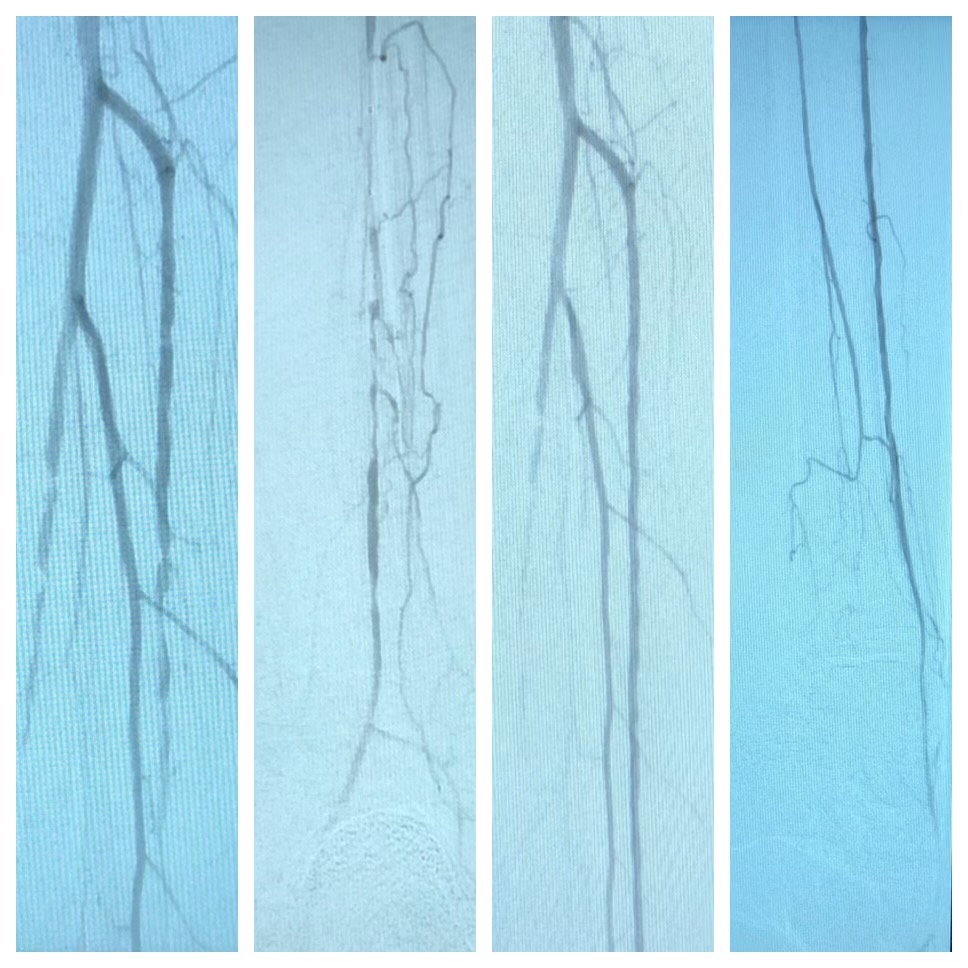
Introduction
Patients with peripheral arterial disease (PAD) have decreased lower extremity arterial perfusion which is commonly referred to as “poor circulation.” In most cases of PAD, atherosclerotic plaques narrow the arterial flow lumen which restricts blood flow to the distal extremity. Reduced blood flow can cause thigh or calf pain with walking due to temporary ischemia of the leg muscles during exertion. Walking pain from PAD is referred to as intermittent claudication which means “to limp.” Many patients with PAD have either no symptoms or atypical complaints that do not strictly conform to the definition of claudication. Others may develop limb-threatening compromise of blood flow, necessitating emergent surgery.
Making the diagnosis of PAD even in asymptomatic patients still, has a significant clinical impact because PAD acts as a marker for systemic atherosclerosis. Patients with PAD have an equivalent cardiovascular risk to patients with previous myocardial infarction and require aggressive risk factor modification to improve their long-term survival. The management of PAD varies depending on the disease severity and symptom status. Treatment options for PAD include lifestyle changes, cardiovascular risk factor reduction, pharmacotherapy, endovascular intervention, and surgery.[1][2][3]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Peripheral artery disease is usually caused by atherosclerosis. Other causes may be inflammation of the blood vessels, injury, or radiation exposure.[4]
Risk factors include:
- Diabetes
- Smoking
- Obesity (a body mass index over 30)
- High blood pressure
- High cholesterol
- Increasing age, especially after reaching 50 years of age
- A family history of peripheral artery disease, heart disease or stroke
- High levels of homocysteine, a protein component that helps build and maintain tissue
Epidemiology
PAD affects over 200 million adults worldwide and the incidence of PAD increases to as high as 20% in people over the age of 70. Although PAD has traditionally been perceived as a disease affecting men, the prevalence of PAD appears to be equal among senior men and women. Under-diagnosis of PAD in the primary care setting may be a significant issue, as most patients with PAD do not present with stereotypical claudication symptoms described in textbooks. Smoking increases the risk of developing PAD fourfold and has the greatest impact on disease severity. Compared to non-smokers, smokers with PAD have shorter life spans and progress more frequently to critical limb ischemia and amputation. Additional risk factors for PAD include diabetes, hyperlipidemia, hypertension, race, and ethnicity.[5]
Pathophysiology
PAD usually involves atherosclerotic disease in the abdominal aorta, iliac, and femoral arteries. The pathophysiology of atherosclerosis involves complex interactions between cholesterol and vascular cells the details of which are beyond the scope of this article. Atherosclerotic plaque builds up slowly on the inside of arteries. In the early stages of PAD, the arteries compensate for the plaque buildup by dilating to preserve flow through the vessel. Eventually, the artery cannot dilate any further, and the atherosclerotic plaque starts to narrow the arterial flow lumen.
In some cases, the cause of sudden ischemia may be emboli either of cardiac origin or from atherosclerotic disease of the aorta. Emboli tend to be most common at sites of arterial bifurcation or where vessel branches have an abrupt takeoff. The femoral artery is the most common site for emboli, followed by the iliac arteries, aorta and the popliteal arteries.
The hemodynamic consequences of atherosclerosis depend on the degree of arterial narrowing. A 50% decrease in vessel diameter corresponds to a 75% loss of cross-sectional area which is usually considered flow limiting. As the narrowing progresses or completely obstructs the artery, blood flow shifts to smaller arteries which parallel the diseased artery. Although this collateral flow preserves distal perfusion, the network of smaller vessels never carries as much blood flow as the main artery. This blood flow restriction represents the hallmark of PAD and its typical symptoms. The muscles of the lower extremity require increased blood flow during ambulation to meet the increased energy demand. Patients with PAD reach a point during walking at which collateral blood flow is maximized and cannot provide any more perfusion to the lower extremity muscles. This supply-demand mismatch causes temporary ischemia of the muscles which manifests as pain, cramping, or fatigue and ultimately makes the patient with PAD slow down or stop walking. Lowering the energy demands of the muscle (by slowing or stopping) allows the blood supply to “catch up,” and the ischemic symptoms resolve. This cycle of blood flow restriction increased energy demand, and temporary muscle ischemia describes the pathophysiology of claudication due to PAD.
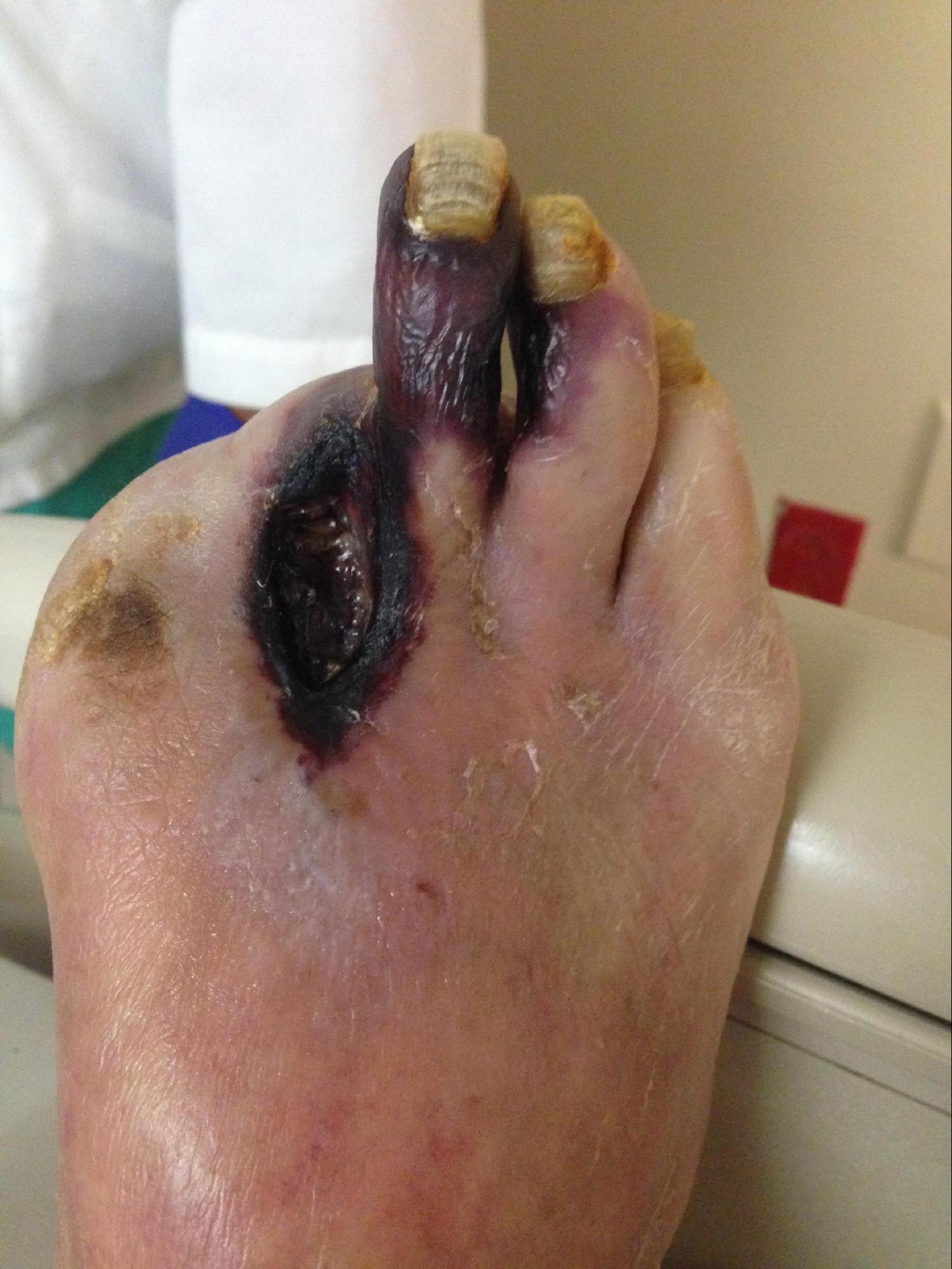
Patients with PAD usually have enough collateral blood flow that they only have symptoms during activities that increase energy demand such as walking. Rarely, the PAD becomes progressively more severe, and the blood flow cannot meet the resting metabolic demands of the lower extremity. Poor perfusion to the nerves can result in ischemic rest pain which is often described as an intractable, burning pain in the soles of the feet. Non-healing wounds and ischemic ulcers represent tissue loss due to poor blood flow. In the most severe cases, the toes or entire forefoot can become black and mummified as gangrene develops. [6]
History and Physical
The most characteristic symptom of PAD is claudication which is a pain in the lower extremity muscles brought on by walking and relieved with rest. Although claudication has traditionally been described as cramping pain, some patients report leg fatigue, weakness, pressure, or aching. Symptoms during walking occur in the muscle group one level distal to the artery narrowed or blocked by PAD. Patients with aortoiliac artery occlusive disease, therefore, have symptoms in the thigh and buttock muscles while patients with femoropopliteal PAD have symptoms in their calf muscles. The walking distance at which symptoms occur depends on multiple factors including disease severity, walking pace, terrain, and incline.
Surprisingly, many patients with PAD have few if any symptoms. The reason for this absence of symptoms usually falls into 1 of 2 broad categories. Some patients with mild or moderate PAD rarely sustain a walking pace that increases the blood flow requirement of the lower extremity muscles. By being physically inactive, these patients avoid the supply-demand mismatch that triggers claudication symptoms. Other patients with PAD have muscle discomfort when they walk but fail to report these symptoms because they attribute them to the natural consequences of aging.
Patients with severe PAD can develop ischemic rest pain. These patients do not walk enough to claudicate because of their severe disease. Instead, they complain of burning pain in the soles of their feet that is worse at night. They cannot sleep due to the pain and often dangle their lower leg over the side of the bed in an attempt to relieve their discomfort. The slight increase in blood flow due to gravity temporarily diminishes the otherwise intractable pain. In some cases, edema from keeping the leg in a dependent position may be mistakenly attributed to venous thrombosis.
Other features of PAD include erectile dysfunction, which is often an early indicator of the disease.
The physical exam may reveal the following:
- Loss of pulses
- Pain on palpation
- Pallor
- Muscle atrophy and loss of hair
- Cool and cyanotic skin
- Presence of bruit
Evaluation
Making the diagnosis of PAD should factor in the patient’s history, physical exam, and objective test results. Key points in the history include an accurate assessment of the patient’s walking ability. Many patients do not explicitly report the classic claudication symptoms of cramping calf pain after walking 2 blocks that resolves with rest. Some patients may walk very little because of other physical ailments or limitations. Other patients may not volunteer any information about walking unless specifically asked. Important aspects of the history also include cardiovascular risk factors such as smoking, hypertension, high cholesterol, and diabetes.[7][8][9]
On physical exam, patients with PAD may have diminished or absent lower extremity pulses. This finding can be confirmed with the ankle-brachial index (ABI), an objective, bedside measure of lower extremity arterial perfusion. As its name implies, the ABI compares the systolic blood pressure at the ankle to the systolic pressure in the arm. A manual blood pressure cuff should be placed just above the ankle while locating the posterior tibial artery or dorsalis pedis artery with a handheld Doppler probe. While listening to the Doppler signal, the blood pressure cuff is inflated until it obliterates the Doppler signal. As the cuff slowly deflates, the pressure at which the Doppler signal returns is recorded as the systolic ankle pressure. The same steps are repeated for the other pedal artery and the other leg. Likewise, the brachial pressure can be measured with a blood pressure cuff on the upper arm and a Doppler probe positioned over the radial or ulnar artery at the wrist. The ABI is the highest systolic pressure measured at each ankle divided by the higher of the two systolic brachial pressures. A normal ABI ranges from 0.9 to 1.3. PAD is defined as an ABI less than 0.9 and most patients with claudication have an ABI between 0.5 and 0.9. Patients with extremely low ABI’s (less than 0.5) usually have ischemic rest pain or tissue loss. An ABI greater than 1.3 indicates arterial wall stiffening which can occur in patients with diabetes or renal failure. If a cuff pressure of 250mmHg does not obliterate the Doppler signal, the ABI is classified as “non-compressible.” Patients with falsely elevated (greater than 1.3) or non-compressible ABI’s require alternative imaging or physiologic studies to confirm the diagnosis of PAD.[10]
A common mistake when taking an ABI involves failure to measure the brachial pressure in both arms. Using the higher brachial pressure as the denominator for both ankle pressures ensures that the ABI will not be underestimated in patients with upper extremity blood pressure discrepancy due to subclavian artery stenosis. Other measures to increase the accuracy of the ABI include having the patient rest supine for at least 5 minutes to allow their blood pressure to stabilize and choosing an appropriately sized blood pressure cuff. The bladder length of the cuff should be 80%, and the width of 40% of the circumference of the extremity.
Blood work may reveal altered renal function and abnormal electrolyte levels. Elevation in CRP, D-dimer and interleukin 6 have been associated with diminished exercise tolerance.
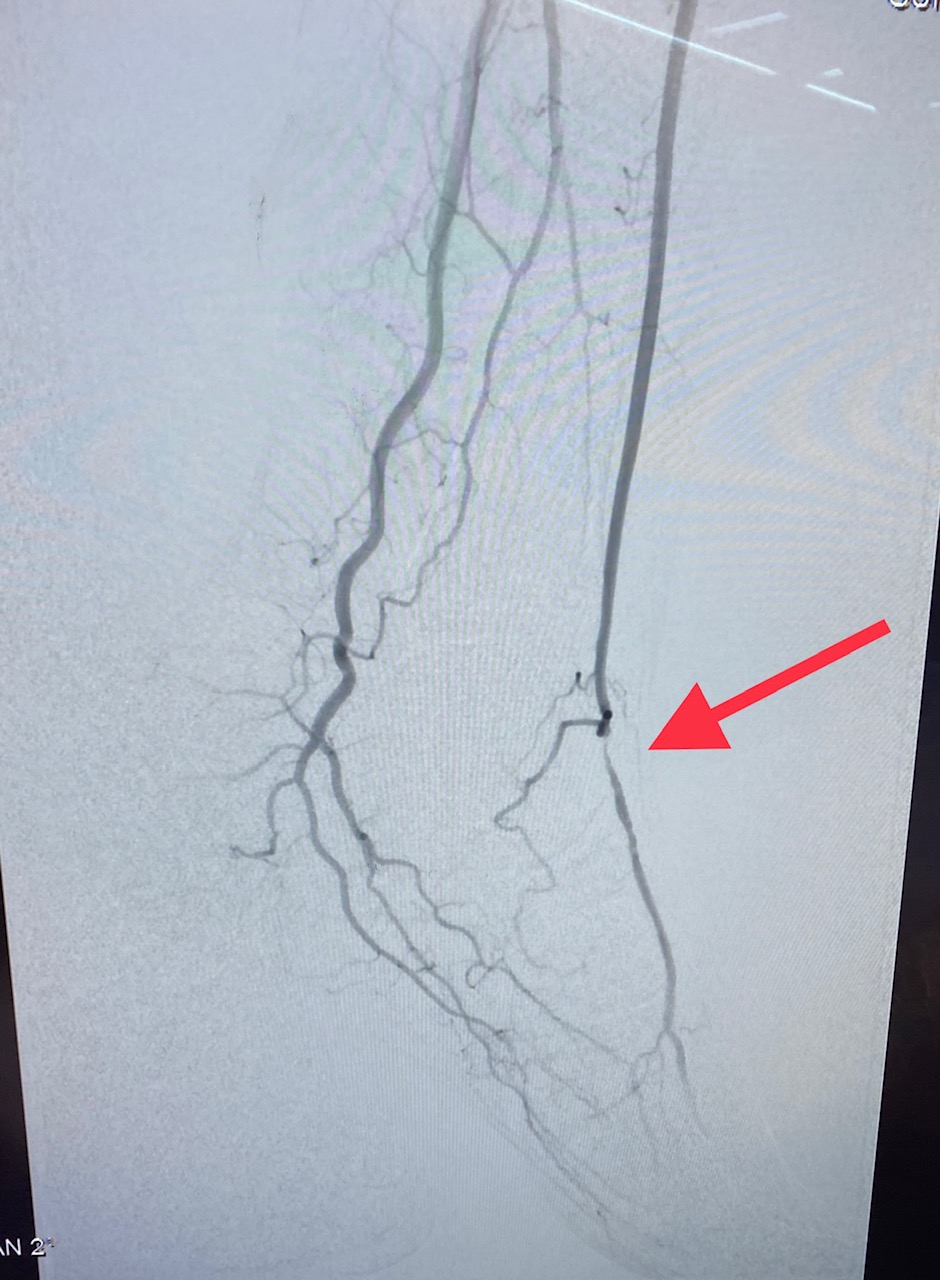
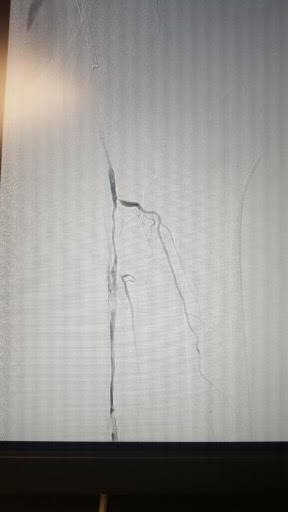
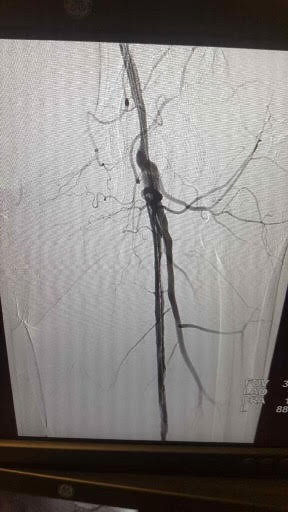
Doppler studies are done to determine the site of blood flow occlusion and flow velocities. In addition, CT angiography and MRA can help determine the sites of occlusion and assess if the patient is a candidate for angioplasty or bypass surgery.
In patients with ulcers, transcutaneous oximetry is a rapid method of assessing perfusion. Finally, an ECG may reveal the presence of an arrhythmia which may be the cause of an embolic event.
Treatment / Management
Management strategies for PAD attempt to achieve two distinct goals: lower cardiovascular risk and improve walking ability. All patients with PAD, regardless of the presence or absence of symptoms, have an increased risk of stroke, myocardial infarction, and thrombosis compared to patients without arterial disease. These cardiovascular events probably account for the shorter life expectancy of patients with PAD. Therefore, all patients diagnosed with PAD should undertake lifestyle changes aimed at lowering their cardiovascular risk profile. Key targets for lifestyle changes include quitting smoking, lowering cholesterol, and controlling hypertension and diabetes.[11][12][13]
Treatment options to improve walking ability vary depending on the patient’s symptoms and disease severity. Exercise therapy involves walking until reaching pain tolerance, stopping for a brief rest, and walking again as soon as the pain resolves. These walking sessions should last 30 to 45 minutes, 3 to 4 times per week for at least 12 weeks. Despite being more effective, supervised exercise programs for PAD are not usually covered by insurance companies. Pharmacotherapy for claudication involves the use of cilostazol, a medication that promotes vasodilation and suppresses the proliferation of vascular smooth muscle cells. Patients who respond to cilostazol usually notice a positive effect within 12 weeks. Since cilostazol is a phosphodiesterase type-3 inhibitor, a history of congestive heart failure precludes patients from receiving this type of therapy. Pentoxifylline, a medication that improves oxygen delivery by its rheolytic effect has been approved for the treatment of claudication. However, it has inconsistent results compared to placebo in walking distance studies. Balloon angioplasty or stent placement provides a minimally invasive, percutaneous treatment option for patients with PAD symptoms that do not respond to exercise or medical therapy. These endovascular treatments achieve the best results when applied to focal occlusive lesions in the iliac and superficial femoral arteries. The technical success and durability of endovascular therapy decrease in patients with long-segment total occlusions and infrapopliteal arterial occlusive disease. Since PAD does not usually represent life or limb-threatening condition, surgery should be reserved for highly selected patients who have significant symptoms despite treatment with non-invasive and endovascular therapy. Surgical options for PAD include bypass grafts to divert flow around the blockage or endarterectomy to segmentally remove the obstructive plaque.[14][15]
There is ample evidence supporting the use of statins to improve the atherosclerotic disease. However, the patient must change lifestyle and reduce the risk factors for atherosclerotic disease.
Differential Diagnosis
- Deep vein thrombosis
- Low back pain
- Superficial thrombophlebitis
- Raynaud phenomenon
- Thromboangiitis obliterans
- Sciatica
Prognosis
Even with treatment, the prognosis of PAD is generally guarded. If the patient does not change his/her lifestyle, the disease is progressive. In addition, most patients with PAD also have coexistence of cerebrovascular or coronary artery disease, which also increases the mortality rate. The outcomes in women tend to be worse than in men, chiefly because of the small diameter of the arteries. In addition, females are more likely to develop complications and embolic events.
Complications
- Ischemia/Gangrene
- Amputation
- Infection
- Ulceration
- Heart attack
- Stroke
- Blood clots
- Erectile Dysfunction
Postoperative and Rehabilitation Care
Once the diagnosis of PVD is made, lifelong follow up is required. The disorder has no cure and if the lifestyle is not modified, the condition progresses.
Consultations
Vascular surgeon
Endocrinologist
Interventional radiologist
Deterrence and Patient Education
- Discontinue smoking
- Control of diabetes
- Maintain a healthy weight
- Begin exercise program
- Control of blood pressure and cholesterol levels
Enhancing Healthcare Team Outcomes
PVD is a systemic disorder and for the most part progressive. The condition is associated with life and limb-threatening complications. Despite many advances in endovascular surgery, amputations of digits and limbs are not uncommon. Thus, the present-day approach is to prevent the disorder in the first place. There is ample evidence that changes in lifestyle can significantly decrease the rate of progression of the disease and improve the quality of life. Besides physicians, nurses and pharmacists have a vital role in preventing PVD. These two healthcare professionals should emphasize the importance of smoking cessation, maintaining a healthy weight and controlling blood sugars. The pharmacist should also ensure that the patient is not on any medications that can lead to vasoconstriction. The patient should be taught about basic foot hygiene and wear protective shoewear. The patient should be educated on signs of vascular disease and seek assistance if any changes occur. Finally, current guidelines indicate that PVD patients should be treated with a statin and either aspirin or cilostazol. An interprofessional approach to patient education and management will improve outcomes. [16][17][18] (Level V)
Outcomes
The outcomes of PVD depend on where the disease is located and gender. Overall, females appear to have worse outcomes compared to males, primarily because they have smaller vessels, which are more likely to become narrowed. When surgery is done in females for peripheral vascular disease, complications are also more frequent and serious. [19][20][21](level V)
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Aysert Yıldız P, Özdil T, Dizbay M, Güzel Tunçcan Ö, Hızel K. Peripheral arterial disease increases the risk of multidrug-resistant bacteria and amputation in diabetic foot infections. Turkish journal of medical sciences. 2018 Aug 16:48(4):845-850. doi: 10.3906/sag-1803-217. Epub 2018 Aug 16 [PubMed PMID: 30119162]
Yuksel A, Velioglu Y, Cayir MC, Kumtepe G, Gurbuz O. Current Status of Arterial Revascularization for the Treatment of Critical Limb Ischemia in Infrainguinal Atherosclerotic Disease. The International journal of angiology : official publication of the International College of Angiology, Inc. 2018 Sep:27(3):132-137. doi: 10.1055/s-0037-1620242. Epub 2018 Jan 22 [PubMed PMID: 30154631]
Tan MNA, Lo ZJ, Lee SH, Teo RM, Tan WLG, Chandrasekar S. Review of Transmetatarsal Amputations in the Management of Peripheral Arterial Disease in an Asian Population. Annals of vascular diseases. 2018 Jun 25:11(2):210-216. doi: 10.3400/avd.oa.17-00123. Epub [PubMed PMID: 30116413]
Simon F, Oberhuber A, Floros N, Düppers P, Schelzig H, Duran M. Pathophysiology of chronic limb ischemia. Gefasschirurgie : Zeitschrift fur vaskulare und endovaskulare Chirurgie : Organ der Deutschen und der Osterreichischen Gesellschaft fur Gefasschirurgie unter Mitarbeit der Schweizerischen Gesellschaft fur Gefasschirurgie. 2018:23(Suppl 1):13-18. doi: 10.1007/s00772-018-0380-1. Epub 2018 Apr 10 [PubMed PMID: 29950791]
Jelani QU, Petrov M, Martinez SC, Holmvang L, Al-Shaibi K, Alasnag M. Peripheral Arterial Disease in Women: an Overview of Risk Factor Profile, Clinical Features, and Outcomes. Current atherosclerosis reports. 2018 Jun 2:20(8):40. doi: 10.1007/s11883-018-0742-x. Epub 2018 Jun 2 [PubMed PMID: 29858704]
Level 3 (low-level) evidenceKim HO, Kim W. Elucidation of the Diagnosis and Treatment of Peripheral Arterial Disease. Korean circulation journal. 2018 Sep:48(9):826-827. doi: 10.4070/kcj.2018.0155. Epub 2018 Jun 11 [PubMed PMID: 30088357]
Rafailidis V, Sidhu PS. Vascular ultrasound, the potential of integration of multiparametric ultrasound into routine clinical practice. Ultrasound (Leeds, England). 2018 Aug:26(3):136-144. doi: 10.1177/1742271X18762250. Epub 2018 Feb 28 [PubMed PMID: 30147737]
Marcadet DM, Pavy B, Bosser G, Claudot F, Corone S, Douard H, Iliou MC, Vergès-Patois B, Amedro P, Le Tourneau T, Cueff C, Avedian T, Solal AC, Carré F. French Society of Cardiology guidelines on exercise tests (part 2): Indications for exercise tests in cardiac diseases. Archives of cardiovascular diseases. 2019 Jan:112(1):56-66. doi: 10.1016/j.acvd.2018.07.001. Epub 2018 Aug 6 [PubMed PMID: 30093255]
Serhal A, Koktzoglou I, Aouad P, Carr JC, Giri S, Morcos O, Edelman RR. Cardiovascular magnetic resonance imaging of aorto-iliac and ilio-femoral vascular calcifications using proton density-weighted in-phase stack of stars. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2018 Aug 6:20(1):51. doi: 10.1186/s12968-018-0479-2. Epub 2018 Aug 6 [PubMed PMID: 30078377]
Santoro L, Flex A, Nesci A, Ferraro PM, De Matteis G, Di Giorgio A, Giupponi B, Saviano L, Gambaro G, Franceschi F, Gasbarrini A, Landolfi R, Santoliquido A. Association between peripheral arterial disease and cardiovascular risk factors: role of ultrasonography versus ankle-brachial index. European review for medical and pharmacological sciences. 2018 May:22(10):3160-3165. doi: 10.26355/eurrev_201805_15076. Epub [PubMed PMID: 29863271]
Debus ES, Kriston L, Schwaneberg T, Hischke S, Rieß HC, Härter M, Marschall U, Federrath H, Behrendt CA. Rationale and methods of the IDOMENEO health outcomes of the peripheral arterial disease revascularisation study in the GermanVasc registry. VASA. Zeitschrift fur Gefasskrankheiten. 2018 Oct:47(6):499-505. doi: 10.1024/0301-1526/a000730. Epub 2018 Aug 16 [PubMed PMID: 30113269]
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for Peripheral Artery Disease and Cardiovascular Disease Risk Assessment With the Ankle-Brachial Index: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 Jul 10:320(2):177-183. doi: 10.1001/jama.2018.8357. Epub [PubMed PMID: 29998344]
Jin J. Screening for Peripheral Artery Disease With Ankle-Brachial Index. JAMA. 2018 Jul 10:320(2):212. doi: 10.1001/jama.2018.9112. Epub [PubMed PMID: 29998339]
Shabani Varaki E, Gargiulo GD, Penkala S, Breen PP. Peripheral vascular disease assessment in the lower limb: a review of current and emerging non-invasive diagnostic methods. Biomedical engineering online. 2018 May 11:17(1):61. doi: 10.1186/s12938-018-0494-4. Epub 2018 May 11 [PubMed PMID: 29751811]
Expert Panel on Vascular Imaging:, Cooper K, Majdalany BS, Kalva SP, Chandra A, Collins JD, Francois CJ, Ganguli S, Gornik HL, Kendi AT, Khaja MS, Minocha J, Norton PT, Obara P, Reis SP, Sutphin PD, Rybicki FJ. ACR Appropriateness Criteria(®) Lower Extremity Arterial Revascularization-Post-Therapy Imaging. Journal of the American College of Radiology : JACR. 2018 May:15(5S):S104-S115. doi: 10.1016/j.jacr.2018.03.011. Epub [PubMed PMID: 29724414]
Kawarada O, Zen K, Hozawa K, Ayabe S, Huang HL, Choi D, Kim SH, Kim J, Kato T, Tsubakimoto Y, Nakama T, Ichihashi S, Fujimura N, Higashimori A, Fujihara M, Sato T, Yan BP, Pang SY, Wongwanit C, Leong YP, Chua B, George RK, Yokoi Y, Motomura H, Obara H. Contemporary critical limb ischemia: Asian multidisciplinary consensus statement on the collaboration between endovascular therapy and wound care. Cardiovascular intervention and therapeutics. 2018 Oct:33(4):297-312. doi: 10.1007/s12928-018-0523-z. Epub 2018 Apr 13 [PubMed PMID: 29654408]
Level 3 (low-level) evidencevan Baal JG, Aan de Stegge WB, Schaper NC. [A multidisciplinary approach in diabetic foot disease is mandatory]. Nederlands tijdschrift voor geneeskunde. 2017:161():D1755 [PubMed PMID: 29057728]
Hartmann B, Fottner C, Herrmann K, Limbourg T, Weber MM, Beckh K. Interdisciplinary treatment of diabetic foot wounds in the elderly: Low risk of amputations and mortality and good chance of being mobile with good quality of life. Diabetes & vascular disease research. 2017 Jan:14(1):55-58 [PubMed PMID: 27941057]
Level 2 (mid-level) evidenceChan AS, Montbriand J, Eisenberg N, Roche-Nagle G. Outcomes of minor amputations in patients with peripheral vascular disease over a 10-year period at a tertiary care institution. Vascular. 2019 Feb:27(1):8-18. doi: 10.1177/1708538118797544. Epub 2018 Aug 29 [PubMed PMID: 30157719]
Prouse AF, Langner P, Plomondon ME, Ho PM, Valle JA, Barón AE, Armstrong EJ, Waldo SW. Temporal trends in the management and clinical outcomes of lower extremity arterial thromboembolism within a national Veteran population. Vascular medicine (London, England). 2019 Feb:24(1):41-49. doi: 10.1177/1358863X18793210. Epub 2018 Aug 14 [PubMed PMID: 30105938]
Level 2 (mid-level) evidenceAkagi D, Hoshina K, Akai A, Yamamoto K. Outcomes in Patients with Critical Limb Ischemia due to Arteriosclerosis Obliterans Who Did Not Undergo Arterial Reconstruction. International heart journal. 2018 Sep 26:59(5):1041-1046. doi: 10.1536/ihj.17-592. Epub 2018 Aug 11 [PubMed PMID: 30101855]