Introduction
Detailed anatomy of the optic canal is important to decipher the various pathologies of the region as well to guide surgical procedures and therapeutic options. The optic canal transmits the optic nerve, ophthalmic artery, and sympathetic nerve fibers.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
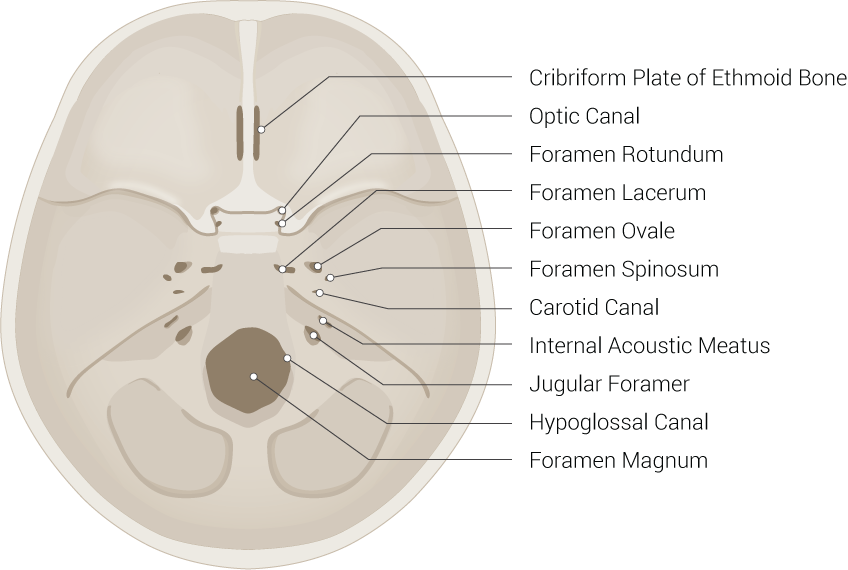
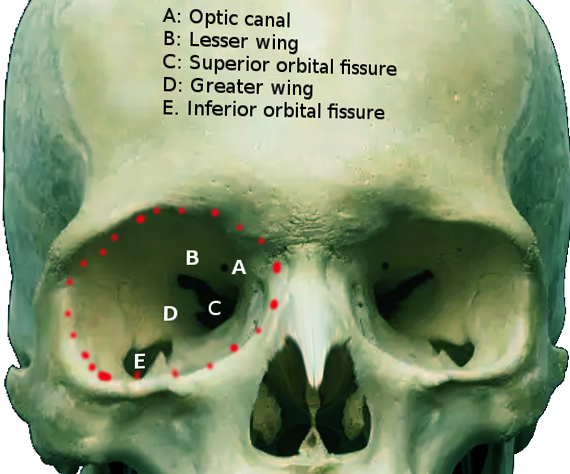
The optic canal is a funnel-like structure as part of the sphenoid bone that extends from the optic foramen to the orbital apex, the posterior-most end of the orbit. The orbital apex consists of the optic canal and the superior orbital fissure. The superior orbital fissure is bordered superomedially by the lesser wing of the sphenoid bone and inferolaterally by the greater wings of the sphenoid bone. The superior orbital fissure is the largest opening that connects the orbit with the middle cranial fossa.[1] The optic canal connects the orbit to the middle cranial fossa and transmits the optic nerve, ophthalmic artery, meningeal sheaths, and sympathetic nerve fibers.
The optic nerve, also known as the cranial nerve II, transmits visual signal from the retina to the visual cortex. The ophthalmic artery, the first branch of the internal carotid artery, arises distal to the cavernous sinus and supplies mainly the orbit but also other structures in the face and meninges.
Embryology
During the third month of gestation, the cartilaginous optic foramen forms. The cartilaginous optic foramen gets ossified between week 12 and 17 of gestation. During the fifth month of fetal development, the bony optic foramen transforms into the bony optic canal. This transformation is dependent on the normal development of the optic strut, the lower part of the sphenoidal wing, into an anterior-inferior and a posterior-superior segment.[2]
Orbital development exhibits a linear growth. The growth rate is different at various stages of fetal growth. The optic canal reaches its peak growth potential when a fetus reaches 400 mm in length.[3] Male fetuses tend to present with a larger diameter of orbit than female counterparts.[3] In a study, researchers determined that the length of the optic canal was 1.61 cm (1.1 to 2.3 cm) in male participants and 1.39 cm (0.7 to 2.0 cm) in female participants.[4]
While the diameter of the cranial opening portion of the optic canal grows the most during the gestational period, this diameter continues to grow during childhood and into adulthood.[5]
Assessing the proper formation and growth of the optic canal is essential when studying the various ocular malformations such as ocular hypertelorism, hypotelorism, anophthalmos, and microphthalmos. These congenital abnormalities affect the shape of the optic canal. Orbital defects in fetuses can be assessed using ultrasound or MRI.[3][6]
Blood Supply and Lymphatics
Understanding blood supply to the optic nerve is vital as the optic nerve is a vulnerable structure to compression within the limited space of the optic canal. The main vascularization of the optic nerve comes from the superior hypophyseal arteries and ophthalmic artery.[7]
The hypophyseal arteries mainly supply the intracranial and intracanalicular part of the optic nerve while the ophthalmic artery supplies the intraorbital portion of the optic nerve through the long ciliary arteries and the central retinal artery.[7]
A critical structure passing through the optical canal is the ophthalmic artery. The ophthalmic artery is the first main branch of the internal carotid artery. It originates from the distal dural ring intracranial, intracanalicular, and intraorbital sections. During its course, it runs inferolateral relative to the optic nerve within the optic canal.[8]
Branches of the ophthalmic artery include the following blood vessels[8][9]:
- Central retinal artery: supplies the inner layer of the retina
- Long posterior ciliary artery: two branches arise that supply the iris through the circulus arteriosus major
- Short posterior ciliary artery: branches pierce the sclera to supply the choroid and the ciliary process.
- Lacrimal artery: supplies the lacrimal gland and the anterior portion of eyeball and a portion of the eyelid
- Anterior ethmoidal artery: gives rise to the anterior meningeal artery. It supplies ethmoidal air cells and the periosteum
- Posterior ethmoidal artery: supplies the ethmoidal air sinuses and part of the nasal mucosa and septum
- Supraorbital artery: supplies part of the orbit and face - terminal branches: include the supratrochlear (or frontal) artery and the dorsal nasal artery, which supply the forehead and scalp
Understanding the lymphatic drainage of the various tissues in the eye are crucial in studying conditions that involve dysfunctional lymphatic systems, including inflammatory diseases, metastatic cancers, transplant rejection, lymphatic malformation, and surgical complications.
While most body tissues have embedded lymphatic drainage system, the ocular lymphatic structure has a heterogeneous appearance. While the cornea, lens, retina, ciliary body, choroid, and sclera are mostly lymphatic-free, other tissues are not. Optic nerve sheath is considered a lymphatic-rich ocular structure.[10] This area is rich with LYVE-1 (lymphatic vessel endothelial hyaluronan receptor-1).[10]
Nerves
Another crucial structure passing through the optical canal is the optic nerve. The optic nerve is the second cranial nerve surrounded by the cranial meninges and responsible for the transmission of sensory information for vision. The retinal ganglion cells receive impulses from the rods and cones and subsequently converge to form the optic nerve. After its formation, it leaves the bony orbit, passes through the optic canal and the sphenoid bone to enter the cranial cavity and run along the middle cranial fossa.
Within the middle cranial fossa, both optic nerves converge to form the optic chiasm. The following optic tracts emerge from the optic chiasm:
- Left optic tract: transmits signal from the left temporal retina and the right nasal retina
- Right optic tract: transmits signal from the right temporal retina and the left nasal retina
All optic tracts synapse at the lateral geniculate nucleus in the thalamus. Axons project from this region into two major optic radiation tracts[11]:
- Upper optic radiation: these fibers travel through the parietal lobe and carry information from the superior retinal quadrants, which correspond to the inferior visual field quadrants.
- Lower optic radiation: these fibers travel through the temporal lobe and carry information from the inferior retinal quadrants, which correspond to the superior visual field quadrants
Each optic tract travels to its corresponding cerebral hemisphere to reach the lateral geniculate nucleus (LGN), a relay system located in the thalamus; the fibers synapse here.
Axons from the LGN then carry visual information via a pathway known as the optic radiation. The pathway itself divides into the following[11]:
- Upper optic radiation – carries fibers from the superior retinal quadrants (corresponding to the inferior visual field quadrants). It travels through the parietal lobe to reach the visual cortex.
- Lower optic radiation– also known as the Meyers loop, carries fibers from the inferior retinal quadrants (corresponding to the superior visual field quadrants). It travels through the temporal lobe, via a pathway known as Meyers loop, to reach the visual cortex.
These optic radiations project to the visual cortex, where sensory data is processed and interpreted.
Muscles
There are seven extraocular muscles within the ocular orbit[12]:
- Levator palpebrae superioris and superior tarsal muscle: levator palpebrae superioris innervated by the oculomotor nerve; superior tarsal muscle innervated by the sympathetic nervous system; both elevate the upper eyelid
- Superior rectus: innervated by the oculomotor nerve; the primary function is the elevation of the eyeball.
- Inferior rectus: innervated by the oculomotor nerve; the primary function is depression of the eyeball.
- Medial rectus: innervated by the oculomotor nerve; the primary function is adduction of the eyeball.
- Lateral rectus: innervated by the abducens nerve; the primary function is the abduction of the eyeball.
- Superior oblique: innervated by the trochlear nerve; the primary function is depression, abduction, and medial rotation of the eyeball.
- Inferior oblique: innervated by the oculomotor nerve; the primary function is elevation, abduction, and lateral rotation of the eyeball.
Physiologic Variants
Optic canal variants include duplicated optical canal and the key-hole anomaly. Duplicated optic canal is a rare finding present in 0.64% of the population. In the key-hole anomaly, the optic canal has a grooved floor with a keyhole appearance in plain film radiographs. This variant was present in 2.65% of studied orbits.[1]
In some cases of the duplicated optic canal, the origin of the ophthalmic artery is the intracavernous part of the internal carotid artery. In this variant, the ophthalmic artery could pass through the superior orbital fissure.[1][13][14]
Surgical Considerations
The optical canal has crucial surgical implications given the important function of the structures within the canal. A significant surgical procedure involving the optic canal is optic nerve decompression. Some of the indications for optic nerve decompression include traumatic optic neuropathy, non-traumatic optic neuropathy (e.g. Graves ophthalmopathy, space-occupying lesions/tumors of the orbit, periorbital sinuses, and the surrounding structures), idiopathic intracranial hypertension (also known as pseudotumor cerebri), neoplasms (sinonasal tumor, meningioma, orbital apex tumor), and osseous lesions.
Decompression of the optic nerve is achievable through several approaches, including transcranial and transsphenoidal routes. Endoscopic optic nerve decompression is an option for both traumatic and non-traumatic optic neuropathies. However, its utilization has been far less common for non-traumatic optic neuropathy.[15] While many approaches exist, the endoscopic endonasal approach has decreased morbidity and recovery time compared to other methods.[16]
Another important structure within the optical canal is the ophthalmic artery. Ophthalmic artery aneurysms pose a serious surgical challenge. By the time patients become symptomatic enough for a diagnosis, they are usually emergency cases. Embolization using the coiling technique is a standard of care. Embolization is contraindicated in cases of large ophthalmic artery aneurysms, atherosclerotic, or hypoplastic internal carotid artery.[17]
Clinical Significance
The optic canal is a very important structure due to the structures that pass through this canal, mainly the optic nerve and the ophthalmic artery. Damage to the optic nerve can lead to permanent loss of vision. Disorders of the optic nerve can be classified into two main categories:
Hereditary optic nerve disorders:
- Autosomal dominant optic atrophy: This is the most common hereditary optic neuropathy. It leads to bilateral optic nerve degeneration. The genetic defect has been attributed to the coding of inner mitochondrial membrane proteins. Some of the genes responsible are OPA1, OPA3, OPA4, OPA5, and OPA8. The disease course is highly variable but it can lead to insidious vision loss, especially during the first decade of life in those with associated extra-ocular features. It can also present with central, centrocecal and paracentral scotomas, color perception deficits. A visual evoked potential test could demonstrate reduced amplitudes and prolonged latencies. Currently, there is no established treatment for this condition.[18]
- Leber hereditary optic neuropathy: an inherited mitochondrial disorder characterized by bilateral, painless vision loss during young adulthood. Vision loss occurs simultaneously or sequentially. There is a severe reduction of visual acuity by central or centrocecal scotoma. Other symptoms include postural tremor, peripheral neuropathy, and myopathy. This condition results from the degeneration of retinal ganglion cells and their projecting axons forming the optic nerve. The diagnosis is through genetic testing. Most commonly identified genes are MT-ND1, MT-ND4, or MT-ND6. There is no cure, and the management is supportive.[19]
Non-hereditary optic nerve disorders:
- Glaucoma: the worldwide leading cause of progressive vision loss. It classifies into open-angle glaucoma and angle-closure glaucoma, with the latter being more common. They can also classify into primary and secondary diseases. Secondary glaucoma could result from trauma, corticosteroids, inflammation, or tumor.[20] Risk factors for primary open-angle glaucoma include increased cup-to-disc ratio and asymmetry, disc hemorrhage, elevated intraocular pressure (IOP), family history of glaucoma, black race, and old age.[21] Most important preventive modality is the reduction of intraocular pressure. In open-angle glaucoma, the resistance to aqueous outflow through the trabecular meshwork becomes increased while in angle-closure glaucoma, the drainage sites get obstructed. The resulted increased in intraocular pressure transmits strain to the posterior structures of the eye, damaging the retinal ganglion cells and the optic nerve in the process. Medications used to lower the IOP include the following:
- Prostaglandin analogs (e.g., latanoprost, travoprost, tafluprost, unoprostone, bimatoprost): Increase outflow of aqueous humor.
- Beta-adrenergic blockers (e.g. timolol, levobunolol, carteolol, metipranolol, betaxolol): Decrease aqueous humor production.
- Alpha-adrenergic agonists (e.g., brimonidine, apraclonidine): Decrease aqueous humor production and increase outflow.
- Carbonic anhydrase inhibitors (e.g., dorzolamide, brinzolamide, acetazolamide): Decrease aqueous humor production.
- Cholinergic agonists (e.g., pilocarpine, carbachol): Increase aqueous humor outflow.
- Optic neuritis: inflammation of the optic nerve. It could be caused by a variety of conditions such as multiple sclerosis, neuromyelitis optica, ischemic optic neuropathy, or infection. Common symptoms include visual loss, periocular pain, and dyschromatopsia. The first line of treatment is intravenous steroids. Spontaneous visual improvement is possible in greater than 90% of the cases.[22]
- Ischemic optic neuropathies: includes anterior ischemic optic neuropathy (AION) affecting the optic disc and posterior ischemic optic neuropathy (PION) affecting the retrobulbar part of the optic nerve. Both types are further subdivided into arteritic and non-arteritic. Arteritic ischemic optic neuropathy is caused by giant cell arteritis mediated by arterial inflammation whereas non-arteritic ischemic optic neuropathy is caused by small arterial occlusive disease. Early treatment with corticosteroid therapy is necessary to avoid permanent vision loss.[23]
- Papilledema: the swelling of the optic disc (most often bilateral) as a result of increased intracranial pressure. Possible underlying causes include space-occupying brain lesions, cerebral hemorrhage, head trauma, hydrocephalus, meningitis, cerebral venous sinus thrombosis, and idiopathic intracranial hypertension. Treatment should be tailored based on the underlying etiology.[24]
- Optic nerve sheath meningiomas (ONSM): a rare finding. ONSM is a benign neoplasm of the meningothelial cells of the meningeal sheath around the optic nerve, either intraorbital or intracanalicularly. While the growth itself is benign, mass compression can lead to compression of the optic nerve or the surrounding blood vessels. This complication can lead to progressive vision loss.[25] Treatment options include observation, radiotherapy, surgery, local chemotherapy, and systemic chemotherapy.[26]
Another essential structure within the optic canal is the ophthalmic artery. Some disorders of the ophthalmic artery include the following:
- Ophthalmic artery occlusion: severe cases can lead to ocular ischemic syndrome.
- Ophthalmic artery aneurysm: 5% of cerebrovascular aneurysms are ophthalmic artery aneurysms. It tends to be asymptomatic until it becomes large enough to become symptomatic. Most common symptoms are diplopia and headache. When symptomatic, they are surgical emergencies in most cases. Embolization techniques are the standard of treatment.[17]
Other Issues
Meningeal sheets cover the optic nerve: pia mater, arachnoid mater and dura mater. Subarachnoid space surrounds the optic nerve. The optic canal is the narrowest point of the optic nerve subarachnoid space. The subarachnoid space surrounding the optic nerve is continuous with the rest of the CSF flow. The CSF flow surrounding the optic nerve can be analyzed using magnetic resonance imaging (MRI).[27] A rise in intracranial pressure is transmitted by the cerebrospinal fluid (CSF) to the back of the eyeball.[28] Therefore, the retinal ganglion cells and their axons have exposure to two types of pressures: intraocular pressure and intracranial pressure. The rise in either pressure can lead to detrimental effects for the retinal ganglion cells and the optic nerve. Increased pressure on optic nerve leads to papilledema. It is important to distinguish papilledema from pseudopapilledema. Pseudopapilledema is the congenital elevation of the optic disc(s). Features of pseudopapilledema include dome-shape disc elevation, lack of edema around the peripapillary nerve fiber layer, and abnormal peripapillary vessels. Ancillary testing may be needed to distinguish one from the other. A-scan ultrasound and optical coherence tomography could be used to differentiate papilledema from pseudopapilledema. Optical coherence tomography can e be useful to measure peripapillary retinal nerve fiber layer thickness. A-scan ultrasound can be used to measure the retrobulbar optic nerve sheath diameter.[29]
Media
(Click Image to Enlarge)
References
Regoli M, Bertelli E. The revised anatomy of the canals connecting the orbit with the cranial cavity. Orbit (Amsterdam, Netherlands). 2017 Apr:36(2):110-117. doi: 10.1080/01676830.2017.1279662. Epub 2017 Mar 3 [PubMed PMID: 28388344]
Kier EL. Embryology of the normal optic canal and its anomalies. An anatomic and roentgenographic study. Investigative radiology. 1966 Sep-Oct:1(5):346-62 [PubMed PMID: 5970634]
Dumic-Cule I, Eljuga D, Izadpanah A, Erjavec I, Prgomet S, Hladnik A, Bicanic I, Rora M, Vinter I, Grgurevic L. Dynamics of optic canal and orbital cavity development revealed by microCT. Surgical and radiologic anatomy : SRA. 2014 Dec:36(10):989-92. doi: 10.1007/s00276-014-1296-4. Epub 2014 Apr 19 [PubMed PMID: 24748403]
Hart CK,Theodosopoulos PV,Zimmer LA, Anatomy of the optic canal: a computed tomography study of endoscopic nerve decompression. The Annals of otology, rhinology, and laryngology. 2009 Dec; [PubMed PMID: 20112517]
Level 2 (mid-level) evidencePrado PA, Ribeiro EC, De Angelis MA, Smith RL. Biometric study of the optic canal during cranial development. Orbit (Amsterdam, Netherlands). 2007 Jun:26(2):107-11 [PubMed PMID: 17613857]
Birnholz JC. Ultrasonic fetal ophthalmology. Early human development. 1985 Nov:12(2):199-209 [PubMed PMID: 3905351]
van Overbeeke J, Sekhar L. Microanatomy of the blood supply to the optic nerve. Orbit (Amsterdam, Netherlands). 2003 Jun:22(2):81-8 [PubMed PMID: 12789588]
Toma N. Anatomy of the Ophthalmic Artery: Embryological Consideration. Neurologia medico-chirurgica. 2016 Oct 15:56(10):585-591 [PubMed PMID: 27298261]
Michalinos A, Zogana S, Kotsiomitis E, Mazarakis A, Troupis T. Anatomy of the Ophthalmic Artery: A Review concerning Its Modern Surgical and Clinical Applications. Anatomy research international. 2015:2015():591961. doi: 10.1155/2015/591961. Epub 2015 Nov 9 [PubMed PMID: 26635976]
Chen L. Ocular lymphatics: state-of-the-art review. Lymphology. 2009 Jun:42(2):66-76 [PubMed PMID: 19725271]
De Moraes CG. Anatomy of the visual pathways. Journal of glaucoma. 2013 Jun-Jul:22 Suppl 5():S2-7. doi: 10.1097/IJG.0b013e3182934978. Epub [PubMed PMID: 23733119]
Shumway CL, Motlagh M, Wade M. Anatomy, Head and Neck, Eye Extraocular Muscles. StatPearls. 2023 Jan:(): [PubMed PMID: 30137849]
Renn WH, Rhoton AL Jr. Microsurgical anatomy of the sellar region. Journal of neurosurgery. 1975 Sep:43(3):288-98 [PubMed PMID: 1151464]
Hayreh SS. Orbital vascular anatomy. Eye (London, England). 2006 Oct:20(10):1130-44 [PubMed PMID: 17019411]
Liu Y, Yu H, Zhen H. Navigation-assisted, endonasal, endoscopic optic nerve decompression for the treatment of nontraumatic optic neuropathy. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2019 Feb:47(2):328-333. doi: 10.1016/j.jcms.2018.12.009. Epub 2018 Dec 13 [PubMed PMID: 30600198]
Chen C, Selva D, Floreani S, Wormald PJ. Endoscopic optic nerve decompression for traumatic optic neuropathy: an alternative. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2006 Jul:135(1):155-7 [PubMed PMID: 16815203]
Level 3 (low-level) evidenceDinca EB, Brehar F, Giovani A, Ciurea AV. Challenges in a case of ophthalmic artery aneurysm associated with abnormal internal carotid arteries. Asian journal of neurosurgery. 2017 Jan-Mar:12(1):106-108. doi: 10.4103/1793-5482.144160. Epub [PubMed PMID: 28413549]
Level 3 (low-level) evidenceLenaers G, Hamel C, Delettre C, Amati-Bonneau P, Procaccio V, Bonneau D, Reynier P, Milea D. Dominant optic atrophy. Orphanet journal of rare diseases. 2012 Jul 9:7():46. doi: 10.1186/1750-1172-7-46. Epub 2012 Jul 9 [PubMed PMID: 22776096]
Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, Yu-Wai-Man P, Chinnery PF. Leber Hereditary Optic Neuropathy. GeneReviews(®). 1993:(): [PubMed PMID: 20301353]
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014 May 14:311(18):1901-11. doi: 10.1001/jama.2014.3192. Epub [PubMed PMID: 24825645]
Hollands H, Johnson D, Hollands S, Simel DL, Jinapriya D, Sharma S. Do findings on routine examination identify patients at risk for primary open-angle glaucoma? The rational clinical examination systematic review. JAMA. 2013 May 15:309(19):2035-42. doi: 10.1001/jama.2013.5099. Epub [PubMed PMID: 23677315]
Level 1 (high-level) evidenceHoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. The open ophthalmology journal. 2012:6():65-72. doi: 10.2174/1874364101206010065. Epub 2012 Jul 24 [PubMed PMID: 22888383]
Hayreh SS. Management of ischemic optic neuropathies. Indian journal of ophthalmology. 2011 Mar-Apr:59(2):123-36. doi: 10.4103/0301-4738.77024. Epub [PubMed PMID: 21350282]
Rigi M, Almarzouqi SJ, Morgan ML, Lee AG. Papilledema: epidemiology, etiology, and clinical management. Eye and brain. 2015:7():47-57. doi: 10.2147/EB.S69174. Epub 2015 Aug 17 [PubMed PMID: 28539794]
Level 2 (mid-level) evidenceSchick U, Jung C, Hassler WE. Primary optic nerve sheath meningiomas: a follow-up study. Central European neurosurgery. 2010 Aug:71(3):126-33. doi: 10.1055/s-0029-1246136. Epub 2010 Feb 1 [PubMed PMID: 20127592]
Level 2 (mid-level) evidenceParker RT,Ovens CA,Fraser CL,Samarawickrama C, Optic nerve sheath meningiomas: prevalence, impact, and management strategies. Eye and brain. 2018; [PubMed PMID: 30498385]
Xie X, Zhang X, Fu J, Wang H, Jonas JB, Peng X, Tian G, Xian J, Ritch R, Li L, Kang Z, Zhang S, Yang D, Wang N, Beijing iCOP Study Group. Noninvasive intracranial pressure estimation by orbital subarachnoid space measurement: the Beijing Intracranial and Intraocular Pressure (iCOP) study. Critical care (London, England). 2013 Jul 24:17(4):R162. doi: 10.1186/cc12841. Epub 2013 Jul 24 [PubMed PMID: 23883736]
Level 2 (mid-level) evidenceZhang X, Lee Y, Olson D, Fleischman D. Evaluation of optic canal anatomy and symmetry using CT. BMJ open ophthalmology. 2019:4(1):e000302. doi: 10.1136/bmjophth-2019-000302. Epub 2019 May 28 [PubMed PMID: 31245611]
Fard MA, Sahraiyan A, Jalili J, Hejazi M, Suwan Y, Ritch R, Subramanian PS. Optical Coherence Tomography Angiography in Papilledema Compared With Pseudopapilledema. Investigative ophthalmology & visual science. 2019 Jan 2:60(1):168-175. doi: 10.1167/iovs.18-25453. Epub [PubMed PMID: 30640969]