Introduction
Noonan syndrome is a genetically inherited disease with heterogeneous, phenotypic manifestations. Gene mutations involve the RAAS/MAPK (mitogen-activated protein kinase) signaling pathway. The patient presentation can range from mild to severe. Thus, Noonan syndrome is typically a clinical diagnosis.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Noonan syndrome (NS) is a pleomorphic autosomal dominant inherited disease. Thus, parents who have Noonan syndrome have a 50% chance of passing the mutation on to the children. Noonan syndrome has been associated with advanced paternal age. Noonan Syndrome can also occur via de novo mutation or sporadic mutation. The mutated genes are involved in the RAS/MAPK cell signaling pathway. For more information on the RAS/MAPK pathway, please refer to the pathology section.[1][2]
Epidemiology
Noonan syndrome occurs in approximately 1 in 1000 to 2500. Since the inheritance pattern is autosomal dominant, it affects both females and males equally. Males may display cryptorchidism, thus making the diagnosis at a younger age. Noonan syndrome occurs across all ethnic groups equally.[1][2]
Pathophysiology
The mutation of the RAS/MAPK pathway involved Noonan syndrome classifies NS as RASopathy. RASopathies are genetic syndromes that involve germline mutations in the Ras/mitogen-activated protein kinase (MAPK) pathway. These syndromes include cardiofaciocutaneous syndrome (CFC), Costello syndrome, Neurofibromatosis type 1 (NF1), and LEOPARD syndrome. The Ras/MAPK pathway is needed for cell division, proliferation, differentiation, and migration. It is vital for healthy development.
The most common mutation in Noonan syndrome occurs in the PTPN11 gene. A smaller portion of mutations occurs in SOS1, RAF1, RIT1. Mutations in PTPN11 can be inherited, autosomal dominant, or occur de novo through sporadic mutation. The mutations involved in Noonan syndrome are considered a gain of function mutation, causing inappropriate prolongation of the RAS/MAPK signaling. The prolongation of the RAS/MAPK pathway gives way to the pleomorphic characteristics found in Noonan syndrome.[1][2]
History and Physical
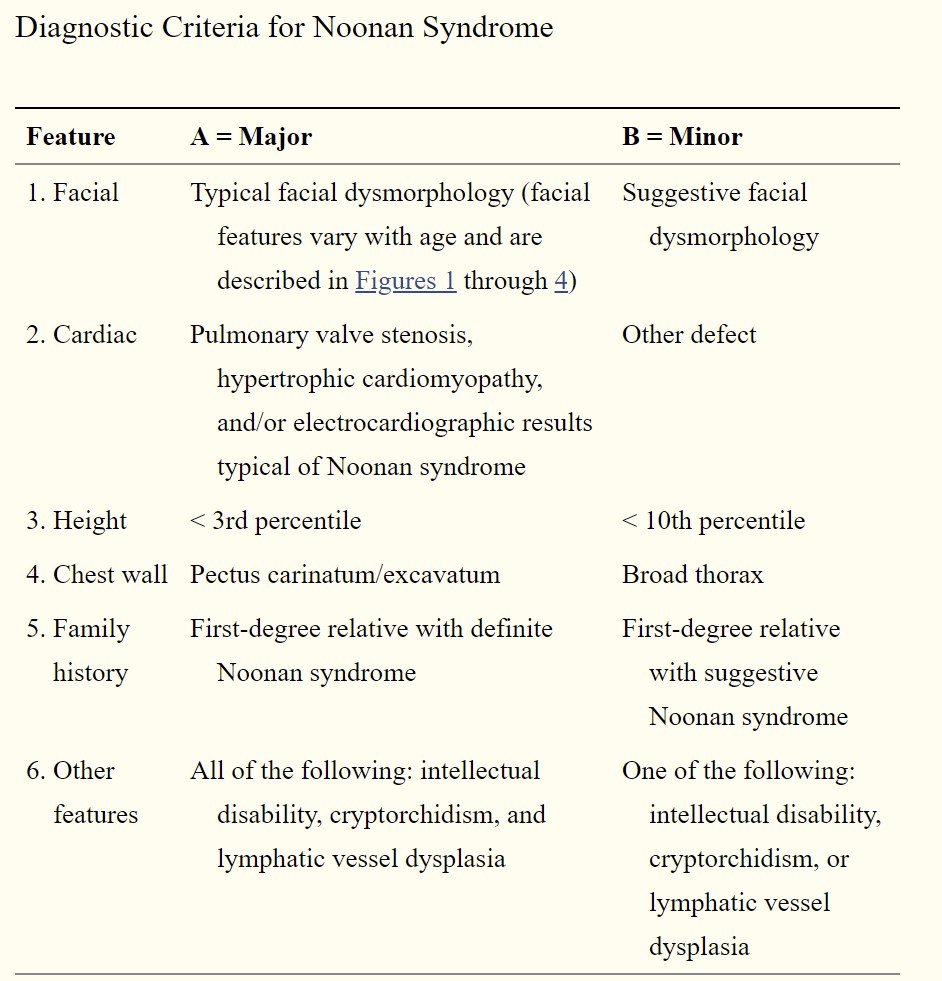
Noonan syndrome has phenotypical heterogeneous manifestations, which change with age. The most consistent features are widely set eyes, low-set ears, short stature, and pulmonic stenosis. Diagnostic criteria have been developed to aid in the diagnosis of Noonan syndrome. Please refer to the attached table for the criteria.
One of the more common features prenatally is increased nuchal translucency in utero. However, increased nuchal translucency is associated with other conditions such as Down syndrome. Other manifestations include polyhydramnios from renal anomalies, congenital heart diseases, mild limb shortening, and fetal macrosomia. As noted before, the vague symptoms present in the prenatal period can elude to a variety of other conditions. Therefore, the index of suspicion for Noonan Syndrome must be relatively high. If karyotyping is done, it will reveal a normal karyotype, which will rule out trisomies. Noonan syndrome should be considered in all fetuses with increased nuchal translucency, polyhydramnios, and cardiac abnormalities with a normal karyotype.
For many with Noonan syndrome, there are no clinical manifestations at birth. Macrosomia and macrocephaly may be present. Cryptorchidism may be present in males. Males have an increased risk of infertility, even if without a previous diagnosis of cryptorchidism. Most females are not at risk for infertility. The most common congenital heart defects are pulmonic stenosis and hypertrophic cardiomyopathy; depending on the severity, it may or may not be detected in a routine physical. The infant may experience feeding difficulties and failure to thrive, hearing loss, and strabismus. Noonan syndrome is associated with intellectual and developmental delays. Patients may display delayed speech or motor milestones, deafness, and short stature.
Distinct facial characteristics are formed in early childhood that change as they age. Features include hypertelorism, low-set ears, blue irises, ptosis, mild neck webbing, high forehead, down-slanting palpebral fissures, and epicanthic folds. In adulthood, facial features show signs of premature aging, and lengthening the jaw occurs to give the face a triangular shape.
Lymphatic dysplasia is common and can lead to early generalized lymphoedema in the extremities and abdomen. A fetal examination may show a cystic hygroma, which is a fluid-filled sac caused by a blockage in the lymphatic system. Patients may experience easy bruising or bleeding due to coagulopathy. The most common etiology is due to factor XI deficiency. Skeletal abnormalities are also common, such as pectus carinatum or pectus excavatum.[3][4][5]
Evaluation
Noonan syndrome remains a clinical diagnosis. Molecular genetic testing can be done to confirm the diagnosis. Upon diagnosis of Noonan syndrome, various organ systems should be evaluated (see Image. Diagnostic Criteria for Noonan Syndrome). Along with a complete physical and neurological exam, the following should be considered:[3][4][5]
- Cardiac evaluation, including echocardiography and electrocardiograph
- Ophthalmologic evaluation
- Audiologic evaluation
- Renal ultrasound
- Coagulation profile for coagulopathies
- Development assessment.
- Imaging of chest and back for skeletal abnormalities
Treatment / Management
There is no cure for Noonan syndrome as it is a genetically inherited disease. Management of Noonan syndrome is targeted toward symptomatic improvement and supportive care. Interprofessional care is often needed; multiple organ systems are to be addressed. Hearing tests and ophthalmic exams are appropriate throughout childhood. In males with cryptorchidism, orchiopexy should be performed when the child is around one-year-old if the testes have not descended to reduce the risk of development of testicular cancer in adulthood.
For congenital heart defects, an echo and ECG should be obtained. Even in patients without a diagnosis of cardiac defects, a cardiac evaluation is needed every five years. Short stature can be evaluated for the treatment of growth hormone. Appropriate supportive measures can be used to target lymphedema.[5][6]
Differential Diagnosis
The differential diagnosis for Noonan syndrome is broad. Many other chromosomal abnormalities can cause a similar constellation of symptoms of congenital heart defect, short stature, and developmental delay.
- Turner syndrome
- Noonan syndrome has been referred to as “pseudo-Turner syndrome” due to the similarities between Turner and Noonan syndromes. Both syndromes display a webbed neck and short stature. However, only females are affected by Turner syndrome (45, X0), as the X chromosome is affected. Both males and females are affected by Noonan syndrome, and the karyotype is normal.
Noonan syndrome is considered a RASopathy since the genetic mutations affect the RAS/MAPK pathway. Other RASopathies exhibit similar features to those in Noonan syndrome. These conditions include:
- Cardiofasciocutaneous syndrome (CFC)
- Costello syndrome
- Neurofibromatosis 1
- Noonan syndrome with multiple lentigines (formerly called LEOPARD syndrome)
Prognosis
Prognosis in those with Noonan Syndrome is dependent on the severity of their phenotype. The severity of the heart defect is linked to the mortality and morbidity of patients. Many patients have an average lifespan and minimal morbidity.[5]
Complications
Complications of the disease include developmental delay with cognitive deficits that limits functionality and require aggressive support services for the affected patient. Physical complications may be due to severe cardiac abnormalities such as valvular stenosis and hypertrophic cardiomyopathy requiring ongoing medical care. Some patients will have bleeding disorders with frequent medical care for the management of bleeding diatheses.[7][8]
Consultations
Genetic counseling can be offered to patients and their families.
Deterrence and Patient Education
Parents and eventually patients will need supportive services to help optimize the development and surroundings of the affected patients. Early referral should be made to the specialized support group called Noonan Syndrome Support Group (NSSG), which is available to all patients.
Enhancing Healthcare Team Outcomes
The diagnosis and management of Noonan syndrome require an interprofessional team that includes a geneticist, pediatrician, nurse practitioner, primary care provider, ENT surgeon, audiologist, ophthalmologist, and a cardiologist.
There is no cure for Noonan syndrome as it is a genetically inherited disease. Management of Noonan syndrome is targeted toward symptomatic improvement and supportive care. Interprofessional care is often needed; multiple organ systems are to be addressed. Hearing tests and ophthalmic exams are appropriate throughout childhood. In males with cryptorchidism, orchiopexy should be performed when the child is approximately one-year-old if the testes have not descended. This procedure is done to reduce the risk of developing testicular cancer in adulthood.
For congenital heart defects, an echo and ECG should be obtained. Even in patients without a diagnosis of cardiac defects, a cardiac evaluation is needed every five years. Short stature can be evaluated for the treatment of growth hormone. Appropriate supportive measures can be used to target lymphedema.[9][10][11][6]
Media
(Click Image to Enlarge)
References
Rauen KA. The RASopathies. Annual review of genomics and human genetics. 2013:14():355-69. doi: 10.1146/annurev-genom-091212-153523. Epub 2013 Jul 15 [PubMed PMID: 23875798]
Level 3 (low-level) evidenceMyers A, Bernstein JA, Brennan ML, Curry C, Esplin ED, Fisher J, Homeyer M, Manning MA, Muller EA, Niemi AK, Seaver LH, Hintz SR, Hudgins L. Perinatal features of the RASopathies: Noonan syndrome, cardiofaciocutaneous syndrome and Costello syndrome. American journal of medical genetics. Part A. 2014 Nov:164A(11):2814-21. doi: 10.1002/ajmg.a.36737. Epub 2014 Sep 22 [PubMed PMID: 25250515]
Level 3 (low-level) evidencevan der Burgt I. Noonan syndrome. Orphanet journal of rare diseases. 2007 Jan 14:2():4 [PubMed PMID: 17222357]
Bhambhani V, Muenke M. Noonan syndrome. American family physician. 2014 Jan 1:89(1):37-43 [PubMed PMID: 24444506]
Roberts AE,Allanson JE,Tartaglia M,Gelb BD, Noonan syndrome. Lancet (London, England). 2013 Jan 26 [PubMed PMID: 23312968]
Moniez S, Pienkowski C, Lepage B, Hamdi S, Daudin M, Oliver I, Jouret B, Cartault A, Diene G, Verloes A, Cavé H, Salles JP, Tauber M, Yart A, Edouard T. Noonan syndrome males display Sertoli cell-specific primary testicular insufficiency. European journal of endocrinology. 2018 Dec 1:179(6):409-418. doi: 10.1530/EJE-18-0582. Epub [PubMed PMID: 30325180]
Briggs B, Savla D, Ramchandar N, Dimmock D, Le D, Thornburg CD. The Evaluation of Hematologic Screening and Perioperative Management in Patients with Noonan Syndrome: A Retrospective Chart Review. The Journal of pediatrics. 2020 May:220():154-158.e6. doi: 10.1016/j.jpeds.2020.01.048. Epub 2020 Feb 25 [PubMed PMID: 32111381]
Level 2 (mid-level) evidenceYamamoto M, Takashio S, Nakashima N, Hanatani S, Arima Y, Sakamoto K, Yamamoto E, Kaikita K, Aoki Y, Tsujita K. Double-chambered right ventricle complicated by hypertrophic obstructive cardiomyopathy diagnosed as Noonan syndrome. ESC heart failure. 2020 Apr:7(2):721-726. doi: 10.1002/ehf2.12650. Epub 2020 Feb 20 [PubMed PMID: 32078254]
Koh AL,Tan ES,Brett MS,Lai AHM,Jamuar SS,Ng I,Tan EC, The spectrum of genetic variants and phenotypic features of Southeast Asian patients with Noonan syndrome. Molecular genetics [PubMed PMID: 30784236]
Gurovich Y, Hanani Y, Bar O, Nadav G, Fleischer N, Gelbman D, Basel-Salmon L, Krawitz PM, Kamphausen SB, Zenker M, Bird LM, Gripp KW. Identifying facial phenotypes of genetic disorders using deep learning. Nature medicine. 2019 Jan:25(1):60-64. doi: 10.1038/s41591-018-0279-0. Epub 2019 Jan 7 [PubMed PMID: 30617323]
Hemmati P, Dearani JA, Daly RC, King KS, Ammash NM, Cetta F, Schaff HV. Early Outcomes of Cardiac Surgery in Patients with Noonan Syndrome. Seminars in thoracic and cardiovascular surgery. 2019 Autumn:31(3):507-513. doi: 10.1053/j.semtcvs.2018.12.004. Epub 2018 Dec 18 [PubMed PMID: 30576779]