Introduction
Myelin sheaths insulate the axons of nerves. These sheaths are a lipid-rich extension of the plasma membrane produced in the peripheral nervous system (PNS) by Schwann cells and by oligodendrocytes in the central nervous system (CNS). The myelin sheath is regularly interrupted along the length of the axon by specialized regions called the nodes of Ranvier, which are necessary for the propagation of an action potential along the axon.[1] The nodes of Ranvier are essential in the speed and timing of delivery of impulses from one neuron to another, and changes in size or function have the potential to jeopardize the effectiveness of a neuron, contributing to the development of neurological disorders.[1]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
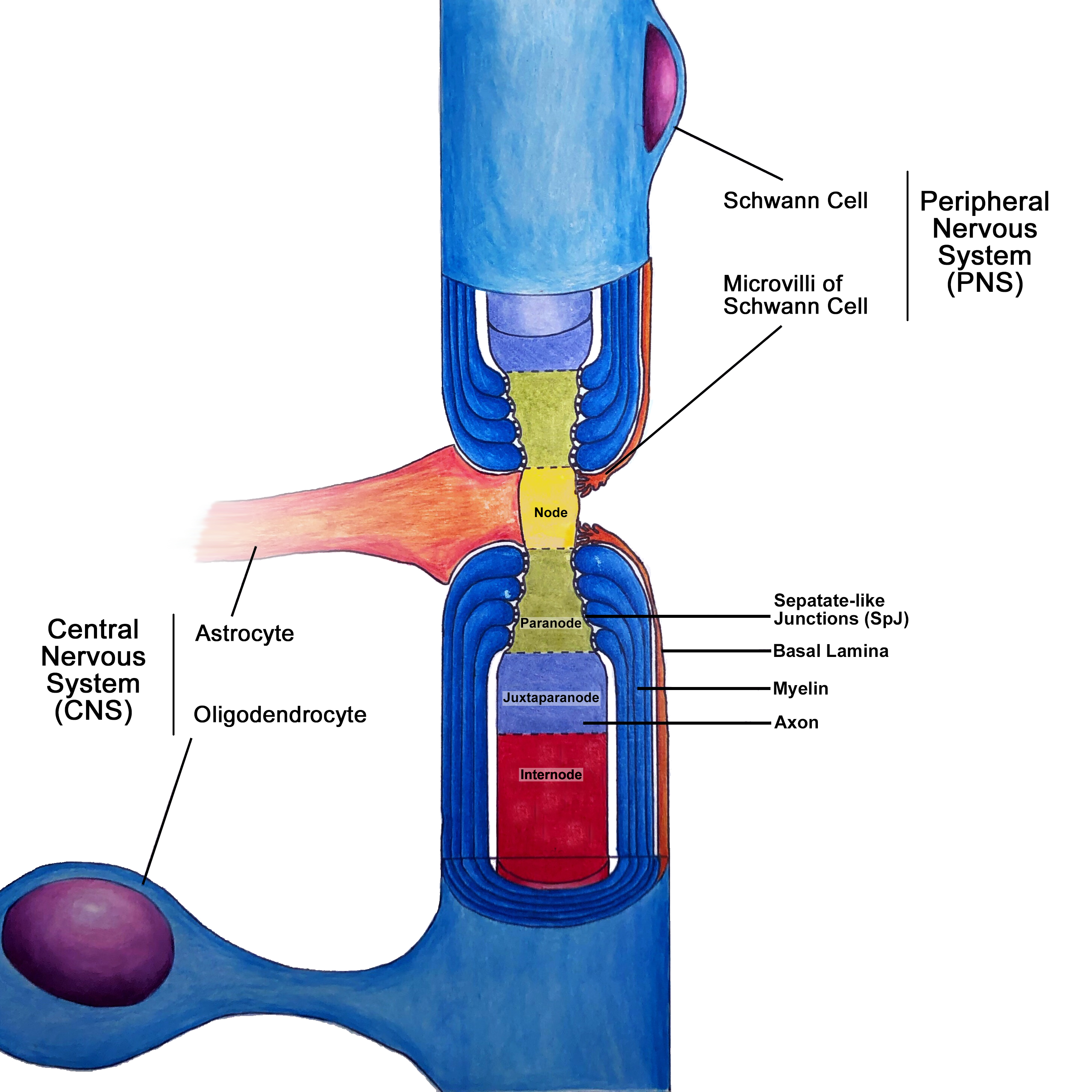
The nodes of Ranvier are characterized by short (1um), specialized regions in the axonal membrane that are not insulated by myelin. Although it is bare of myelin at the node, the axon is in direct contact with the microvilli of the Schwann cells in the PNS, or with processes of astrocytes in the CNS (Figure 1).[2] The nodes contain high concentrations of voltage-gated sodium ion channels, responsible for raising membrane voltage during the creation of an all-or-nothing action potential. In the regions directly adjacent to the nodes, termed the paranodes, loops of myelin form a tight, septate-like junction (SpJ) with the axonal membrane.[3] This junction provides scaffolding within the axon to compartmentalize molecules within the axon and restrict the movement of ion channels within the axonal membrane.[4] On the side of this junction opposite the node, termed juxtaparanode, there is a high concentration of voltage-gated potassium ion channels that facilitate the return of the membrane voltage to baseline following an action potential. For efficient propagation of the action potential, it is crucial that the voltage-gated sodium and potassium channels remain in distinct regions.
The long, myelin-covered section of axon between nodes is the internode. The purpose of the myelin sheath is to increase the speed of transmission by acting as an electrical insulator. In non-myelinated axons, the action potential must propagate continuously along the plasma membrane. Under myelin, there is increased resistance to movement of charges across the membrane, resulting in rapid propagation of the voltage signal within the axonal cytoplasm to the next node. The action potential must be ‘amplified’ at each node by the influx of sodium ions as the voltage signal dissipates along the length of the myelinated axon.
The nodes of Ranvier, including adjacent regions, contain a high concentration of the structural proteins ankyrins, which are responsible for the anchoring of proteins to the axonal cytoskeleton.[2] Pathological distribution of essential nodal proteins, including ion channels and cell adhesion molecules, can lead to dysfunction in the propagation of nerve impulses.[3] For example, the ganglioside GM1 is instrumental in tethering the molecular components of the node within sub-domains of the nodal plasma membrane called lipid rafts. Damage to GM1 by the production of anti-ganglioside antibodies is observed in most autoimmune neuropathies, resulting in loss of conduction efficacy.[5] Similarly, damage to proteins within the paranode results in the loss of paranodal septate-like junction (SpJ) and subsequent myelin retraction. Appropriate localization of ion channels requires the primary adhesion molecule in the paranode, Neurofascin 155 (NF155). The disassociation of glial NF155 with axonal adhesion molecules following neonatal hypoxia and during autoimmune disorders starts the degradation of the paranode structure and may lead to myelin retraction.[6] Impaired myelin maintenance by dysfunctional oligodendrocytes may also result in myelin retraction, resulting in the uncovering of voltage-gated potassium channels in the juxtaparanode. If exposed these channels do not function properly and present in multiple neurological diseases including stroke, multiple sclerosis, and in traumatic or hypoxic brain injuries.[1]
Embryology
During development, nodes form relatively densely along the axon, then the internode length increase as the embryo grows and the axons stretch along their length. In humans, peak myelination occurs in the first year of life, beginning in the peripheral nervous system, followed later by myelination in the brain and spinal cord. The largest diameter axons are also the first to be myelinated.[7]
The assembly process of nodes of Ranvier requires 1) the expression of specific structural proteins within the axon and 2) interactions with glial signals. First, the neuronal expression of ankyrin (ankG) structurally interacts with voltage sodium channels and other nodal proteins. Secondly, sodium channels concentrate within axonal membrane adjacent to new myelin sections, termed heminodes. The ion channels, restricted in movement by the paranodal SpJ, are clustered as two neighboring heminodes merge to form a node of Ranvier. In the CNS, there is also a redundant, independent mechanism of node formation by glial signals. Node formation in the CNS is also mediated through interactions with oligodendrocyte-secreted proteins such as chondroitin sulfate proteoglycans.[4]
Surgical Considerations
Disruption of myelin sheath integrity leads to disruptions in nerve conduction and, if severe enough, complete conduction block. Minor acute damage to peripheral nerves will often resolve unaided. Conduction velocity may still be slower after recovery because Schwann cell-mediated remyelination occurs with thinner myelin and with nodes more closely spaced along the axon.[8] However, more severe peripheral nerve injury, associated with neurotmesis, requires surgical intervention, usually end-to-end nerve repair. Following removal of necrotic nerve ends and the realignment of the nerve and blood supply, sutures to the epineurium maintain the nerve repair. Age is the most critical factor in recovery, accounting for 50% of the variance in outcome.[9]
Clinical Significance
Disruption of the nodes of Ranvier and myelin may also occur as a result of autoimmune disorders, including Multiple sclerosis (MS), Guillain-Barré syndrome (GBS), and lupus. Injuries to the white matter such as during neonatal hypoxia or following traumatic brain injury may also result in subsequent demyelination.[6][1]
Multiple sclerosis characteristically presents with multiple lesions to the white-matter that develop across time. Although reduction of conduction velocity, or complete conduction block, due to demyelination is the primary pathology associated with MS, the long-term progression of the disease symptoms also correlates with axonal damage caused by direct immune interactions with nodal/paranodal proteins. For example, disruption of paranodal septate-like junctions due to loss of NF155 disrupts conduction by permitting extracellular current flow and the inappropriate redistribution of potassium channels into the node.[10][1] Therefore, the pharmacological blockade of voltage-gated potassium channels has been demonstrated to attenuate MS symptoms such as difficulties with walking or vision.[11] Patients presenting with symptoms of MS should undergo blood and CSF analysis to rule out infections by other illnesses with similar symptoms. During the active phase of MS, an MRI of the patient’s brain and spinal cord may reveal white matter lesions when accompanied by intravenous gadolinium contrast material. An MRI of the spinal cord can also rule out spinal stenosis or a tumor.[12] Acute treatment with aggressive concentrations of corticosteroids, such as oral prednisone or IV methylprednisolone, reduces nerve inflammation. To limit the chronic progression of the disease, subcutaneous or intramuscular injections of beta interferon decrease expression of inflammatory cytokines and concurrently promote expression of anti-inflammatory and pro-survival factors.[13]
Guillain-Barre syndrome is an immune-mediated neuropathy that is mediated by antibodies that cross-react with endogenous proteins enriched in peripheral nerves, such as the ganglioside GM1 or members of the neurofascin adhesion molecule family, such as NF155.[5][14] GBS often manifests post-infection, as a patient’s antibodies against the infection are cross-reactive with endogenous gangliosides such as GM1.[15] Patients present with symmetrical flaccid weakness, usually accompanied by areflexia or hyporeflexia.[5] Approximately 25% of patients will require the use of a respiratory ventilator during their episode. A subtype of GBS, acute motor axonal neuropathy, has two distinct prognoses: rapid recovery of motor function upon acute resolution of conduction block, or a long-term deficit in function associated with Wallerian degeneration and chronic injury to the nerve.[16] Typically, clinical evaluation for GBS necessitates lumbar puncture to detect albuminocytologic dissociation (high CSF protein levels), and electrophysiological measurements of nerve conduction and electromyography. Blood sample analysis will usually reveal anti-ganglioside antibodies.[15] Standard treatment is similar to MS, including corticosteroid immunotherapy and/or plasma exchange.[17]
Age-dependent changes to the molecular distributions of nodal and paranodal proteins may be implicated in cognitive decline. In aged rodent and primate nerves, there is an increase in the inappropriate distribution of voltage-gated potassium channels into the nodal and paranodal regions.[18] This redistribution of channels would serve to maintain the node at a more hyperpolarized voltage, thus impeding action potential conduction. Indeed, older patients have higher thresholds for electrical stimuli to induce action potentials than do younger patients. Similarly, older humans had longer latencies, smaller amplitudes, and slower conduction velocities when compared with younger patients.[19]
Media
References
Arancibia-Carcamo IL, Attwell D. The node of Ranvier in CNS pathology. Acta neuropathologica. 2014 Aug:128(2):161-75. doi: 10.1007/s00401-014-1305-z. Epub 2014 Jun 10 [PubMed PMID: 24913350]
Level 3 (low-level) evidenceDavis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. The Journal of cell biology. 1996 Dec:135(5):1355-67 [PubMed PMID: 8947556]
Level 3 (low-level) evidencePoliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nature reviews. Neuroscience. 2003 Dec:4(12):968-80 [PubMed PMID: 14682359]
Level 3 (low-level) evidenceRasband MN, Peles E. The Nodes of Ranvier: Molecular Assembly and Maintenance. Cold Spring Harbor perspectives in biology. 2015 Sep 9:8(3):a020495. doi: 10.1101/cshperspect.a020495. Epub 2015 Sep 9 [PubMed PMID: 26354894]
Level 3 (low-level) evidenceNguyen TP, Taylor RS. Guillain-Barre Syndrome. StatPearls. 2023 Jan:(): [PubMed PMID: 30335287]
Zhang YP, Huang QL, Zhao CM, Tang JL, Wang YL. GM1 improves neurofascin155 association with lipid rafts and prevents rat brain myelin injury after hypoxia-ischemia. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2011 Jun:44(6):553-61 [PubMed PMID: 21670940]
Level 3 (low-level) evidenceSnaidero N, Simons M. Myelination at a glance. Journal of cell science. 2014 Jul 15:127(Pt 14):2999-3004. doi: 10.1242/jcs.151043. Epub [PubMed PMID: 25024457]
Level 3 (low-level) evidenceFex Svennigsen A, Dahlin LB. Repair of the Peripheral Nerve-Remyelination that Works. Brain sciences. 2013 Aug 2:3(3):1182-97. doi: 10.3390/brainsci3031182. Epub 2013 Aug 2 [PubMed PMID: 24961524]
M F G, M M, S H, Khan WS. Peripheral nerve injury: principles for repair and regeneration. The open orthopaedics journal. 2014:8():199-203. doi: 10.2174/1874325001408010199. Epub 2014 Jun 27 [PubMed PMID: 25067975]
Howell OW, Palser A, Polito A, Melrose S, Zonta B, Scheiermann C, Vora AJ, Brophy PJ, Reynolds R. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain : a journal of neurology. 2006 Dec:129(Pt 12):3173-85 [PubMed PMID: 17041241]
Griggs RB, Yermakov LM, Susuki K. Formation and disruption of functional domains in myelinated CNS axons. Neuroscience research. 2017 Mar:116():77-87. doi: 10.1016/j.neures.2016.09.010. Epub 2016 Oct 4 [PubMed PMID: 27717670]
Traboulsee AL, Li DK. The role of MRI in the diagnosis of multiple sclerosis. Advances in neurology. 2006:98():125-46 [PubMed PMID: 16400831]
Level 3 (low-level) evidenceKieseier BC. The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS drugs. 2011 Jun 1:25(6):491-502. doi: 10.2165/11591110-000000000-00000. Epub [PubMed PMID: 21649449]
Kira JI, Yamasaki R, Ogata H. Anti-neurofascin autoantibody and demyelination. Neurochemistry international. 2019 Nov:130():104360. doi: 10.1016/j.neuint.2018.12.011. Epub 2018 Dec 22 [PubMed PMID: 30582947]
van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nature reviews. Neurology. 2014 Aug:10(8):469-82. doi: 10.1038/nrneurol.2014.121. Epub 2014 Jul 15 [PubMed PMID: 25023340]
Hiraga A, Mori M, Ogawara K, Kojima S, Kanesaka T, Misawa S, Hattori T, Kuwabara S. Recovery patterns and long term prognosis for axonal Guillain-Barré syndrome. Journal of neurology, neurosurgery, and psychiatry. 2005 May:76(5):719-22 [PubMed PMID: 15834034]
Kieseier BC, Mathey EK, Sommer C, Hartung HP. Immune-mediated neuropathies. Nature reviews. Disease primers. 2018 Oct 11:4(1):31. doi: 10.1038/s41572-018-0027-2. Epub 2018 Oct 11 [PubMed PMID: 30310069]
Mel'nikov RA, Goshchitskiĭ LG, Kovalev VK. [Clinical manifestations of squamous cell carcinoma of the rectum]. Voprosy onkologii. 1984:30(8):76-83 [PubMed PMID: 6485288]
Level 3 (low-level) evidencePalve SS, Palve SB. Impact of Aging on Nerve Conduction Velocities and Late Responses in Healthy Individuals. Journal of neurosciences in rural practice. 2018 Jan-Mar:9(1):112-116. doi: 10.4103/jnrp.jnrp_323_17. Epub [PubMed PMID: 29456354]