Introduction
The papillary muscles of the heart are pillar-like muscles seen within the cavity of the ventricles, attached to their walls. They have an integral role in proper cardiac valvular function. They arise from the inner walls of the left and right ventricle and attach to mitral and tricuspid valve leaflets respectively via chordae tendinae. Historical documentation of the existence of papillary muscles as a component of cardiac anatomy exists at least as early as the 16th century.[1] This article will describe the structure, function, embryology, blood supply, lymphatics, nerves, physiologic variants, surgical considerations, and clinical significance of the papillary muscles of the heart.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
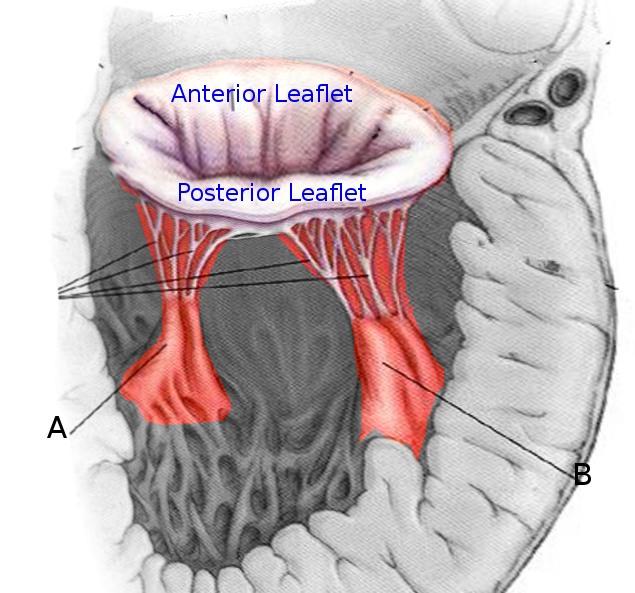
The papillary muscles arise from the walls of the left and right cardiac ventricles. The classic description of the left cardiac ventricle is as containing two papillary muscles: the anterolateral and posteromedial.[2] The anterolateral arises from the sternocostal wall, and the posteromedial papillary muscle arises from the diaphragmatic wall of the ventricle. The right ventricle contains three papillary muscles, classically described as anterior, posterior, and septal.[3] The anterior is larger and arises from the anterior wall, the posterior arises from the inferior wall, and the smallest of them all, the septal arises from the inter-ventricular septum. Chordae tendinae form the thin fibrous connection between the papillary muscles and the mitral and tricuspid valve leaflets.[4]
The general understanding is that the papillary muscles arise uniformly from the smooth surface of the ventricles. Nevertheless, a recent cardiac magnetic resonance (CMR) study has identified a 'cypress-tree' root-like structure at the base of the papillary muscle of the left ventricle with many thin projections which coalesce before forming into a thick muscle pillar.[5]
The primary function of these muscles is the proper functioning of the valves, i.e., opening and closer of the atrioventricular orifice. When the ventricles contract during the systole, simultaneous contraction of the papillary muscles taut the chordae tendinae. This arrangement prevents prolapse as well as the inversion of the cusps of the mitral and tricuspid valves.[6] Any morphological changes in these muscles and loss of contractility like in myocardial infarction with muscle fibrosis or ischemia can result in a malfunction of these valves leading to regurgitation or other conditions that are described later in this article.[2][7]
Embryology
Embryologic development of the papillary muscles begins at approximately week five of development and ends at about week nineteen of development.[8] The development of left ventricular papillary muscles starts with the emergence of a muscular trabecular ridge in the left ventricular wall from approximately weeks five to seven.[8] This ridge is oriented in a posteroanterior direction and is continuous with the atrial myocardium. The aortic leaflet of the mitral valve forms from atrioventricular cushion tissue between the anterior and posterior sections of the ridges at approximately week seven. From weeks eight to ten, papillary muscles emerge from the anterior and posterior segments of the ridge, with increased mobility and emergence of valves leaflets and precursors to chordae tendinae noted at approximately week twelve. Papillary muscle development becomes further refined with valve completion and chordae tendinae development between weeks fourteen to nineteen.[8]
Blood Supply and Lymphatics
The blood supply to the left anterolateral papillary muscles derives from branches of the left coronary artery. The blood supply to the left posteromedial papillary muscles most commonly derives from the right coronary artery. In some cases, posteromedial papillary muscles receiving blood supply from branches of the left circumflex artery.[9] The dominant circulation explains this variance in supply in the heart. The article “Papillary Muscle Perfusion Pattern, A Hypothesis for Ischemic Papillary Muscle Dysfunction” by Voci et al. (1995) found the posteromedial papillary muscle to have perfusion by only one vessel in 63% of patients, while the anterolateral papillary muscle more often demonstrated perfusion by multiple vessels.[9]
The blood supply to the anterior papillary muscle of the right ventricle most commonly originates from vessels branching from the left and right coronary arteries, with some anatomical variants receiving blood supply originating only from the left coronary artery.[10] The current belief is that each papillary muscles mostly receive blood supply from more than one segmental artery with wide anatomical variations. Thus they present with wide function variations in ischemic heart diseases.[11]
The lymphatic supply of the papillary muscles forms from lymphatic plexuses with a range of lymphatic vessel sizes.[12]
Nerves
Post-ganglionic axons arising from cervical, thoracic and mediastinal ganglia provide sympathetic motor innervation to the ventricular myocardium.[13] The heart is supplied by the cardiac plexus, which contains both the sympathetic and parasympathetic components. Cardiac plexus fibers innervate and regulate the pacemaker of the heart, the SA node, and through it to the AV node. The AV bundles carrying impulses from the AV node cross the fibrous ring to enter the interventricular septum, and divides into the right and left bundles of His. Right bundle of His supply, the musculature of the right ventricle, and the left bundle supply the left ventricle. Each of these bundles further divides into thin fibers called Purkinje fibers at the base of papillary muscles. Thus all the impulses spread into ventricles from the base of the papillary muscles and so the force of contraction.[5]
Further information about specific innervation of the papillary muscles is not very well described in the available literature.
Physiologic Variants
Physiologic variants of the papillary muscles can include modifications in muscle morphology, ventricular wall origin, and chordae attachment. Morphological variants can be described based on the number of papillary muscle heads and the presence of shared or separate basal origins.[14] Papillary muscles also vary by shape, which may be described as conical, flattop, truncated, bifurcated, and trifurcated.[2] The number of chordae tendinae attached to the papillary muscles of the left ventricle ranges from 2 to 20 chordae per muscle.[2]
Usually, the left ventricles have two papillary muscles, but studies indicate that 46% of hypoplastic and 18% of the borderline left ventricle cases had just a single papillary muscle. In these cases, it was also observed that the supporting pedicle had a more extensive attachment over the ventricular wall.[15] The variations of the papillary muscle structure can range from developmental anomalies to neoplasms. They can be asymptomatic throughout life or present with associated symptoms like outflow obstruction and sometimes can even lead to death.
Surgical Considerations
Surgical considerations regarding the papillary muscles include pathologic papillary muscle abnormalities leading to left ventricular outflow tract obstruction and papillary muscle rupture. Left ventricular outflow tract obstruction secondary to papillary muscle pathology that is amenable to surgical resection has been established in multiple case reports, with significant clinical improvement described post-intervention.[16][17] Papillary muscle realignment via pledget mattress stitching is another surgical treatment possibility for left ventricular outflow tract obstruction caused by pathologic papillary muscle orientation.[18]
Pathologic papillary muscle anatomy has been described as a contributing factor to left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. It may require modifications to surgical techniques generally used for the treatment of hypertrophic cardiomyopathy.[19]
Surgical intervention, such as mitral valve replacement or repair, is the recommended treatment for treatment following papillary muscle rupture after myocardial infarction.[20][21] Mitral valve repair with papillary muscle reimplantation is a surgical possibility for the treatment of papillary muscle rupture following acute myocardial infarction.[22]
Clinical Significance
The papillary muscles are integral to proper valvular coordination and alignment. Papillary muscle dysfunction can occur due to congenital abnormalities resulting in left ventricular outflow tract obstruction as well as from injury to the papillary muscles from ischemia or ventricular remodeling. Given the integral role of the papillary muscles in valvular coaptation, clinically significant valvular abnormalities correlate with these derangements.
Congenital abnormalities of the papillary muscles include pathological insertion of the papillary muscles and abnormal chordae tendinae attachment. Direct papillary muscle leaflet insertion has been described in case reports and is associated with hypertrophic cardiomyopathy and left ventricular outflow tract obstruction.[23] Abnormal chordae attachment can occur in the parachute mitral valve, a condition in which all mitral chordae tendinae attach to a single papillary muscle.[14] Parachute mitral valve often results in mitral stenosis but can be associated with mitral regurgitation or normal valvular function.[14]
Left ventricular remodeling due to cardiomyopathy or cardiac ischemia can lead to secondary (functional) mitral regurgitation. In contrast to primary mitral regurgitation, in which intrinsic defects of the mitral valve apparatus lead to insufficiency, secondary mitral regurgitation results from disease of the atrium or ventricle without the mitral valvular disease.[24] Displacement of the papillary muscles because of changes in ventricular size leads to improper application of tension on valve leaflets, leading to poor coaptation and regurgitation.
Other Issues
Rupture of the papillary muscle can occur following acute myocardial infarction as well as following traumatic injury.
As previously described, the posteromedial papillary muscle often has perfusion by only one vessel. Accordingly, the rupture of the posteromedial papillary muscle occurs more commonly than that of the anterolateral papillary muscle.[9][25] Given that the isolated vessel perfusion of the posterior-medial papillary muscle arises from the RCA, and that RCA occlusion is involved in inferior wall myocardial infarctions, rupture of the posteromedial papillary muscle can occur following inferior wall myocardial infarctions.
Blunt trauma leading to papillary muscle rupture has appeared in multiple case reports. It should be a consideration when a new murmur or elevated cardiac enzymes present following severe trauma.[26][27] The rupture of papillary muscles should receive immediate treatment; not doing so can be fatal.
This research was supported (in whole or in part) by HCA and/or an HCA-affiliated entity. The views expressed in this publication represent those of the author(s) do not necessarily represent the official views of HCA or any of its affiliated entities.
Media
(Click Image to Enlarge)
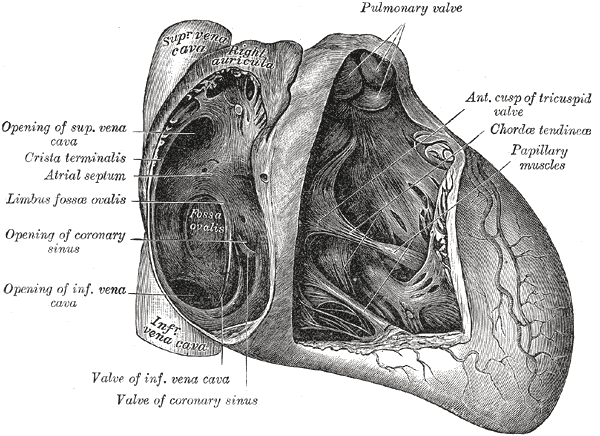
Anatomy of the Heart, Pulmonary valve, Anterior cusp of tricuspid valve, Chordae tendineae, Papillary muscles, Valve of coronary sinus, Valve of Inferior vena cava, Coronary sinus, Limbus fossae ovalis, Crista terminalis, Atrial Septum, Superior Vena cava
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
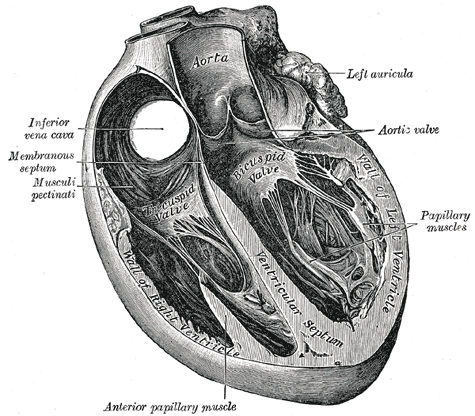
Trans Sagittal Cross section of the Heart, Aorta, Left Auricula, Aortic Valve, Papillary muscles, Left Ventricle, Bicuspid Valve, Ventricular Septum, Inferior Vena Cava, Membranous septum, Musculi pectinati, Anterior Papillary Muscles, Tricuspid Valve
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
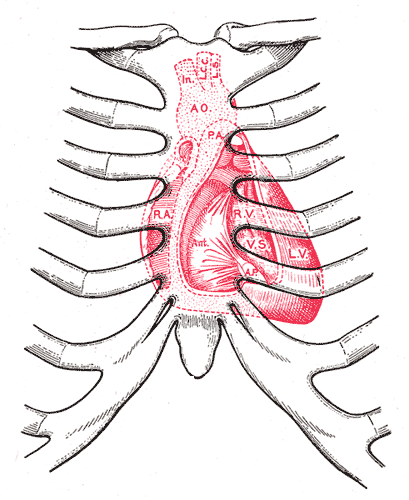
Surface markings of the Thorax, Diagram showing relations of opened heart to front of thoracic wall, Ant; Anterior segment of tricuspid valve, AO; Aorta, AP; Anterior papillary muscle, IN; Innominate artery, LCC; Left common carotid artery, LS; Left subclavian artery, LV; Left ventricle, PA; Pulmonary artery, RA; Right atrium, RV; Right ventricle, VS; Ventricular septum
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
References
Bestetti RB, Restini CB, Couto LB. Development of anatomophysiologic knowledge regarding the cardiovascular system: from Egyptians to Harvey. Arquivos brasileiros de cardiologia. 2014 Dec:103(6):538-45. doi: 10.5935/abc.20140148. Epub 2014 Oct 10 [PubMed PMID: 25590934]
Saha A, Roy S. Papillary muscles of left ventricle-Morphological variations and it's clinical relevance. Indian heart journal. 2018 Nov-Dec:70(6):894-900. doi: 10.1016/j.ihj.2017.12.003. Epub 2017 Dec 11 [PubMed PMID: 30580862]
Saha A, Roy S. Papillary muscles of right ventricle-morphological variations and its clinical relevance. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2018 May-Jun:34():22-27. doi: 10.1016/j.carpath.2018.01.007. Epub 2018 Feb 9 [PubMed PMID: 29525728]
Gunnal SA, Wabale RN, Farooqui MS. Morphological study of chordae tendinae in human cadaveric hearts. Heart views : the official journal of the Gulf Heart Association. 2015 Jan-Mar:16(1):1-12. doi: 10.4103/1995-705X.152994. Epub [PubMed PMID: 25838872]
Khan MS, Biederman R. Dynamic cardiac anatomy: the "cypress tree" papillary muscle root. Journal of cardiovascular and thoracic research. 2018:10(3):138-143. doi: 10.15171/jcvtr.2018.22. Epub 2018 Sep 30 [PubMed PMID: 30386533]
Schubert SA, Mehaffey JH, Charles EJ, Kron IL. Mitral Valve Repair: The French Correction Versus the American Correction. The Surgical clinics of North America. 2017 Aug:97(4):867-888. doi: 10.1016/j.suc.2017.03.009. Epub [PubMed PMID: 28728720]
Mihos CG, Yucel E, Santana O. The role of papillary muscle approximation in mitral valve repair for the treatment of secondary mitral regurgitation. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2017 Jun 1:51(6):1023-1030. doi: 10.1093/ejcts/ezw384. Epub [PubMed PMID: 28040676]
Oosthoek PW, Wenink AC, Wisse LJ, Gittenberger-de Groot AC. Development of the papillary muscles of the mitral valve: morphogenetic background of parachute-like asymmetric mitral valves and other mitral valve anomalies. The Journal of thoracic and cardiovascular surgery. 1998 Jul:116(1):36-46 [PubMed PMID: 9671895]
Level 3 (low-level) evidenceVoci P, Bilotta F, Caretta Q, Mercanti C, Marino B. Papillary muscle perfusion pattern. A hypothesis for ischemic papillary muscle dysfunction. Circulation. 1995 Mar 15:91(6):1714-8 [PubMed PMID: 7882478]
Zajączkowski MA, Gajić A, Kaczyńska A, Zajączkowski S, Kobiela J, Kamiński R, Kosiński A. Individual variability of vascularization of the anterior papillary muscle within the right ventricle of human heart. PloS one. 2018:13(10):e0205786. doi: 10.1371/journal.pone.0205786. Epub 2018 Oct 15 [PubMed PMID: 30321241]
DiDio LJ, Rodrigues H, Baptista CA. The papillary muscles of the left ventricle and the cardiac segments. Surgical and radiologic anatomy : SRA. 1990:12(4):281-5 [PubMed PMID: 2096463]
Ratajska A, Gula G, Flaht-Zabost A, Czarnowska E, Ciszek B, Jankowska-Steifer E, Niderla-Bielinska J, Radomska-Lesniewska D. Comparative and developmental anatomy of cardiac lymphatics. TheScientificWorldJournal. 2014:2014():183170. doi: 10.1155/2014/183170. Epub 2014 Jan 27 [PubMed PMID: 24592145]
Level 3 (low-level) evidenceHanna P, Rajendran PS, Ajijola OA, Vaseghi M, Andrew Armour J, Ardell JL, Shivkumar K. Cardiac neuroanatomy - Imaging nerves to define functional control. Autonomic neuroscience : basic & clinical. 2017 Nov:207():48-58. doi: 10.1016/j.autneu.2017.07.008. Epub 2017 Jul 29 [PubMed PMID: 28802636]
Rajiah P, Fulton NL, Bolen M. Magnetic resonance imaging of the papillary muscles of the left ventricle: normal anatomy, variants, and abnormalities. Insights into imaging. 2019 Aug 19:10(1):83. doi: 10.1186/s13244-019-0761-3. Epub 2019 Aug 19 [PubMed PMID: 31428880]
Velasco Forte MN, Nassar M, Byrne N, Silva Vieira M, Pérez IV, Ruijsink B, Simpson J, Hussain T. Morphological three-dimensional analysis of papillary muscles in borderline left ventricles. Cardiology in the young. 2017 Sep:27(7):1369-1376. doi: 10.1017/S1047951117000439. Epub [PubMed PMID: 28782496]
Nomura T, Harada Y, Suzaki Y, Hayashi H, Tanaka H, Shiraishi J, Komatsu S, Hosomi Y, Hirano S, Yaku H, Kitamura N. Left ventricular outflow tract obstruction due to anomalous insertion of papillary muscle. Circulation journal : official journal of the Japanese Circulation Society. 2004 Dec:68(12):1219-22 [PubMed PMID: 15564711]
Level 3 (low-level) evidenceCosta MACD, Wippich AC. Correction of Left Ventricular Outflow Tract Obstruction Caused by Anomalous Papillary Muscle and Subaortic Membrane. Brazilian journal of cardiovascular surgery. 2018 Nov-Dec:33(6):634-637. doi: 10.21470/1678-9741-2017-0046. Epub [PubMed PMID: 30652755]
Bryant R 3rd, Smedira NG. Papillary muscle realignment for symptomatic left ventricular outflow tract obstruction. The Journal of thoracic and cardiovascular surgery. 2008 Jan:135(1):223-4. doi: 10.1016/j.jtcvs.2007.08.034. Epub [PubMed PMID: 18179954]
Level 3 (low-level) evidenceMaron BJ, Nishimura RA, Danielson GK. Pitfalls in clinical recognition and a novel operative approach for hypertrophic cardiomyopathy with severe outflow obstruction due to anomalous papillary muscle. Circulation. 1998 Dec 8:98(23):2505-8 [PubMed PMID: 9843454]
Level 3 (low-level) evidenceBouma W, Wijdh-den Hamer IJ, Koene BM, Kuijpers M, Natour E, Erasmus ME, Jainandunsing JS, van der Horst IC, Gorman JH 3rd, Gorman RC, Mariani MA. Long-term survival after mitral valve surgery for post-myocardial infarction papillary muscle rupture. Journal of cardiothoracic surgery. 2015 Jan 27:10():11. doi: 10.1186/s13019-015-0213-1. Epub 2015 Jan 27 [PubMed PMID: 25622516]
Bouma W, Wijdh-den Hamer IJ, Klinkenberg TJ, Kuijpers M, Bijleveld A, van der Horst IC, Erasmus ME, Gorman JH 3rd, Gorman RC, Mariani MA. Mitral valve repair for post-myocardial infarction papillary muscle rupture. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2013 Dec:44(6):1063-9. doi: 10.1093/ejcts/ezt150. Epub 2013 Mar 21 [PubMed PMID: 23520228]
Park WK, Kim JB, Choo SJ. Repair of Acute Post Infarction Mitral Regurgitation with Papillary Muscle Reimplantation - A case report -. The Korean journal of thoracic and cardiovascular surgery. 2011 Aug:44(4):285-7. doi: 10.5090/kjtcs.2011.44.4.285. Epub 2011 Aug 18 [PubMed PMID: 22263170]
Level 3 (low-level) evidenceKorabathina R, Chiu K, van Gelder HM, Labovitz A. Anomalous Papillary Muscle Insertion Causing Dynamic Left Ventricular Outflow Tract Obstruction without Hypertrophic Obstructive Cardiomyopathy. Case reports in cardiology. 2017:2017():9878049. doi: 10.1155/2017/9878049. Epub 2017 May 15 [PubMed PMID: 28589043]
Level 3 (low-level) evidenceEl Sabbagh A, Reddy YNV, Nishimura RA. Mitral Valve Regurgitation in the Contemporary Era: Insights Into Diagnosis, Management, and Future Directions. JACC. Cardiovascular imaging. 2018 Apr:11(4):628-643. doi: 10.1016/j.jcmg.2018.01.009. Epub [PubMed PMID: 29622181]
Level 3 (low-level) evidenceJayawardena S, Renteria AS, Burzyantseva O, Lokesh G, Thelusmond L. Anterolateral papillary muscle rupture caused by myocardial infarction: A case report. Cases journal. 2008 Sep 20:1(1):172. doi: 10.1186/1757-1626-1-172. Epub 2008 Sep 20 [PubMed PMID: 18803861]
Level 3 (low-level) evidenceNabzdyk CS, Tabrizi MB. In-Hospital Diagnosis of Tricuspid Papillary Muscle Rupture in an Asymptomatic Patient after Blunt Chest Trauma. Case reports in critical care. 2019:2019():1890640. doi: 10.1155/2019/1890640. Epub 2019 May 9 [PubMed PMID: 31210992]
Level 3 (low-level) evidenceHazan E, Guzeloglu M, Sariosmanoglu N, Ugurlu B, Keskin V, Unal N. Repair of isolated mitral papillary muscle rupture consequent to blunt trauma in a small child. Texas Heart Institute journal. 2009:36(3):252-4 [PubMed PMID: 19568400]
Level 3 (low-level) evidence