Introduction
Neurons are electrically excitable cells that transmit signals throughout the body. Neurons employ both electrical and chemical components in the transmission of information. Neurons are connected to other neurons at synapses and connected to effector organs or cells at neuroeffector junctions. A typical multipolar neuron is comprised of soma or cell body, an axon, and dendrites. The axon is thought of as the part transmitting efferent signals, while the dendrites are receiving afferent signals from their surroundings.[1]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Neurons are unique in their ability to receive and transmit information. Neurons are characterized by the long processes which extend out from the cell body or soma. Dendrites receive afferent signals. Axons carry efferent signals. Neurons exist in a variety of forms including multipolar, bipolar, pseudounipolar, and anaxonic which differ primarily in their number and arrangement of axons and dendrites. The soma contains the nucleus and other organelles necessary for neuronal function. There may be one or many dendrites associated with a single neuron depending on its function and location. In addition to afferent signaling, dendrites can be involved in protein synthesis and independent signaling functions with other neurons. Axons typically end in an axon terminal at which neurotransmitters, neuromodulators, or neurohormones are released in the conversion of the electrical signal to a chemical signal which can cross the synapse or neuromuscular junction. Axonal transport is carried out by proteins such as kinesin and dynein. Neurons propagate their potentials by ion movement through voltage-gated ion channels (though calcium channels are largely voltage-independent) across their membranes. Potassium, sodium, and chloride ions are the greatest contributors to the membrane potential of the common neuron. The resting membrane potential of typical neurons is around -70 mV. As a depolarizing threshold stimulus occurs, an action potential that is consistent in amplitude is generated and travels down the axon to the terminal. Graded potentials are also important to note as they vary in strength, and lose amplitude throughout their transmission. The variety of interactions among neurons enables the transmission of impulses to perpetrate diverse functions within the body. Mature neurons are unable to divide, so their destruction may lead to a neurological deficit. However, neural progenitors that are capable of participation in neurogenesis are present in certain regions such as the dentate gyrus of the rat, and in the subependymal of rodents. There is a continuing interest in potential therapeutic uses for neural progenitor cells following injury.[2][3][4][5][6]
Nerves
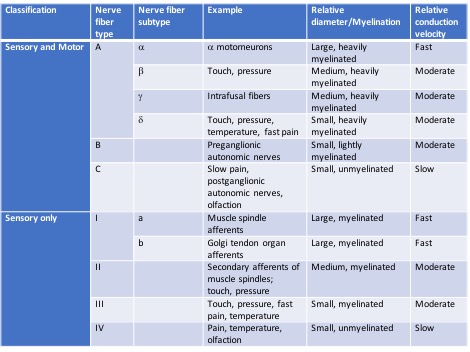
In the periphery, individual nerve fibers are surrounded by delicate connective tissue called the endoneurium. The endoneurial-surrounded fibers are grouped together into fascicles which are surrounded by the perineurium. The perineurium is also made up of connective tissue that is arranged in a lamellar manner. The cells comprising the perineurium are epithelioid myofibroblasts. The outermost layer of connective tissue surrounding peripheral nerves is called the epineurium. The epineurium commonly surrounds multiple fascicles and the blood vessels supplying the nerve. The epineurium is formed by arachnoid and dura invagination as the nerves exit the vertebral canal.
There are a number of different nerve fiber types that have been classified. Some are constituents of both motor and sensory pathways, and others serve only sensory pathways.[7]
Muscles
Muscles require innervation to contract and to maintain tone. Motor neurons in vertebrates secrete acetylcholine into the neuromuscular junction. When an action potential reaches the axon terminal of the motor neuron, voltage-dependent calcium channels open and accommodate the influx of calcium. This permits the fusion of neurotransmitter vesicles with the axon terminal membrane to cause acetylcholine release into the junction. Acetylcholine molecules then bind their receptors on the sarcolemma to cause the opening of sodium channels which results in the production of an action potential and its propagation to the myofibril to cause muscle contraction.
Somatic nerves and autonomic nerves both act on muscles. Somatic innervation is the cause of voluntary muscle function, whereas, autonomic innervation controls the involuntary muscle contraction and relaxation, and glandular secretion that enables the body to function and adapt without conscious thought. A notable example of autonomic innervation is the parasympathetic innervation of the detrusor muscle of the bladder and the internal urethral sphincter to accommodate micturition. Sympathetic innervation in the pelvic region inhibits the functions of the parasympathetic innervation with the relaxation of the detrusor muscle and constriction of the internal urethral sphincter. Somatic innervation to the external urethral sphincter is also involved in the process.
Surgical Considerations
General Considerations
The technical considerations regarding repairing an injured nerve are constantly evolving. Commonly cited factors influencing nerve healing potential following repair include:
- Age: the most important factor. Younger patients have more positive outcomes (compared to adults) and the recovery time is quicker
- Studies have shown that, even in the pediatric population, patients < 6 years of age recovery more quickly compared to their adolescent counterparts
- Level of injury: distal injuries have a better chance of recovery compared to more proximal injuries
- Injury pattern: sharp lacerations/transections do better than crush injuries
- Delay in repair: chronic injuries do poorly
- The technique is critical:
- Secondary tissue damage occurs with excessive handling
- Nerve endings should be properly aligned (and not under tension) during a direct repair
- Excessive suture material used during the repair risks the development of a neuroma at the site of repair
- The general consensus in the literature favors utilizing an epineurial repair
- Epineurial repair ensures the proper orientation of the repair and reduces tension at the repair site
- Traditionally, techniques also included the incorporation of a perineurial repair in addition to an epineurial repair
- Outcome studies comparing epineurial alone versus perineal and epineurial repair techniques often report equivalent results; consideration should be given to excessive suture utilization as this can result in a neuroma at the repair site
- The general consensus in the literature favors utilizing an epineurial repair
End-to-end Repair
The gold standard for microsurgical nerve injury reconstruction techniques remains the end-to-end (ETE) direct repair of the nerve stumps without tension. Difficulty with ETE is recognized in the following scenarios:
- Chronicity of the injury (leading to limited nerve stump excursion, neural tissue degeneration, and fibrosis/scarring in the region affected)
- The distance of the nerve injury from its specific muscle targets (i.e. distance from the muscle(s) it innervates)
- Significant gaps separating the injured nerve stumps from each other -- including the surgical inaccessibility of one of the nerve endings
End-to-side Repair
End-to-side (ETS) is a technique that can be utilized when the surgeon is concerned regarding the reparability and usability of the injured nerve's proximal stump. There is some literature supporting nerve fiber regeneration and collateral axonal sprouting following ETS repair. However, the efficacy of ETS remains controversial.
ETS is most often given consideration in the following settings:
- Upper extremity nerve injuries
- Facial reanimation
- Nerve reconstruction following tumor surgeries and ablation procedures
- Neuroma formation prevention
Sutureless Repair Techniques
Sutureless techniques have been becoming increasingly popular over the last 10 to 15 years. Evolving techniques include:
Clinical Significance
Many conditions can affect neurons and their transmission. Some of these include neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) or Lou Gehrig disease, some dementias, Huntington disease, spinal and bulbar muscular atrophies, as well as lesions of the motor neurons.
ALS is characterized by the clinical presentation of both upper and lower motor neuron lesion symptoms. The disease includes the destruction of motor neurons in the motor cortex, the brainstem, and the spinal cord. Symptoms of ALS include fasciculations, weakness, spasticity, muscle cramps, and difficulty speaking, swallowing, and breathing. Some patients exhibit cognitive decline. Most patients with ALS die of respiratory arrest.
Dementia describes a vast array of conditions with the most common forms being Alzheimer disease and frontotemporal dementia. The onset of symptoms of dementia is often gradual, and its effects are chronic. Dementia can cause a variety of cognitive and behavioral deficits depending on the location and extent of damage in the brain.
Huntington disease is an autosomal dominant condition that is characterized by the death of neurons caused by the huntingtin protein. Symptoms can present at any time, but most commonly appear when the patient is in the third or fourth decade of life. Huntington disease can cause changes in personality, cognition, and movement with the readily noticeable symptoms of jerky, uncontrollable movements, typical of chorea.
Atrophy of the spinal cord or pons can present in a variety of ways, but there is typically muscular discomfort and progressive weakness. There is also the potential for the involvement of various cranial nerves if the lesion is in the brain stem. Signs of upper motor neuron and lower motor neuron disease may be observed depending on the condition.
Upper motor neuron lesions are characterized by muscle weakness, spasticity (typically not immediately following an acute event), clasp-knife response, hyperreflexia, pronator drift, and positive Babinski sign. Lower motor neuron lesions are accompanied by weakness and paresis, hypotonia, hyporeflexia, fasciculations, and absent Babinski reflex.[11][12]
Media
(Click Image to Enlarge)
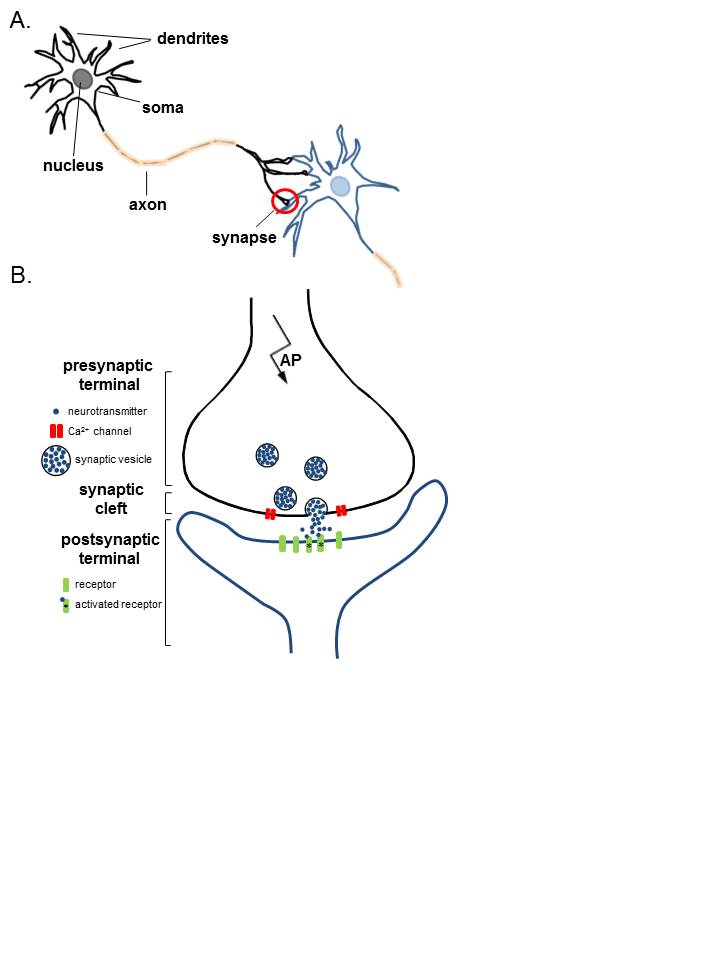
Anatomy of Neurons. A. Two connected neurons. Neurons have a soma that contains a nucleus, an axon, and a dendritic tree. A single synapse (red circle) is formed at the point where an axon's neuron (black) connects to another neuron's dendrite, soma, or axon (blue). B. A magnified view of a single synapse. On the arrival of an action potential at the presynaptic terminal, calcium triggers the release of neurotransmitters from the synaptic vesicles into the synaptic cleft. Neurotransmitters diffuse across the synaptic cleft to activate postsynaptic receptors.
Contributed Image by Karin Aubrey
References
Orgel MG. Epineurial versus perineurial repair of peripheral nerves. Clinics in plastic surgery. 1984 Jan:11(1):101-4 [PubMed PMID: 6368091]
Level 3 (low-level) evidenceAltman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. The Journal of comparative neurology. 1965 Jun:124(3):319-35 [PubMed PMID: 5861717]
Level 3 (low-level) evidenceAltman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. The Journal of comparative neurology. 1966 Mar:126(3):337-89 [PubMed PMID: 5937257]
Level 3 (low-level) evidenceHauke C, Ackermann I, Korr H. Cell proliferation in the subependymal layer of the adult mouse in vivo and in vitro. Cell proliferation. 1995 Nov:28(11):595-607 [PubMed PMID: 8555372]
Level 3 (low-level) evidenceCarpenter MK, Cui X, Hu ZY, Jackson J, Sherman S, Seiger A, Wahlberg LU. In vitro expansion of a multipotent population of human neural progenitor cells. Experimental neurology. 1999 Aug:158(2):265-78 [PubMed PMID: 10415135]
Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science (New York, N.Y.). 1982 May 21:216(4548):890-2 [PubMed PMID: 7079742]
Level 3 (low-level) evidenceMorshead CM, van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992 Jan:12(1):249-56 [PubMed PMID: 1729437]
Level 3 (low-level) evidenceLois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proceedings of the National Academy of Sciences of the United States of America. 1993 Mar 1:90(5):2074-7 [PubMed PMID: 8446631]
Level 3 (low-level) evidenceBordoni B, Reed RR, Tadi P, Varacallo M. Neuroanatomy, Cranial Nerve 11 (Accessory). StatPearls. 2023 Jan:(): [PubMed PMID: 29939544]
Bodman MA, Varacallo M. Peripheral Diabetic Neuropathy. StatPearls. 2024 Jan:(): [PubMed PMID: 28723038]
Munakomi S, Foris LA, Varacallo M. Spinal Stenosis and Neurogenic Claudication. StatPearls. 2023 Jan:(): [PubMed PMID: 28613622]
Nguyen V, Reddy V, Varacallo M. Neuroanatomy, Cranial Nerve 6 (Abducens). StatPearls. 2023 Jan:(): [PubMed PMID: 28613463]