Introduction
A neurolytic block is the targeted destruction of a nerve or nerve plexus. Frequently, the term neuroablation is used to also describe the physical interruption of pain either chemically, thermally, or surgically. All neurolytic techniques cause Wallerian degeneration of the nerve axon distal to the lesion.[1] Chemicals were extensively utilized in the past for neurolysis in the early 20 century. The first report of chemical neurolysis was made in 1863 by Luton who delivered irritant chemicals subcutaneously in patients with sciatic neuralgia, offering them significant alleviation of pain. [Luton A, generales de medecin, 1863] The advent of newer and safer modalities have been introduced into pain practice including radiofrequency ablation (RFA),[2] cryoablation,[3] and neurosurgical procedures.[4] The advancements in imaging modalities such as fluoroscopy have improved the precision and efficiency of targeted neurolysis.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Anatomy is dependent on the location of the targeted peripheral nerve or nerve plexus, and careful needle advancement is employed with guidance through ultrasonography, fluoroscopy, and/or nerve stimulation.
Indications
There are no consensus indications for neurolysis as targeted nerve destruction can apply to a variety of pain conditions. However, appropriate patient selection is important before interventional pain treatment. Generally, an interventional approach is taken after conservative options fail. In addition to obtaining a thorough history, physical exam, and complete diagnostic laboratory workup, a recent radiological evaluation is often necessary to identify the cause of the pain and to prevent complications associated with the interventional neurolytic technique. A psychological assessment is frequently performed to determine if the patient is suitable for intervention, especially if a neuromodulatory approach is taken.
Celiac plexus neurolytic blocks are frequently performed for visceral pain originating from upper abdominal malignancy, especially pancreatic cancer.[5] Superior hypogastric plexus neurolysis may be attempted for patients with pelvic visceral pain.[6] Trigeminal neuropathy may be alleviated with neurolysis of the trigeminal nerve.[7] Intercostal nerve neurolysis can treat pain from fractured ribs, cancer metastasis, and post-thoracotomy pain. Sympathetic plexus neurolysis can also be performed, particularly in cancer pain patients if they manifest with neuropathic pain syndromes (e.g., thoracic plexus, lumbosacral plexus, stellate plexus) or visceral pain (e.g., celiac, hypogastric, and ganglion impart blocks) from damage to a sympathetic plexus. Facetogenic and vertebral pain may be alleviated with neurolysis of the medial branch of the primary dorsal ramus.[1] Neuraxial administration of alcohol and phenol has fallen out of favor due to significant side effects and is typically described in spasticity disorders and the end-stage cancer population.[8][9]
Contraindications
Absolute contraindications to targeted neurolysis include patient refusal, active infection at the site of injection, and allergy to a chemical neurolytic agent. Bleeding disorders or anticoagulation treatment is considered a contraindication, particularly if injection occurs at non-compressible sites. If a pacemaker is in place and radiofrequency ablation is planned, the proceduralist should consult pacemaker interrogation services.
Equipment
The necessary equipment includes the following:
- Chlorhexidine gluconate or povidone iodine
- Ultrasound probe with a sterile probe cover and gel (if applicable)
- Fluoroscopy equipment (if applicable)
- Nerve stimulator (if applicable)
- Local anesthetic, typically 1% lidocaine, for superficial layer local anesthesia
- Regional block local anesthetic test solution (2% lidocaine or 1.5% mepivacaine)
- A 10- to 20-mL syringe with extension tubing
- Block needle (length-variable depending on depth of targeted peripheral nerve) or spinal/epidural needle if neuraxial approach (needle characteristics based on body habitus and provider preference)
- Chemical neurolytic agent (alcohol, phenol), if applicable
- Radiofrequency probe, if applicable
- Cryo machine and cryoprobe with cooling agents (i.e. nitrous oxide, carbon dioxide), if applicable
Agents used for chemical neurolysis primarily include 50-100% alcohol and 5% to 15% phenol, although the use of other agents including hypertonic saline, glycerol, ammonium salt solutions, and chlorocresol has also been reported.[10] The mechanism of action of alcohol neurolysis is axonal and Schwann cell destruction from phospholipid extraction in the cell membrane and lipoprotein precipitation.[11] Phenol infiltration causes damage from protein coagulation and degeneration.[12]
Radiofrequency probes are frequently used for nerve ablation of the medial branch nerve, percutaneous cordotomy, thermocoagulation of Gasserian ganglion, percutaneous rhizotomy, and percutaneous radiofrequency sympathectomy.[8][9] Conventional RFA generates a current with an oscillating frequency, producing heat that creates circumscript lesions used for selective nerve lesioning. Generally, heat over 60 C is used to create lesions. Pulsed RFA is similar to conventional thermal ablation although it uses a higher voltage in a pulsatile manner, permitting energy to dissipate easier and generating less heat. Cooled RFA involves active cooling with a continuous flow of water that prevents the current from reaching high temperatures experienced in conventional ablation; this allows for higher temperatures and more spherical and larger lesioning.[8][9]
Cryoprobes attached to a cryo machine can be used to target nerves with extreme cold temperature exposure.[8] Cryoablation disrupts the vasa nervorum leading to axonal destruction, and may be associated with decreased incidence of post-procedural hyperalgesia and formation of neuromas when compared to conventional RFA.[13]
Finally, surgical neurolysis or neurectomy involves directly severing a nerve, and is generally reserved for rare cases with a poor prognosis. This modality carries a high risk of deafferentation pain, which is a complication due to loss of neuronal input leading to spontaneous firing within the spinothalamic tract.[14] This pain may manifest more severely than the original symptoms prior to neurolysis.
Personnel
A pain medicine specialist with training in ultrasound- and fluoroscopic-guided nerve injections is preferable. Additional nursing staff with training in sedation anesthesia may assist.
Preparation
The proceduralist should obtain informed consent. The patient is positioned appropriately depending on the location of the targeted nerve. Minimal or no sedation is typically required. However, moderate sedation may be considered in certain instances, such as chemical neurolysis with alcohol which may be painful, or during RFA. Aseptic technique should be maintained throughout the procedure.
Technique or Treatment
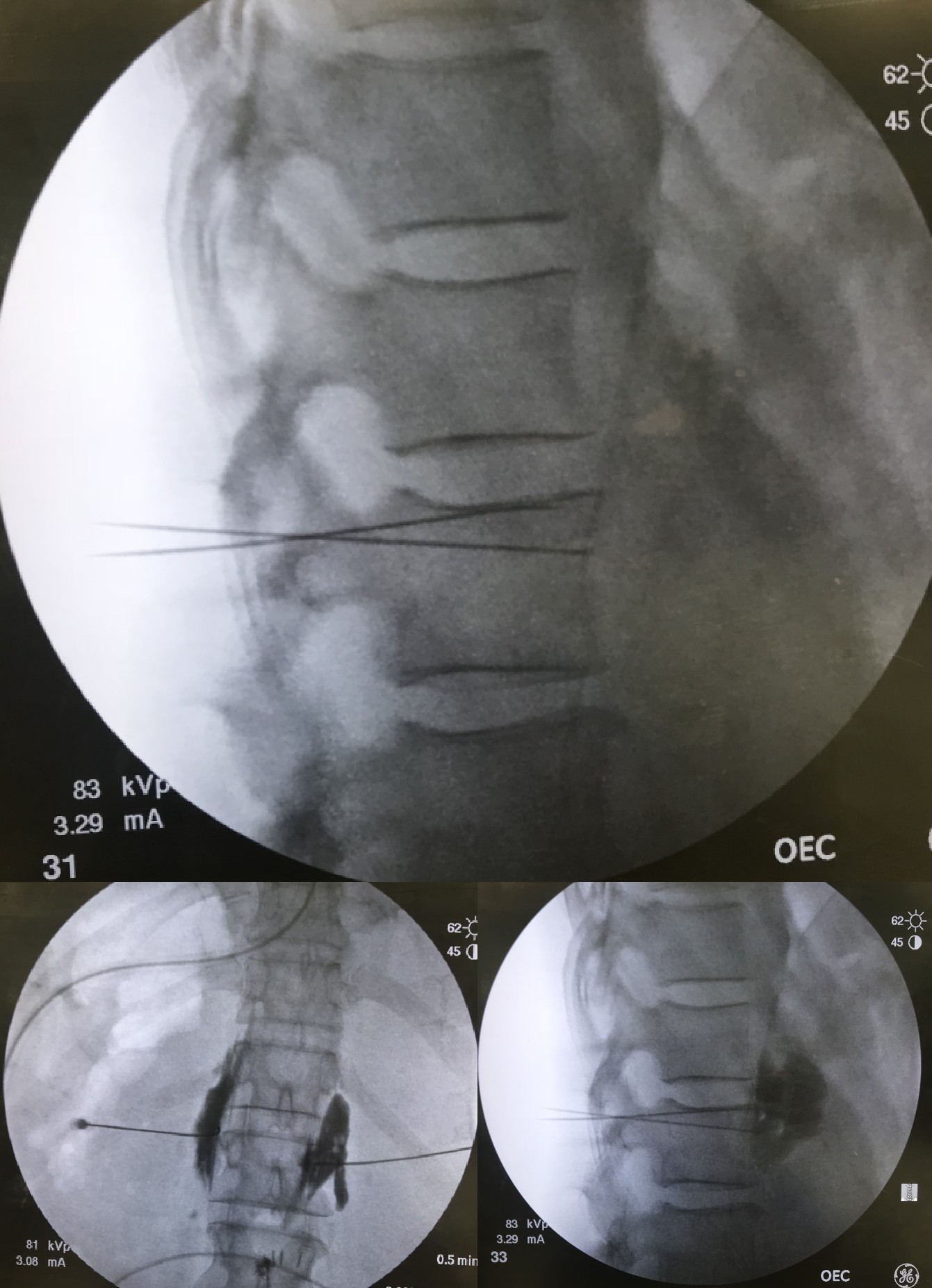
Under aseptic precaution, the target nerve is identified using fluoroscopy, ultrasound, or nerve stimulation. A local anesthetic is infiltrated at the skin. The needle, radiofrequency probe, or cryoprobe is advanced toward the target nerve, and after a preliminary aspiration, a diagnostic block with a local anesthetic may be performed to confirm position, and subsequently, neurolysis (e.g., injection of alcohol or phenol, RFA, among others) can be performed. Typically, diagnostic blocks are performed before neurolytic intervention to allow better prediction of efficacy. Cardiovascular monitoring with resuscitative backup should be readily available. Patients should be counseled that the therapeutic effect from neurolysis, particularly chemical neurolysis, may not be evident for 3 to 7 days. See Image. Neurolytic Celiac Plexus Block, Radiograph.
Complications
Bleeding, infection, pain, and damage to surrounding tissue may occur as with any interventional procedure. Intravascular injection of a neurolytic agent may result in systemic toxicity depending on the chemical agent used. Superficial cryoablation may lead to skin damage, manifesting with alopecia, hypo- or hyperpigmentation.[15] Less frequently, neuritis may occur after partial denervation with a neurolytic agent, with subsequent nerve regeneration and hyperesthesia worse than the original pain. If neurolysis of a motor nerve occurs, prolonged motor paralysis may result; similarly, bowel, bladder, and sexual dysfunction may result from denervation.
Clinical Significance
A neurolytic block is used to target the destruction of a nerve or nerve plexus. Chemicals, radiofrequency ablation (RFA), cryoablation, and neurosurgical procedures are often used with fluoroscopy to improve the precision and efficiency of targeted neurolysis. Patients often received substantial reductions in pain.
Enhancing Healthcare Team Outcomes
There are many case series, observational trials, and few randomized controlled trials investigating outcomes in neurolysis, but due to heterogeneity of nerve targets and a large variety of tools for neurolysis, large data pools have not been studied.
In terms of chemical neurolysis, phenol neurolysis may provide therapeutic relief for 8 to 12 weeks, while alcohol neurolysis generally lasts longer from 12 to 24 weeks. RFA of peripheral nerves may last 3 to 12 months until axonal regeneration occurs.[16] Cryoanalgesia may provide variable relief lasting weeks to months.[15]
Of studies looking at chemical neurolysis, those targeting the celiac plexus for pancreatic malignancy have been extensively described. A meta-analysis demonstrated that neurolytic celiac plexus block in patients with unresectable pancreatic cancer was associated with improved pain scores, reduced opioid use, and decreased constipation.[17]
Of all studies looking at non-chemical interventional approaches to neurolysis of peripheral nerve targets, RFA of the medial branch of the dorsal primary rami that innervate facet joints has been most studied. A prospective 10-year clinical trial investigating radiofrequency neurotomy of the lumbar facet joints for relief of chronic low back pain showed that over 68% of patients reported good (greater than 50%) to excellent (greater than 80%) pain relief lasting from 6 to 24 months.[18] Furthermore, Lee and colleagues performed a meta-analysis of randomized controlled trials comparing the efficacy of conventional RFA versus control treatment (sham or epidural block), which showed a greater improvement in back pain scores in the RFA group at a 1-year follow-up.[19] Studies have also compared the efficacy of RFA and chemical neurolysis. An RCT comparing RFA and chemical neurolysis of thoracic splanchnic nerves for abdominal malignancy pain demonstrated that RFA of the splanchnic nerves at the T10 and T11 levels are more effective compared to using alcohol neurolysis solely. Patients in the RFA arm also reported faster and longer-acting duration of analgesia with a better safety profile.[20] Factors associated with a poor response to radiofrequency facet denervation include long duration of pain, previous back surgery, depression, and the number of treated joints.[21][22]
Despite these meta-analyzed data, controversy still exists on the long-term efficacy of RFA for low back pain. For example, a recent study that included three separate randomized controlled trials performed by Juch and colleagues included patients with chronic low back pain from facet joints, sacroiliac joints, or intervertebral disks. They reported that RFA combined with exercise compared with exercise alone resulted in no difference in pain intensity after 3 months.[23]
A systematic review on the efficacy of cryoablation in the cancer pain population demonstrated that cryoablation decreased pain scores by over 60% at 24 hours post-procedure, by 70% at 3 months, and by over 80% at 6 months.[24] Furthermore, after 4 weeks and 8 weeks post-procedure, cryoablation was associated with about a 44% and 60% improvement in the quality of life, respectively. The need for opioids also decreased significantly by about 60% at 3 months.
Data on surgical neurectomy are even more limited, and typically involve peripheral nerves at the trunk. A study investigating neurectomies of the ilioinguinal, iliohypogastric, genotifemoral, lateral-femoral cutaneous, and intercostal nerves demonstrated this as a reasonable modality for treatment of chronic postoperative neurogenic pain, leading to significant improvement in pain scores and quality of life.[25] Of all peripheral nerve neurectomies, ilioinguinal and iliohypogastric neurectomy have been most extensively studied, primarily in patients with postherniorraphy inguinal neuralgia. A tailored neurectomy approach of the ilioinguinal and iliohypogastric nerves can be 3 times more effective compared to tender point infiltration in alleviating chronic inguinodynia after anterior hernia mesh repair.[26] Several randomized controlled trials intervening with prophylactic planned ilioinguinal neurectomy at the time of inguinal hernia repair have also been reported to lead to a decrease in the incidence of chronic postoperative pain.[27]
Outcomes data on neurolysis for pain relief remains inconsistent due to variable patient selection, diagnostic criteria, type of intervention, and outcome measures. Due to the absence of compelling evidence and the paucity of data suggesting low-risk long-term benefits, neurolysis should be cautiously considered only after a failure of other modalities including pharmacologic, physical, psychiatric, and non-destructive interventional therapy.[8]
Media
(Click Image to Enlarge)
References
Van Zundert J, Vanelderen P, Kessels A, van Kleef M. Radiofrequency treatment of facet-related pain: evidence and controversies. Current pain and headache reports. 2012 Feb:16(1):19-25. doi: 10.1007/s11916-011-0237-8. Epub [PubMed PMID: 22090264]
Shealy CN. Percutaneous radiofrequency denervation of spinal facets. Treatment for chronic back pain and sciatica. Journal of neurosurgery. 1975 Oct:43(4):448-51 [PubMed PMID: 125787]
Loev MA, Varklet VL, Wilsey BL, Ferrante FM. Cryoablation: a novel approach to neurolysis of the ganglion impar. Anesthesiology. 1998 May:88(5):1391-3 [PubMed PMID: 9605700]
Level 3 (low-level) evidenceMazal PR, Millesi H. Neurolysis: is it beneficial or harmful? Acta neurochirurgica. Supplement. 2005:92():3-6 [PubMed PMID: 15830957]
Brown DL, Bulley CK, Quiel EL. Neurolytic celiac plexus block for pancreatic cancer pain. Anesthesia and analgesia. 1987 Sep:66(9):869-73 [PubMed PMID: 3619093]
Bosscher H. Blockade of the superior hypogastric plexus block for visceral pelvic pain. Pain practice : the official journal of World Institute of Pain. 2001 Jun:1(2):162-70 [PubMed PMID: 17129292]
Ko AL, Ozpinar A, Lee A, Raslan AM, McCartney S, Burchiel KJ. Long-term efficacy and safety of internal neurolysis for trigeminal neuralgia without neurovascular compression. Journal of neurosurgery. 2015 May:122(5):1048-57. doi: 10.3171/2014.12.JNS14469. Epub 2015 Feb 13 [PubMed PMID: 25679283]
Level 2 (mid-level) evidenceJackson TP, Gaeta R. Neurolytic blocks revisited. Current pain and headache reports. 2008 Jan:12(1):7-13 [PubMed PMID: 18417017]
Erdine S. Neurolytic blocks: when, how, why. Agri : Agri (Algoloji) Dernegi'nin Yayin organidir = The journal of the Turkish Society of Algology. 2009 Oct:21(4):133-40 [PubMed PMID: 20127532]
Swerdlow M. Intrathecal neurolysis. Anaesthesia. 1978 Sep:33(8):733-40 [PubMed PMID: 581435]
Choi EJ, Choi YM, Jang EJ, Kim JY, Kim TK, Kim KH. Neural Ablation and Regeneration in Pain Practice. The Korean journal of pain. 2016 Jan:29(1):3-11. doi: 10.3344/kjp.2016.29.1.3. Epub 2016 Jan 4 [PubMed PMID: 26839664]
Halpern D. Histologic studies in animals after intramuscular neurolysis with phenol. Archives of physical medicine and rehabilitation. 1977 Oct:58(10):438-43 [PubMed PMID: 907449]
Level 3 (low-level) evidencePrologo JD, Gilliland CA, Miller M, Harkey P, Knight J, Kies D, Hawkins CM, Corn D, Monson DK, Edalat F, Dariushnia S, Brewster L. Percutaneous Image-Guided Cryoablation for the Treatment of Phantom Limb Pain in Amputees: A Pilot Study. Journal of vascular and interventional radiology : JVIR. 2017 Jan:28(1):24-34.e4. doi: 10.1016/j.jvir.2016.09.020. Epub 2016 Nov 23 [PubMed PMID: 27887967]
Level 3 (low-level) evidencevan Assen T, Boelens OB, van Eerten PV, Scheltinga MR, Roumen RM. Surgical options after a failed neurectomy in anterior cutaneous nerve entrapment syndrome. World journal of surgery. 2014 Dec:38(12):3105-11. doi: 10.1007/s00268-014-2737-2. Epub [PubMed PMID: 25189442]
Level 2 (mid-level) evidenceIlfeld BM, Gabriel RA, Trescot AM. Ultrasound-guided percutaneous cryoneurolysis providing postoperative analgesia lasting many weeks following a single administration: a replacement for continuous peripheral nerve blocks?: a case report. Korean journal of anesthesiology. 2017 Oct:70(5):567-570. doi: 10.4097/kjae.2017.70.5.567. Epub 2017 Feb 3 [PubMed PMID: 29046778]
Level 3 (low-level) evidenceMcCormick ZL, Marshall B, Walker J, McCarthy R, Walega DR. Long-Term Function, Pain and Medication Use Outcomes of Radiofrequency Ablation for Lumbar Facet Syndrome. International journal of anesthetics and anesthesiology. 2015:2(2):. pii: 028. Epub [PubMed PMID: 26005713]
Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. The American journal of gastroenterology. 2007 Feb:102(2):430-8 [PubMed PMID: 17100960]
Level 1 (high-level) evidenceGofeld M, Jitendra J, Faclier G. Radiofrequency denervation of the lumbar zygapophysial joints: 10-year prospective clinical audit. Pain physician. 2007 Mar:10(2):291-300 [PubMed PMID: 17387351]
Lee CH, Chung CK, Kim CH. The efficacy of conventional radiofrequency denervation in patients with chronic low back pain originating from the facet joints: a meta-analysis of randomized controlled trials. The spine journal : official journal of the North American Spine Society. 2017 Nov:17(11):1770-1780. doi: 10.1016/j.spinee.2017.05.006. Epub 2017 May 30 [PubMed PMID: 28576500]
Level 1 (high-level) evidenceAmr SA, Reyad RM, Othman AH, Mohamad MF, Mostafa MM, Alieldin NH, Hamed FA. Comparison between radiofrequency ablation and chemical neurolysis of thoracic splanchnic nerves for the management of abdominal cancer pain, randomized trial. European journal of pain (London, England). 2018 Nov:22(10):1782-1790. doi: 10.1002/ejp.1274. Epub 2018 Jul 11 [PubMed PMID: 29975804]
Level 1 (high-level) evidenceStreitberger K, Müller T, Eichenberger U, Trelle S, Curatolo M. Factors determining the success of radiofrequency denervation in lumbar facet joint pain: a prospective study. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2011 Dec:20(12):2160-5. doi: 10.1007/s00586-011-1891-6. Epub 2011 Jun 30 [PubMed PMID: 21717237]
Cohen SP, Hurley RW, Christo PJ, Winkley J, Mohiuddin MM, Stojanovic MP. Clinical predictors of success and failure for lumbar facet radiofrequency denervation. The Clinical journal of pain. 2007 Jan:23(1):45-52 [PubMed PMID: 17277644]
Juch JNS, Maas ET, Ostelo RWJG, Groeneweg JG, Kallewaard JW, Koes BW, Verhagen AP, van Dongen JM, Huygen FJPM, van Tulder MW. Effect of Radiofrequency Denervation on Pain Intensity Among Patients With Chronic Low Back Pain: The Mint Randomized Clinical Trials. JAMA. 2017 Jul 4:318(1):68-81. doi: 10.1001/jama.2017.7918. Epub [PubMed PMID: 28672319]
Level 1 (high-level) evidenceFerrer-Mileo L, Luque Blanco AI, González-Barboteo J. Efficacy of Cryoablation to Control Cancer Pain: A Systematic Review. Pain practice : the official journal of World Institute of Pain. 2018 Nov:18(8):1083-1098. doi: 10.1111/papr.12707. Epub 2018 Jun 7 [PubMed PMID: 29734509]
Level 1 (high-level) evidenceNagarkar P, Ramanadham S, Chamseddin K, Chhabra A, Rozen SM. Neurectomy for the Treatment of Chronic Postoperative Pain after Surgery of the Trunk. Plastic and reconstructive surgery. 2017 Jan:139(1):204-211. doi: 10.1097/PRS.0000000000002892. Epub [PubMed PMID: 28027249]
Verhagen T, Loos MJA, Scheltinga MRM, Roumen RMH. The GroinPain Trial: A Randomized Controlled Trial of Injection Therapy Versus Neurectomy for Postherniorraphy Inguinal Neuralgia. Annals of surgery. 2018 May:267(5):841-845. doi: 10.1097/SLA.0000000000002274. Epub [PubMed PMID: 28448383]
Level 1 (high-level) evidenceJohner A, Faulds J, Wiseman SM. Planned ilioinguinal nerve excision for prevention of chronic pain after inguinal hernia repair: a meta-analysis. Surgery. 2011 Sep:150(3):534-41. doi: 10.1016/j.surg.2011.02.024. Epub 2011 May 24 [PubMed PMID: 21605884]
Level 1 (high-level) evidence