral granules are used. In the absence of clinical data on efficacy and safety in patients younger than two years of age with seasonal allergic rhinitis, montelukast is not indicated for these patients.
Specific Patient Population
Hepatic Insufficiency: No dosage adjustment is needed in patients with mild-to-moderate hepatic insufficiency. According to product labeling, the pharmacokinetics of montelukast in patients with more severe hepatic impairment have not been assessed.
Renal Insufficiency: Montelukast and its metabolites are excreted via the bile. The pharmacokinetics of montelukast has not been evaluated in patients with renal insufficiency. Therefore, no dosage adjustment is recommended for these patients.
Pregnancy Considerations: Available data from published studies with montelukast use in pregnant women have not established a drug-associated risk of major congenital disabilities.
Breastfeeding Considerations: Low levels of montelukast appear in breast milk. Montelukast has been used in neonates in dosages far greater than the amounts in breast milk. Amounts ingested by the infant are not expected to cause any adverse effects in breastfed infants. No special precautions are required.[7]
Adverse Effects
Neuropsychiatric events have been reported in patients receiving montelukast. These events have been noted in adults, teenagers, and younger patients. They include, among others: anxiety, depression, aggressiveness, agitation, attention and memory impairment, sleeping disorders (insomnia, somnambulism, dream anomalies), seizures, paresthesia, hypoesthesia, as well as suicidal thoughts and behavior.[7][8]
During treatment with montelukast, some patients with asthma may develop systemic eosinophilia, sometimes associated with vasculitis, consistent with Churg-Strauss syndrome (rare). This event may be associated with a decrease in oral corticosteroid doses. However, montelukast is the causative agent of these systemic manifestations has not been established.
Other adverse effects of montelukast include (among others):
- Headaches, fever, fatigue
- Upper respiratory signs (rhinorrhea, pharyngitis, laryngitis, sinusitis, epistaxis)
- Auricular signs: otitis
- Lower respiratory signs: a cough, pneumonia, wheezing
- Ocular signs: conjunctivitis
- Gastrointestinal signs (nausea, diarrhea, vomiting, abdominal pain, dyspepsia, pancreatitis). Some of these may be secondary to lactose that is compounded with montelukast.
- Infections (influenza, varicella)
- Dermatologic manifestations (pruritus, eczema, atopic dermatitis, angioedema, urticaria, skin rash, bruising, erythema multiforme, erythema nodosum, toxic epidermal necrolysis, and Stevens-Johnson syndrome)
- Musculoskeletal signs: Arthralgia, myalgia
- Hypersensitivity manifestations: anaphylaxis, eosinophilic infiltration of the liver
- Hepatotoxicity: In clinical trials, mild elevations in serum aminotransferase levels were found in 1% to 2% of patients taking montelukast chronically, but similar rates are reported in matched placebo recipients. The enzyme elevation pattern is usually mixed, including hepatocellular and cholestatic patterns. Allergic features and autoantibody formation were rare. The injury is usually resolved within 1 to 4 months of stopping the drug. Likelihood score: B (rare but likely cause of clinically apparent liver injury).[9]
Contraindications
Montelukast is contraindicated in patients with a history of hypersensitivity to the drug or its components. In addition, for patients with phenylketonuria (PKU), caution should be exercised with phenylalanine-containing formulations.
Boxed Warning: Neuropsychiatric Events
Neuropsychiatric events have been described with the use of montelukast sodium. These postmarketing reports have been highly variable, including agitation, aggressive behavior, anxiousness, depression, disorientation, disturbance in attention, irritability, memory impairment, obsessive-compulsive symptoms, hallucinations, insomnia, restlessness, suicidal thoughts, and behavior (including suicide). Neuropsychiatric events have been documented in patients with and without a history of psychiatric disorders. Based on risk-benefit considerations, the asthma indication has not been changed.[10]
Monitoring
Patients taking montelukast should be regularly monitored for mood or behavior changes, including suicidal thinking or behavior. In addition, clinicians should advise their patients to report any neuropsychiatric signs.
Toxicity
In clinical studies, montelukast has been used at high doses in adult patients (up to 200 mg daily for 22 weeks and up to 900 mg daily for about a week) without significant adverse effects. Cases of acute overdosage with montelukast have been reported in adults and children with doses as high as 1000 mg. However, clinical and biological signs in such cases were relatively benign and included headaches, thirst, somnolence or hyperactivity, vomiting, and abdominal pain.[11]
In case of overdose with montelukast, classical supportive therapies such as gastric lavage, adsorption with activated carbon, clinical monitoring, and, if necessary, supportive therapy may be used. Unfortunately, there is no known antidote for montelukast overdosage. In addition, no data exist concerning the efficiency of hemodialysis and peritoneal dialysis for removing montelukast from the body.
Montelukast has no known carcinogenic or mutagenic effects. In addition, no fertility impairment or teratogenic effect has been reported with this molecule. Dose adjustment is not necessary for renal failure or mild-to-moderate hepatic insufficiency.
Enhancing Healthcare Team Outcomes
Montelukast is an effective prophylactic agent for asthma. However, prescribers, including nurse practitioners, pharmacists, internists, and primary care providers, must be aware that the drug can cause neuropsychiatric alterations in young people, including suicidal thoughts. It is, therefore, necessary to adopt an interprofessional team approach to montelukast therapy. This healthcare team includes clinicians (MDs, DOs, NPs, PAs), nurses, respiratory therapists, and pharmacists, all contributing from their expertise and openly sharing information and patient status among the team. Young patients may need to be closely monitored by a mental health nurse while on treatment.[12] Using this interprofessional paradigm will result in improved outcomes with fewer adverse events. [Level 5]
Media
References
Sánchez G, Buitrago D. Effect of Montelukast 10 mg in Elderly Patients with Mild and Moderate Asthma Compared with Young Adults. Results of a Cohort Study. The open respiratory medicine journal. 2018:12():67-74. doi: 10.2174/1874306401812010067. Epub 2018 Nov 14 [PubMed PMID: 30988828]
Sun W, Liu HY. Montelukast and Budesonide for Childhood Cough Variant Asthma. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP. 2019 Apr:29(4):345-348. doi: 10.29271/jcpsp.2019.04.345. Epub [PubMed PMID: 30925958]
de Benedictis FM, Vaccher S, de Benedictis D. Montelukast sodium for exercise-induced asthma. Drugs of today (Barcelona, Spain : 1998). 2008 Nov:44(11):845-55. doi: 10.1358/dot.2008.44.11.1297498. Epub [PubMed PMID: 19180262]
Krishnamoorthy M,Mohd Noor N,Mat Lazim N,Abdullah B, Efficacy of Montelukast in Allergic Rhinitis Treatment: A Systematic Review and Meta-Analysis. Drugs. 2020 Nov [PubMed PMID: 32915441]
Level 1 (high-level) evidenceCastro-Rodriguez JA, Rodriguez-Martinez CE, Ducharme FM. Daily inhaled corticosteroids or montelukast for preschoolers with asthma or recurrent wheezing: A systematic review. Pediatric pulmonology. 2018 Dec:53(12):1670-1677. doi: 10.1002/ppul.24176. Epub 2018 Nov 5 [PubMed PMID: 30394700]
Level 1 (high-level) evidencePizzichini E, Leff JA, Reiss TF, Hendeles L, Boulet LP, Wei LX, Efthimiadis AE, Zhang J, Hargreave FE. Montelukast reduces airway eosinophilic inflammation in asthma: a randomized, controlled trial. The European respiratory journal. 1999 Jul:14(1):12-8 [PubMed PMID: 10489822]
Level 1 (high-level) evidence. Montelukast. Drugs and Lactation Database (LactMed®). 2006:(): [PubMed PMID: 30000548]
Farah RI,Damkier P,Christiansen A,Henriksen DP, Early Discontinuation of Montelukast Treatment; A Danish Nationwide Utilization Study. Basic [PubMed PMID: 29438596]
. Montelukast. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012:(): [PubMed PMID: 31643588]
Clarridge K, Chin S, Eworuke E, Seymour S. A Boxed Warning for Montelukast: The FDA Perspective. The journal of allergy and clinical immunology. In practice. 2021 Jul:9(7):2638-2641. doi: 10.1016/j.jaip.2021.02.057. Epub 2021 Mar 17 [PubMed PMID: 33744471]
Level 3 (low-level) evidenceLassila T, Hokkanen J, Aatsinki SM, Mattila S, Turpeinen M, Tolonen A. Toxicity of Carboxylic Acid-Containing Drugs: The Role of Acyl Migration and CoA Conjugation Investigated. Chemical research in toxicology. 2015 Dec 21:28(12):2292-303. doi: 10.1021/acs.chemrestox.5b00315. Epub 2015 Nov 11 [PubMed PMID: 26558897]
Schwimmbeck F, Staffen W, Höhn C, Rossini F, Renz N, Lobendanz M, Reichenpfader P, Iglseder B, Aigner L, Trinka E, Höller Y. Cognitive Effects of Montelukast: A Pharmaco-EEG Study. Brain sciences. 2021 Apr 27:11(5):. doi: 10.3390/brainsci11050547. Epub 2021 Apr 27 [PubMed PMID: 33925326]