Introduction
The Organ of Corti is an organ of the inner ear located within the cochlea which contributes to audition. The Organ of Corti includes three rows of outer hair cells and one row of inner hair cells. Vibrations caused by sound waves bend the stereocilia on these hair cells via an electromechanical force. The hair cells convert mechanical energy into electrical energy that is transmitted to the central nervous system via the auditory nerve to facilitate audition.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The primary function of the organ of Corti is the transduction of auditory signals. Sound waves enter the ear via the auditory canal and cause vibration of the tympanic membrane. Movement of the tympanic membrane causes subsequent vibrations within the ossicles, the three bones of the middle ear, which transfers the energy to the cochlea through the oval window. As the oval window moves, waves propagate through the perilymph fluid inside the scala tympani and then the scala vestibuli of the cochlea. When fluid moves through these structures, the basilar membrane (located between the scala media and scala tympani) shifts respectively to the tectorial membrane (see Image. Organ of Corti, A). The organ of Corti is an organ of the inner ear contained within the scala media of the cochlea. It resides on the basilar membrane, a stiff membrane separating the scala tympani and scala media. The scala me dia is a cavity within the cochlea that contains endolymph which has a high (150 mM) K+ concentration. The endolymph helps to regulate the electrochemical impulses of the auditory hair cells.[1]
The organ of Corti is composed of both supporting cells and mechanosensory hair cells. The arrangement of mechanosensory cells is comprised of inner and outer hair cells along rows (see Image. Organ of Corti, B). There is a single row of inner hair cells and three rows of outer hair cells which are separated by the supporting cells. The supporting cells are also named Dieters' or phalangeal cells.
The hair cells within the organ of Corti have sterocilia that attach to the tectorial membrane. Shifts between the tectorial and basilar membranes move these sterocilia and activate or deactivate receptors on the hair cell surface. When cation channels open on the hair cells, potassium ions flow into the hair cells, the cells depolarize, and the depolarization causes voltage-gated calcium channels to open. The calcium influx results in glutamate release from the hair cells into the auditory nerve. The auditory nerve then sends a stimulus resulting from the sound wave to the brain, which recognizes it as sound.
Inner hair cells function primarily as the sensory organs for audition. They provide input to 95% of the auditory nerve fibers that project to the brain.[1] The stiffness and size of the hair cell arrangement throughout the cochlea enable hair cells to respond to a variety of frequencies from low to high. Cells at the apex to respond to lower frequencies while hair cells at the base of the cochlea (near the oval window) respond to higher frequencies, which creates a tonotopic gradient throughout the cochlea.[2][1]
While inner hair cells are the output center of the cochlea, the outer hair cells are the input center. They receive descending inputs from the brain to assist with the modulation of inner hair cell function (i.e., modulating tuning and intensity information). Unlike other regions of the brain, the modulation of inner hair cells by outer hair cells is not electrical but mechanical. Activation of outer hair cells changes the stiffness of their cell bodies; this manipulates the resonance of perilymph fluid movement within the scala media and allows for fine-tuning of inner hair cell activation.[1]
Inner and outer hair cells are distinctly different in structure. Both types of hair cells have stereocilia on the apical surface; however, the arrangement of sterocilia and their connection to the tectorial membrane are distinctly different. For both types of hair cells, the mechanical bending of the sterocilia opens potassium channels at the tips of the sterocilia that allow hyperpolarization of the cells.[1] The tallest of the stereocilia of outer hair cells are embedded into the tectorial membrane. These stereocilia get displaced as the basilar membrane moves with the tectorial membrane. The stereocilia of inner hair cells are free-floating. Movement of the viscous perilymph fluid provides the mechanical force to open these channels.[1]
Inner hair cell activation is much more complicated than outer hair cell activation. The movement of fluid within the scala media relies on the resonance (vibration) of both the tectorial membrane and organ of Corti. Cells within the organ of Corti are much more flexible than cells within the basilar membrane. Alterations in the stiffness of these cells change the resonance of the organ of Corti and subsequently the movement of fluid within the scala media.[1]
The outer hair cells alter the stiffness of the organ of Corti through a motor protein, prestin, located on the lateral membrane of these cells. These proteins vary in shape in response to voltage changes. Depolarization of the outer hair cells causes prestin to shorten, shifting the basilar membrane and increasing the membrane deflection, thereby intensifying the effect on the inner hair cells.[3][4][5][6]
Embryology
The inner ear originates from the invagination of the otic placodes during the fourth week of development. The otic placodes are sensory placodes, which are a series of transiently thickened surface ectodermal patches that form pairs rostrocaudally in the region of the developing head. Sensory placodes are involved in the development of special sensory systems like vision, olfaction, and hearing. The otic placodes are one of the first sensory placodes to form and contribute to the formation of the inner ear structures associated with hearing and balance. Otic placode induction is dependent on Wnts and FGFs provided by the hindbrain and surrounding head mesenchyme. The otic placodes are located behind the second pharyngeal arch and give rise to the otic pits by invaginating into the mesenchyme adjacent to the rhombencephalon during the fourth week of development.
Near the end of the fourth week, the otic pits break off from the surface ectoderm to form a hollow piriform shaped structure lined with columnar epithelium called the otic vesicle. At this point, the otic vesicle lies beneath the surface ectoderm enveloped in the mesenchyme, forming the otic capsule. The statoacoustic ganglion also forms during the formation of the otic vesicle and splits into cochlear and vestibular portions. The otic vesicle differentiates to form all the components of the membranous labyrinth and ultimately gives rise to the inner ear structures associated with hearing and balance. As the otic vesicle develops into the membranous labyrinth, its epithelium undergoes variations in thickness and begins to distort. The otic vesicle divides into a dorsal utricular portion and ventral saccular portion, with the dorsal utricular portion giving rise to the vestibular system and the ventral saccular portion giving rise to inner ear structures like the organ of corti that are involved in hearing. The ventral saccular portion develops into the cochlear duct (which houses the organ of corti) and saccule. The dorsal utricular portion forms into the utricle, semicircular canals, and endolymphatic tube.
In the 6th week of development, the ventral saccular component of the otic vesicle penetrates the surrounding mesenchyme in a spiraling fashion. It completes two and a half turns to form the cochlear duct by the end of the 8th week. At this point, the saccule connects to the utricle via the ductus reuniens and mesenchyme surrounds the entire cochlear duct. The mesenchyme surrounding the cochlear duct forms cartilage. During the tenth week of development, this cartilaginous shell undergoes vacuolization to create the two perilymphatic spaces of the cochlea, the scala vestibule, and the scala tympani. Two membranes separate the cochlear duct proper, which is also known as the scala media, from the scala tympani and scala vestibule. The basilar membrane demarcates the scala media from the scala tympani, while the vestibular membrane separates the scala media from the scala vestibule. Laterally, the cochlear duct is attached to the surrounding cartilage via a connective tissue structure called the spiral ligament.
The Organ of Corti is located within the scala media of the cochlear duct and resides on the basilar membrane. The capsular cartilage that surrounds the membranous labyrinth becomes ossified between 16 and 23 weeks gestation to form the true bony labyrinth. The organ of Corti is the sensory portion of the cochlear duct located between the scala media and scala tympani. There is one row of inner hair cells and three rows of outer hair cells which are surrounded by supporting cells. Inner and outer hair cells form, differentiate, and separate into their respective rows prior to the formation of the Deiters or supporting cells. Several genes share links with the embryological development of the organ of Corti. Cochlear duct growth and hair cell formation have specifically been linked to identified genes. In terms of neural development, the spiral ganglion, which innervates the organ of Corti, forms from the primitive otocyst preceding the organ’s development.[7]
Blood Supply and Lymphatics
The labyrinthine artery is the main supplier of oxygenated blood to the cochlea and therefore to the organ of Corti. This artery is also known as the auditory artery, or the internal auditory artery. The labyrinthine artery most commonly originates from the anterior inferior cerebellar artery (AICA). AICA most commonly originates from the basilar artery. Occasionally, about 15% of the time, the auditory or labyrinthine artery, can branch off directly from the basilar artery. Less commonly, this artery may originate from the superior cerebellar or vertebral artery.
The labyrinthine artery follows the vestibulocochlear nerve from its point of origin into the internal acoustic meatus where it further divides into two branches, the anterior vestibular artery, and the common cochlear artery. The common cochlear artery will then divide into two more arteries, the proper cochlear artery, and the vestibulocochlear artery. The vestibulocochlear artery then gives off the vestibular ramus and the cochlear ramus.[8][9]
Nerves
Inner hair cells are mechanoreceptor cells. They transmit information about acoustic stimuli directly to the type I spiral ganglion neurons (i.e., auditory nerve, radial afferents). These afferents synapse within the cochlear nucleus within the brain. Input from the inner hair cells to the type I spiral ganglion neurons can be altered by lateral olivocochlear efferent nerves that have axodendritic synapses onto these type I ganglion neurons. The lateral olivocochlear nerves do not synapse onto inner hair cells. They terminate on the auditory nerve fibers within the cochlea.[10][1][11]
Outer hair cells have both efferent and afferent connections. They receive input from medial olivocochlear neurons directly onto their cell bodies, axosomatic synapses. These efferent connections form feedback loops that manipulate the stiffness of the Organ of Corti and therein the activity of the inner hair cells. The outer hair cells synapse onto type II spiral ganglion neurons.[1][11] The function of these afferents is still unknown. These neurons do not respond to auditory stimuli.[1]
Physiologic Variants
Individual variations exist in terms of the size and shape of the cochlea. These variations can have wide-ranging effects on surgical outcomes.
Common variations include:
- The length of the outer cochlear wall
- The diameter of the cochlear tube (first turn of the cochlea)
- Length of the pars ascendens (first turn of the cochlea)
- The width of the scala tympani (the ascending portion of the cochlear duct)
Individuals with narrow scala tympani or small cochlear size are at potential risk of experiencing trauma during electrode implantation. Also, the variation in diameter of the cochlear tube may result in limitations with conventional cochlear implants. Some of the smaller diameter tubes may not be large enough for the surgeries in some individuals.[12][13]
Surgical Considerations
Electrode array placement into the cochlea is a current treatment option for high-grade sensorineural hearing loss. Surgical complications are typically associated with surgical technique or device failure. Complications classified as either minor or major. The most common minor complications include infections, vestibular problems, and persistent tinnitus. Major complications include more serious infections such as coalescent mastoiditis or meningitis, damage to middle or inner ear structures, and device failure problems.[14] These types of complications have dramatically decreased in recent years with the use of small surgical incisions, the development of smaller implants, and the use of more biocompatible implants.[15]
During electrode array surgery, surgeons should be aware of the location of the petrous portion of the internal carotid artery as well as the jugular bulb. These structures lie near the tympanic cavity and should be avoided during surgery. Although not common, anatomical variations for both of these structures occur including the absence of the bone dividing these vessels and the middle ear and positioning of one or more vessel in either a superior or lateral direction from normal. During surgery, these structures can be damaged, or damage may occur due to post-surgical inflammation. To avoid these complications, CT or MRI imaging of the region should be performed prior to surgery to identify anatomical abnormalities. If imaging reveals anomalies, surgery within the tympanic cleft should be avoided.[16]
Clinical Significance
Sensorineural hearing loss is the most commonly reported cause of auditory deficits. This type of hearing loss often results from exposure to either loud sounds or ototoxic drugs. Exposure to loud noises causes the vibrational shift between the tectorial and basilar membranes to increase. This shift can damage the stereocilia of the outer hair cells. When damage occurs to the outer hair cells, the stiffness of the organ of Corti decreases which in turn increases vibrational forces on the inner hair cells. Damage to the outer hair cells decreases the protection of inner hair cells and causes them to become more sensitive. Over time, the inner hair cells will also become damaged and audition affected.[1]
Aminoglycoside antibiotics are an example of ototoxic drugs. These drugs are K+ channel blockers. As such, they block the ability of both inner and outer hair cells to depolarize. These types of drugs can also change the concentration of ions within the perilymph which can lead to damage or death of both inner and outer hair cells; destruction of the hair cells causes permanent auditory deficits because they do not regenerate.[1]
Media
(Click Image to Enlarge)
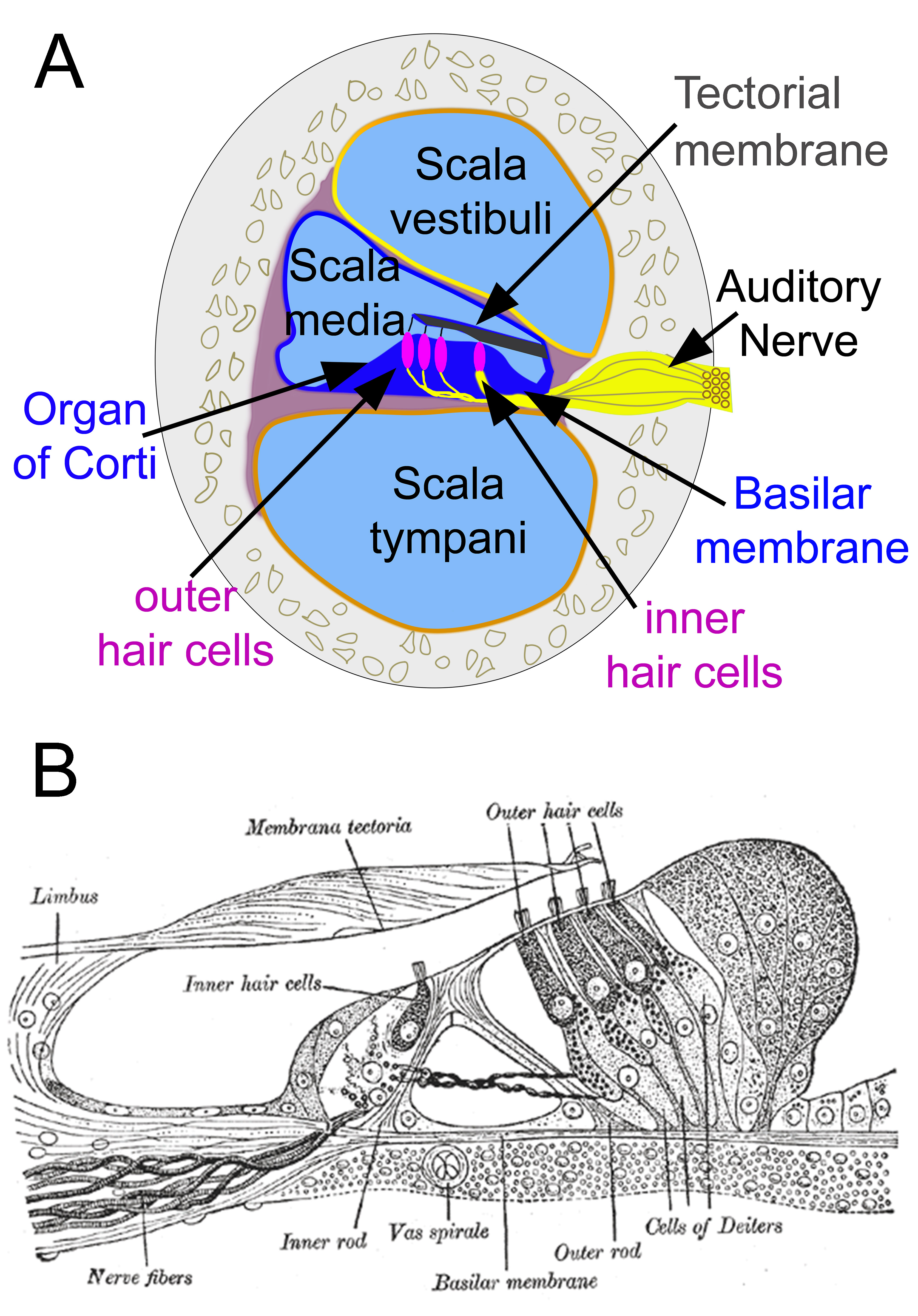
Organ of Corti. Cross-section of the cochlea (A). Section through the organ of Corti (B).
(A) Contributed by D Peterson, MD
(B) Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Patuzzi R, Robertson D. Tuning in the mammalian cochlea. Physiological reviews. 1988 Oct:68(4):1009-82 [PubMed PMID: 3054945]
Level 3 (low-level) evidencePeterson DC, Reddy V, Launico MV, Hamel RN. Neuroanatomy, Auditory Pathway. StatPearls. 2024 Jan:(): [PubMed PMID: 30335344]
Lim DJ. Functional structure of the organ of Corti: a review. Hearing research. 1986:22():117-46 [PubMed PMID: 3525482]
Level 3 (low-level) evidenceWright A. Scanning electron microscopy of the human organ of Corti. Journal of the Royal Society of Medicine. 1983 Apr:76(4):269-78 [PubMed PMID: 6341584]
Ashmore JF. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. The Journal of physiology. 1987 Jul:388():323-47 [PubMed PMID: 3656195]
Level 3 (low-level) evidenceAshmore J. Cochlear outer hair cell motility. Physiological reviews. 2008 Jan:88(1):173-210. doi: 10.1152/physrev.00044.2006. Epub [PubMed PMID: 18195086]
Level 3 (low-level) evidenceFritzsch B, Jahan I, Pan N, Kersigo J, Duncan J, Kopecky B. Dissecting the molecular basis of organ of Corti development: Where are we now? Hearing research. 2011 Jun:276(1-2):16-26. doi: 10.1016/j.heares.2011.01.007. Epub 2011 Jan 21 [PubMed PMID: 21256948]
Level 3 (low-level) evidenceWende S,Nakayama N,Schwerdtfeger P, The internal auditory artery: (embryology, anatomy, angiography, pathology). Journal of neurology. 1975 Aug 4 [PubMed PMID: 51066]
Matsunaga T, Igarashi M, Kanzaki J. The course of the internal auditory artery and its branches. Computer-aided three-dimensional reconstructions. Acta oto-laryngologica. Supplementum. 1991:487():54-60 [PubMed PMID: 1843586]
Nadol JB Jr. Comparative anatomy of the cochlea and auditory nerve in mammals. Hearing research. 1988 Aug:34(3):253-66 [PubMed PMID: 3049492]
Level 3 (low-level) evidenceAshmore JF. The G.L. Brown Prize Lecture. The cellular machinery of the cochlea. Experimental physiology. 1994 Mar:79(2):113-34 [PubMed PMID: 8003297]
Level 3 (low-level) evidenceErixon E, Högstorp H, Wadin K, Rask-Andersen H. Variational anatomy of the human cochlea: implications for cochlear implantation. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2009 Jan:30(1):14-22. doi: 10.1097/MAO.0b013e31818a08e8. Epub [PubMed PMID: 18833017]
Biedron S, Prescher A, Ilgner J, Westhofen M. The internal dimensions of the cochlear scalae with special reference to cochlear electrode insertion trauma. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2010 Jul:31(5):731-7. doi: 10.1097/MAO.0b013e3181d27b5e. Epub [PubMed PMID: 20142798]
Level 2 (mid-level) evidenceFarinetti A, Ben Gharbia D, Mancini J, Roman S, Nicollas R, Triglia JM. Cochlear implant complications in 403 patients: comparative study of adults and children and review of the literature. European annals of otorhinolaryngology, head and neck diseases. 2014 Jun:131(3):177-82. doi: 10.1016/j.anorl.2013.05.005. Epub 2014 Jun 2 [PubMed PMID: 24889283]
Level 2 (mid-level) evidenceCohen NL, Hoffman RA. Complications of cochlear implant surgery in adults and children. The Annals of otology, rhinology, and laryngology. 1991 Sep:100(9 Pt 1):708-11 [PubMed PMID: 1952660]
Di Lella F, Falcioni M, Piccinini S, Iaccarino I, Bacciu A, Pasanisi E, Cerasti D, Vincenti V. Prevention and management of vascular complications in middle ear and cochlear implant surgery. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2017 Nov:274(11):3883-3892. doi: 10.1007/s00405-017-4747-9. Epub 2017 Sep 20 [PubMed PMID: 28932983]