Introduction
Blood transfusion practices have evolved since the first attempt to administer the treatment in the 17th century. The therapy has transitioned from whole blood to component therapy, including packed red blood cells, platelets, white blood cells, frozen plasma, and plasma-derived products. Platelets' integral role in hemostasis became apparent in the 1950s and 1960s from studies of severe hemorrhagic complications in leukemia patients undergoing chemotherapy.[1]
Blood collection using glass bottles in the mid-20th century often resulted in platelet depletion during storage. The advent of plastic bags revolutionized blood storage, as plastic's gas permeability was found to be essential for preserving functional platelets. Separation technique advances enabled the development of apheresis for high-yield platelet component collection. Safety protocol enhancements have decreased adverse transfusion outcomes.[2] Ongoing efforts aim to improve blood transfusion safety measures further.[3]
Platelet concentrates are widely used for treating severe thrombocytopenia, which may occur in patients with hematologic malignancy, bone marrow failure, and other immune and nonimmune causes of platelet destruction. Transfusion with normal platelet counts is rare. Platelets are a scarce resource, partly because of their short shelf life of 5 days. Thus, the World Health Organization includes these blood products in its Essential Medicines list.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Platelets are anucleated discoid cells with diameters ranging from 2.0 to 5.0 μm, thickness of 0.5 μm, and mean cell volume ranging from 6 to 10 fL. Platelets are produced in the bone marrow through megakaryopoiesis from hematopoietic stem cells under the influence of thrombopoietin and other growth factors. An average of 1011 platelets are produced daily and released into circulation.[4] These platelets have an average life span of 8 to 10 days. The liver recognizes surface structural changes accumulated within that period, allowing the organ to clear senescent platelets from circulation.[5]
Platelets' main roles in hemostasis include adhesion to damaged endothelial tissue, secretion of mediators from granules to facilitate aggregation and coagulation, and contraction to retract the clot along with fibrin and other components. Platelets also engage in inflammation, wound healing, mitogenesis, and bolstering antimicrobial defenses.
Structurally, the platelet can be divided into 3 zones as follows:
- Peripheral zone: This zone is primarily involved in adhesion and aggregation. The important components of the peripheral zone include the glycocalyx, unit membrane, and submembrane area.
- The glycocalyx, a thick carbohydrate-rich structure found on the exterior surface of platelets, is the primary contact site during the hemostatic response, consisting of major and minor glycoproteins. The glycoprotein Ib-IX-V complex is involved in adhesion at the vascular injury site. Glycoprotein IIb-IIIa facilitates aggregation by binding to fibrinogen and linking platelets together. The unit membrane is comprised of a lipid bilayer and an open canalicular system. This component is critical in accelerating coagulation. The unit membrane uses phosphatidylserine, an anionic phospholipid present on activated platelet surfaces during clot initiation, to convert prothrombin to thrombin.
- The submembrane area facilitates signal transmission from the surface to the cytoplasmic organelles, regulating platelet activation.
- Sol-gel zone: The Sol-gel zone comprises microtubules and microfilaments, which are vital components for platelet structure and support. This region is responsible for various shape changes on activation during hemostasis and ex vivo storage. Organelles are embedded within this matrix.
- Organelle zone: This region contains alpha and dense granules, mitochondria, glycogen, lysosomes, and peroxisomes. Alpha granules store fibrinogen, factor V, von Willebrand factor, platelet-derived growth factor, cytokines, chemokines, transforming growth factor beta 1, and vascular endothelial growth factor. Dense granules store calcium, adenine nucleotides, polyphosphate, pyrophosphate, serotonin, and histamine.[6] Mitochondria are the powerhouses of platelets. Glycogen provides carbohydrates for fuel. Lysosomes and peroxisomes play vital roles in cellular defense mechanisms (see Image. An Overview of Platelet Structure).
Platelet activation triggers the release of substances from both alpha and dense granules, enhancing platelet activation and aggregation while exerting immune-mediated effects.[7]
Indications
Normal human platelet counts range from 150,000 to 450,000 cells/μL. Platelet transfusion is mainly indicated to treat or prevent bleeding in patients with thrombocytopenia or platelet function disorder.
Platelet Transfusion Thresholds in Bleeding Patients
Platelet transfusion thresholds guide transfusion decisions based on severity and clinical context. The treatment is indicated in the following bleeding cases:
- Less than 50,000 cells/μL with severe bleeding, including disseminated intravascular coagulation
- Less than 30,000 cells/μL when bleeding is not life-threatening or considered severe
- Less than 100,000 cells/μL for bleeding in the context of multiple trauma or intracranial bleeding [8]
Adherence to platelet transfusion thresholds, tailored to bleeding severity and the circumstances, optimizes patient care and outcomes.
Prophylactic Transfusion Thresholds
Platelet transfusion is recommended when the platelet count falls below a certain threshold, either as a preventive measure before specific procedures or to prevent spontaneous bleeding. The prophylactic transfusion thresholds are outlined in the table below.
Specific Prophylactic Transfusion Thresholds
| Clinical Context | Platelet Transfusion Threshold |
| Spontaneous bleeding prevention |
<10,000 cells/μL Some authors recommend a threshold of 5000 cells/μL. |
|
Before neurosurgery or ocular surgery |
<100,000 cells/μL |
| Before major surgery | <50,000 cells/μL |
| Disseminated intravascular coagulation | <50,000 cells/μL |
| Before central line placement | <20,000 cells/μL [9] |
| Before epidural anesthesia | <80,000 cells/μL |
| Before bronchoalveolar lavage | <20,000-30,000 cells/μL [10] |
| Before endoscopic procedures |
<50,000 cells/μL for therapeutic procedures <20,000 cells/μL for low-risk diagnostic procedures |
| During parturition |
<30,000 cells/μL for a vaginal delivery <50,000 cells/μL for a cesarean section [11] |
| Before lumbar puncture |
<10,000-20,000 cells/μL in hematologic malignancies <40,000-50,000 cells/μL if without a hematologic malignancy |
Platelet transfusion is not routinely indicated before bone marrow biopsy, peripheral or central catheter insertion, traction removal of tunneled central venous catheters, and cataract removal.[12]
Platelet Transfusion in Specific Settings
Platelet transfusion practices vary across specific clinical settings. Transfusion is typically avoided in idiopathic thrombocytopenic purpura unless severe bleeding occurs. In malignancy and chemotherapy, platelet transfusion thresholds generally adhere to the levels mentioned above, except in acute promyelocytic leukemia, where increased bleeding risk necessitates transfusion at less than 30,000 cells/μL. Chemotherapy is carried out at counts less than 20,000 cells/μL.
Patients undergoing cardiac surgery with perioperative bleeding and thrombocytopenia are suitable candidates for platelet transfusions. Prophylactic transfusion is not recommended for individuals without thrombocytopenia undergoing cardiopulmonary bypass.[13]
Inherited and acquired platelet disorders warrant transfusion only in the presence of bleeding symptoms. Glanzmann's thrombasthenia and Bernard-Soulier syndrome are examples of congenital platelet disorders. Acquired types arise in the presence of conditions such as uremia and drug-induced platelet dysfunction.
Pediatric transfusion guidelines largely align with adult recommendations, as demonstrated by the PLADO study, with exceptions for neonates and infants based on clinical stability and specific criteria such as birth weight and age. Platelet transfusion is administered to clinically stable neonates when their platelet count falls below 30,000 cells/μL. The threshold is 50,000 cells/μL for neonates with birth weights under 1500 g and who are younger than 7 days old and infants who are clinically unstable, undergoing surgical procedures, and experiencing significant hemorrhage or concurrent coagulopathy.[14] Infants undergoing extracorporeal membrane oxygenation may receive platelet transfusion at platelet counts below 100,000 cells/μL.
In the PlaNeT-2 randomized controlled trial, platelet transfusion thresholds of 50,000 cells/μL versus 25,000 cells/μL were compared in preterm infants born at less than 34 weeks of gestational age. The study found a higher rate of major bleeding or death in the group receiving platelet transfusion with a threshold of 50,000 cells/μL compared to the group with a threshold of 25,000 cells/μL.[15]
Contraindications
The only widely accepted platelet transfusion contraindication is thrombotic thrombocytopenic purpura due to its heightened thrombosis risk. However, studies on outcomes and mortality show mixed results.[16][17] Platelet transfusion is strictly reserved for instances of life-threatening bleeding.
Platelet transfusion in the context of heparin-induced thrombocytopenia was previously believed to elevate thrombosis risk, but recent studies have shown no such correlation.[18] Platelet transfusion in heparin-induced thrombocytopenia is reserved only before or during a procedure or in the presence of severe bleeding. Prophylactic transfusion is not recommended for patients with heparin-induced thrombocytopenia.
Equipment
Platelets are transfused at the bedside through intravenous tubing with an in-line filter. This filter typically has a pore size of 170 to 260 μm, allowing it to effectively trap fibrin clots and large debris. The tubing may be primed with normal saline or the blood product itself. Emergency necessities, including 0.9% normal saline, oxygen, and anaphylaxis medications, should be available in case of a transfusion reaction.
Personnel
Platelet transfusion requires the collaboration of professionals from various disciplines, including clinicians to ancillary medical services. The clinician assesses the patient's condition and determines the need for platelet transfusion, issuing the necessary orders. The actual administration of the platelets is often carried out by a transfusionist, who may be a nurse specially trained in transfusion procedures. The transfusionist verifies the patient's identity and matches the units before the therapy starts.
Preparation
Preparation for platelet transfusion starts with producing quality-approved platelet concentrates in blood banks. Platelet concentrates may be prepared from whole blood or by apheresis. The usual dose in adults for prophylactic therapy is 4 to 6 units of whole blood unit–derived platelets, which is equal to one apheresis platelet unit. This dose contains 3 to 4×1011 platelets.[13] The usual pediatric dose is 5 to 10 mL/kg. The shelf life of platelet concentrates is 5 days.
The blood bank verifies the clinician's transfusion request alongside the pretransfusion sample, confirming ABO and Rh blood grouping. Platelet concentrates are issued based on demand specified in the blood request form. Group-specific platelets are typically recommended, but cross-group transfusion may be allowed if necessary, particularly in emergencies such as traumatic bleeding. Serologic crossmatching is rarely required unless platelet concentrates have high red blood cell content.
Patient consent must be obtained before sending a request to the blood bank. The intravenous line must be prepared before the blood bank issues the platelet concentrates. The issue counter staff checks for details before issuing the units, including patient identification, unit numbers, blood group, and abnormal appearance or clumps suggestive of infection in the platelet bag.
Clinicians should indicate on the blood request forms if a patient requires special blood product procedures. For example, leukoreduction minimizes human leukocyte antigen alloimmunization and cytomegalovirus transmission. Irradiation prevents transfusion-associated graft-versus-host disease.
Technique or Treatment
The patient should have an appropriate intravenous cannula ranging from 14 to 26 gauge. An 18- to 22-gauge cannula is typically used in adults, whereas pediatric patients typically require 25- to 26-gauge cannulas. Transfusion may also proceed intraosseously in rare cases where intravenous access cannot be established.
The transfusionist records the patient's pretransfusion vital signs. The platelet concentrate bag is then connected by aseptically spiking the blood transfusion set to the intravenous line. A standard blood transfusion set with an inline filter pore size of 170 to 260 μm is used. Transfusion is generally administered over 30 to 60 minutes but may be faster if necessary. Slower flow rates are used in patients at risk of fluid overload. The patient is closely monitored during the transfusion, with the vitals recorded every 15 minutes. If a transfusion reaction is suspected at any point, the transfusion is disrupted immediately, and the appropriate management protocol is initiated.
Complications
Platelet transfusion may precipitate benign to life-threatening complications.[19] These adverse effects and the corresponding management approaches are explained below.
Febrile Nonhemolytic Transfusion Reaction
Febrile nonhemolytic transfusion reaction is a relatively common complication of platelet transfusion, with a frequency of 0.09% to 27%.[20][21] According to the revised 2021 criteria from the Centers for Disease Control and Prevention (CDC) Biovigilance Network, definitive febrile nonhemolytic transfusion reaction is characterized by a reaction occurring during or within 4 hours of transfusion cessation, with no other attributable causes, marked by fever (≥38 °C or 100.4 °F oral, with a ≥1 °C or 1.8 °F change from baseline) or chills and rigors.
Nonimmune-mediated febrile nonhemolytic transfusion reaction arises from exposure to white blood cell cytokines accumulating during product storage.[22] Biological response modifiers such as sCD40L, interleukin-6 (IL-6), IL-7, IL-8, IL-27, and transforming growth factor-β may be released during platelet storage. Elevated biological response modifier concentrations are typically observed after prolonged storage, particularly from the third day onward, potentially correlating with recipient adverse events.[23]
Immune-mediated febrile nonhemolytic transfusion reaction results from endogenous pyrogen release from the donor or recipient's white blood cells due to a reaction between donor antigens and recipient antibodies.[24] Antibodies targeting human leukocyte antigens or leukocyte antigens in donor plasma are most commonly implicated. This interaction leads to donor white cell antigens binding with recipient antibodies, triggering the release of pyrogens and cytokines such as TNF-α, IL-1, and IL-6.
Febrile nonhemolytic transfusion reaction is a diagnosis of exclusion, determined based on specific characteristics and by excluding other causes of fever through additional investigations and laboratory findings. Febrile nonhemolytic transfusion reaction risk can be reduced by 50% through prestorage leukodepletion.[25] Platelets stored in platelet additive solution replace most of the plasma, reducing allergen content and the likelihood of reactions.[26]
Management includes stopping the transfusion, obtaining lab tests to rule out a hemolytic transfusion reaction, evaluating other fever causes, and administering antipyretics and meperidine or pethidine for chills and rigors.[22] Premedications aiming at preventing febrile nonhemolytic transfusion reactions have shown no benefit.[27]
Allergic and Anaphylactic Transfusion Reactions
An allergic reaction is one of the most common transfusion reactions, with a 1% to 3% incidence. This type 1 hypersensitivity reaction manifests as pruritus, hives or urticaria, or localized angioedema within 4 hours of transfusion. Pathogenesis is heterogeneous in origin, with donor, product, and recipient factors impacting the condition. Plasma proteins commonly contribute to the etiology. Others include antibodies, cytokines, and biological response modifiers released during storage. Management includes transfusion cessation and H1-antihistamine administration. Famotidine, hydroxyzine, or methylprednisolone may be used for severe allergic reactions refractory to H1-antihistamines. Allergic reactions may be minimized by depleting plasma in platelet preparations.
The reported incidence of anaphylactic reactions is 1 in 30,000 to 1 in 42,000 per unit of blood product transfused. The condition may occur in patients with immunoglobulin A (IgA) deficiency, as anti-IgA antibodies target IgA-containing platelets. Anaphylactic transfusion reactions develop rapidly. Patients present with hypotension, shock, angioedema, and bronchospasm, leading to respiratory distress and wheezing. Anaphylaxis is a medical emergency requiring prompt evaluation and treatment. This transfusion reaction may be prevented by administering platelets that are washed or obtained from IgA-deficient donors.[28][29]
Transfusion-Associated Graft-versus-Host Disease
Transfusion-associated graft-versus-host disease is a rare but fatal complication characterized by fever and systemic manifestations affecting organs such as the skin, gastrointestinal tract, liver, and bone marrow. The condition arises from transfused viable lymphocytes thriving in the recipient's body, leading to tissue attack. Transfusion-associated graft-versus-host disease occurs within 1 to 6 weeks post-transfusion, primarily affecting hosts with immunosuppression, congenital T-cell defects, low genetic diversity (homozygous human leukocyte antigen) issues, or first-degree relatives with similar episodes. Diagnosis relies on exclusion, molecular testing, and microscopy. Prevention is achieved through platelet irradiation.[30]
Transfusion-Related Acute Lung Injury
Transfusion-related acute lung injury is a life-threatening complication that presents as acute lung injury with hypoxemia (PaO2/FiO2 ≤300 mm Hg or oxygen saturation <90% on room air) during transfusion or within 6 hours of cessation with no evidence of acute lung injury before the therapy.[31] Radiographic evidence comprises bilateral lung infiltrates without circulatory overload.
Neutrophils have been considered crucial in transfusion-related acute lung injury development. Neutrophil activation releases reactive oxygen species, resulting in pulmonary endothelial cell damage and lung leak.[32] Factors activating host neutrophils may include blood product antibodies targeting recipient antigens or bioactive lipids that can potentially trigger neutrophil activation. All other acute lung injury causes must be ruled out before considering transfusion-related acute lung injury. This reaction may be averted by reducing plasma in platelets and resuspending in a platelet additive solution. Some centers test selected platelet donors, such as multiparous females, for anti-human leukocyte antigen antibodies.
Posttransfusion Purpura
Posttransfusion purpura is a rare transfusion reaction manifesting as severe thrombocytopenia, which may cause symptoms such as purpura, petechiae, and bleeding. Thrombocytopenia due to passive antiplatelet antibody transfer typically begins within hours and resolves within a few days.[33] Posttransfusion purpura resulting from an alloantigen on transfused platelets typically starts around 5 to 12 days posttransfusion, with the associated thrombocytopenia potentially persisting for days to weeks. Posttransfusion purpura may be diagnosed by demonstrating alloantibodies directed against human platelets or other platelet-specific antigens. Intravenous immunoglobulin 1 to 2 g/kg/d for 2 to 5 days is the preferred treatment for posttransfusion purpura. Patients typically respond favorably within 2 days of intravenous immunoglobulin treatment.[34]
Transfusion-Related Immunomodulation
Leukocytes and biological response modifiers released during storage have been implicated in immunomodulation. The condition leads to a modified response of T-helper cell types 1 and 2, predisposing the patient to infection and delayed postsurgical recovery. Transfusion-related immunomodulation may also be prevented by prestorage leukoreduction and depleting plasma from platelets.[35]
Platelet Refractoriness
Platelet refractoriness occurs when posttransfusion platelet recovery is not as expected. The condition may arise from various causes, ranging from immune to nonimmune, although a multi-transfused patient often develops antibodies against specific human platelets or human leukocyte antigens. The antibodies trigger the immune system to destroy donor platelets. Platelet crossmatching can reduce platelet refractoriness. Human leukocyte antigen–matched platelets or a matching human platelet antigen is recommended, especially if an anti-human platelet antibody is involved.
Transfusion-Associated Circulatory Overload
The CDC Biovigilance Network in the United States defines definitive transfusion-associated circulatory overload as a new episode or exacerbation of 3 or more of the following within 12 hours of transfusion cessation:
- Evidence of acute or worsening respiratory distress
- Radiographic or clinical evidence of acute or worsening pulmonary edema
- Elevated brain natriuretic peptide or N-terminal pro-brain natriuretic peptide relevant biomarker
- Evidence of cardiovascular system changes unexplained by any underlying medical condition, such as elevated central venous pressure; evidence of left heart failure, including the development of tachycardia; hypertension; widened pulse pressure; jugular venous distension; enlarged cardiac silhouette; or peripheral edema
- Evidence of fluid overload
Transfusion-associated circulatory overload is a multifactorial condition. Patients with preexisting cardiac and renal dysfunction are at an increased risk.[36] Management includes transfusion disruption, oxygen supplementation hypoxemia, diuretic administration, and, if necessary, ventilatory support.
Physical Injury
Physical injuries may arise from intravenous cannulation-related trauma. Potential complications may include hematoma or nerve injury.
Sepsis and Bacterial Infection
Bacterial infection or sepsis in patients receiving platelet transfusion may be categorized as possible or definitive. Possible cases include individuals with unexplained clinical illness temporally linked to transfusion, with no detected pathogen after ruling out other adverse reactions. Definitive cases involve laboratory confirmation of pathogen presence in recipients. Severity ranges from requiring medical intervention with no permanent organ damage to life-threatening conditions. Staphylococcus and Streptococcus species are the most common pathogens.[37]
Infections may develop from donor phlebotomy site issues, such as improper cleansing, or asymptomatic bacteremia. Equipment or collection set contamination and collection bag damage are other sources. The documented incidence of bacterial contamination in platelets varies from 1 in 1000 to 1 in 2500 units.[38] Prevention includes adequate donor screening and sterile technique adherence during collection. Leukoreduction may decrease transfusion-related infection rates. Pathogen reduction technology helps minimize infections in plasma and platelets.[39]
Viral Infection Transmission
All blood donations are screened for HIV and hepatitis B and C viruses. Screening for other viruses may be indicated, depending on geographical location. Residual transmission risk remains because not all infected donors test positive within the window period. The transmission of viruses that are not routinely screened in the blood bank is also possible, particularly emerging infections. Pathogen reduction technology has been used to reduce this risk but is ineffective against all viruses.
Hypotensive Reaction
This condition is characterized by hypotension developing during transfusion or within an hour after the transfusion is completed. All other adverse reactions presenting with hypotension should be excluded before confirming this diagnosis. Patients on angiotensin-converting enzyme inhibitors who receive bedside leukoreduction are at risk of hypotension. Exposure of the blood component to the filter's negatively charged surface produces vasoactive bradykinin-related peptides within 5 minutes of transfusion. Angiotensin-converting enzyme inhibitors prevent the metabolism of these peptides, leading to hypotension.[40] A detailed pretransfusion medication history is thus critical in preventing this adverse reaction.
Clinical Significance
Platelets are irreplaceable blood components due to their intricate functions. Platelet collection, processing, storage, and transfusion are interprofessional processes requiring skill and precision. Given transfusion therapy's associated risks, a thorough risk-versus-benefit assessment is crucial before requesting a platelet transfusion. The field of transfusion medicine is constantly evolving to minimize these risks and enhance patient outcomes.
Enhancing Healthcare Team Outcomes
Considering the scarcity of platelets, clinicians should reserve platelet requests for necessary cases. Special requests such as irradiation, leukoreduction, or plasma reduction should be discussed with transfusion specialists, as these modifications have specific indications and potentially burden the transfusion services staff. Regular blood usage audits enhance patient outcomes. Continuing technical and nursing staff education on transfusion safety may improve transfusion services and patient care. The healthcare team must remember that each transfusion carries additional potential risks for the patient.
Every country or region should coordinate with a hemovigilance network to record all adverse events. The data may be used as an information source for improving regional guidelines and transfusion policies. Establishing robust hemovigilance systems is crucial, especially in regions where they are lacking. Platelet transfusion guidelines have been formulated based on randomized controlled trials and meta-analyses and should be implemented in our clinical practice to enhance patient care.
Nursing, Allied Health, and Interprofessional Team Interventions
Nurses are integral to any clinical practice and are at the forefront of blood product transfusion. Adequate training and regular education are essential for maintaining transfusion safety and overcoming barriers to identifying errors or adverse events, ultimately ensuring optimal patient care.
Media
(Click Image to Enlarge)
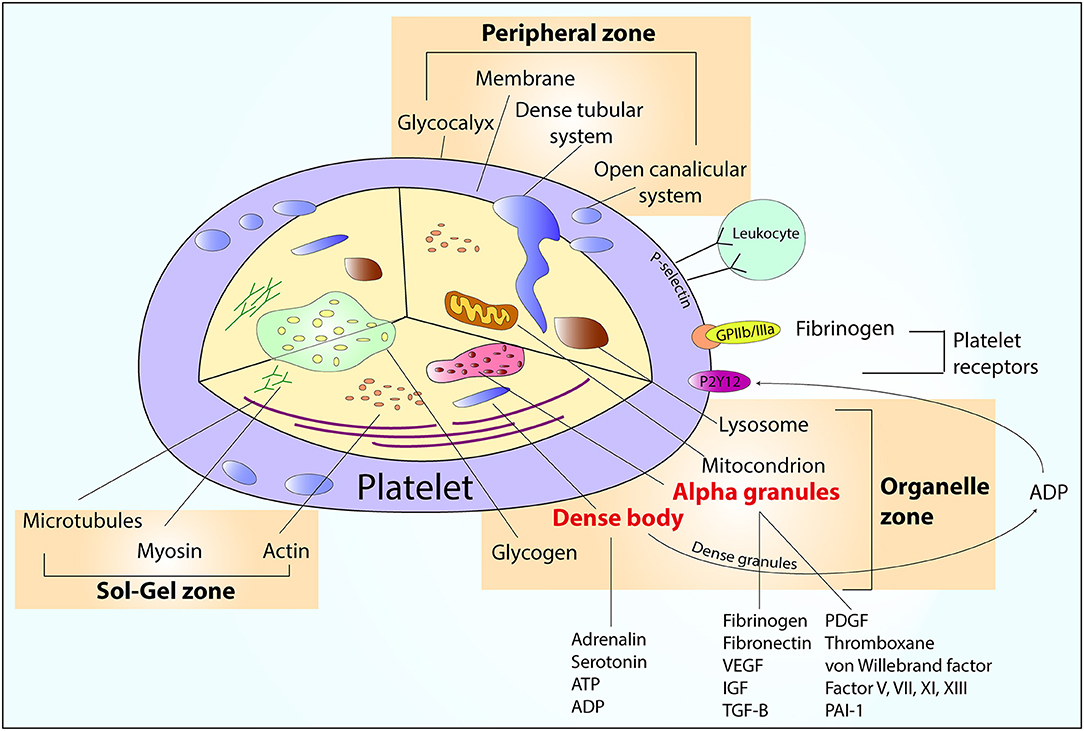
An Overview of Platelet Structure. The image shows the contents of the platelet, including peripheral, sol-gel, and organelle zones. Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; GP IIb/IIIa, glycoprotein IIb/IIIa; IGF, insulin-like growth factor; PAI-1, plasminogen activator inhibitor; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor.
Ostrowska M, Kubica J, Adamski P, et al. Stratified approaches to antiplatelet therapies based on platelet reactivity testing. Front Cardiovasc Med. 2019;6:176. doi: 10.3389/fcvm.2019.00176.
References
Freireich EJ. Origins of platelet transfusion therapy. Transfusion medicine reviews. 2011 Jul:25(3):252-6. doi: 10.1016/j.tmrv.2011.01.003. Epub 2011 Mar 2 [PubMed PMID: 21371858]
Level 3 (low-level) evidenceHeuft HG, Mende W, Blasczyk R. A general change of the platelet transfusion policy from apheresis platelet concentrates to pooled platelet concentrates is associated with a sharp increase in donor exposure and infection rates. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2008 Apr:35(2):106-13. doi: 10.1159/000117788. Epub 2008 Mar 10 [PubMed PMID: 21512637]
Solves Alcaina P. Platelet Transfusion: And Update on Challenges and Outcomes. Journal of blood medicine. 2020:11():19-26. doi: 10.2147/JBM.S234374. Epub 2020 Jan 24 [PubMed PMID: 32158298]
Kaushansky K. The molecular mechanisms that control thrombopoiesis. The Journal of clinical investigation. 2005 Dec:115(12):3339-47 [PubMed PMID: 16322778]
Level 3 (low-level) evidenceThon JN, Italiano JE. Platelets: production, morphology and ultrastructure. Handbook of experimental pharmacology. 2012:(210):3-22. doi: 10.1007/978-3-642-29423-5_1. Epub [PubMed PMID: 22918725]
Level 3 (low-level) evidenceSharda A, Flaumenhaft R. The life cycle of platelet granules. F1000Research. 2018:7():236. doi: 10.12688/f1000research.13283.1. Epub 2018 Feb 28 [PubMed PMID: 29560259]
Behnke O. The morphology of blood platelet membrane systems. Series haematologica (1968). 1970:3(4):3-16 [PubMed PMID: 4107203]
Yan M, Lin Y, Callum J. British Committee for Standards in Haematology guidelines for aplastic anaemia: single centre retrospective review finds no compelling evidence for the recommended higher platelet count threshold of 20 × 10(9) /l. British journal of haematology. 2018 Jul:182(2):284-286. doi: 10.1111/bjh.14767. Epub 2017 Jul 5 [PubMed PMID: 28677840]
Level 2 (mid-level) evidenceZeidler K, Arn K, Senn O, Schanz U, Stussi G. Optimal preprocedural platelet transfusion threshold for central venous catheter insertions in patients with thrombocytopenia. Transfusion. 2011 Nov:51(11):2269-76. doi: 10.1111/j.1537-2995.2011.03147.x. Epub 2011 Apr 22 [PubMed PMID: 21517892]
Nandagopal L, Veeraputhiran M, Jain T, Soubani AO, Schiffer CA. Bronchoscopy can be done safely in patients with thrombocytopenia. Transfusion. 2016 Feb:56(2):344-8. doi: 10.1111/trf.13348. Epub 2015 Oct 7 [PubMed PMID: 26446048]
Cines DB, Levine LD. Thrombocytopenia in pregnancy. Hematology. American Society of Hematology. Education Program. 2017 Dec 8:2017(1):144-151. doi: 10.1182/asheducation-2017.1.144. Epub [PubMed PMID: 29222249]
Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, Mumford AD, Stanworth SJ, Tinegate H, British Committee for Standards in Haematology. Guidelines for the use of platelet transfusions. British journal of haematology. 2017 Feb:176(3):365-394. doi: 10.1111/bjh.14423. Epub 2016 Dec 23 [PubMed PMID: 28009056]
Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, Cipolle MD, Cohn CS, Fung MK, Grossman BJ, Mintz PD, O'Malley BA, Sesok-Pizzini DA, Shander A, Stack GE, Webert KE, Weinstein R, Welch BG, Whitman GJ, Wong EC, Tobian AA, AABB. Platelet transfusion: a clinical practice guideline from the AABB. Annals of internal medicine. 2015 Feb 3:162(3):205-13. doi: 10.7326/M14-1589. Epub [PubMed PMID: 25383671]
Level 1 (high-level) evidenceSparger K, Deschmann E, Sola-Visner M. Platelet Transfusions in the Neonatal Intensive Care Unit. Clinics in perinatology. 2015 Sep:42(3):613-23. doi: 10.1016/j.clp.2015.04.009. Epub 2015 May 27 [PubMed PMID: 26250921]
Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C, Deary A, Hodge R, Hopkins V, Lopez Santamaria B, Mora A, Llewelyn C, D'Amore A, Khan R, Onland W, Lopriore E, Fijnvandraat K, New H, Clarke P, Watts T, PlaNeT2 MATISSE Collaborators. Randomized Trial of Platelet-Transfusion Thresholds in Neonates. The New England journal of medicine. 2019 Jan 17:380(3):242-251. doi: 10.1056/NEJMoa1807320. Epub 2018 Nov 2 [PubMed PMID: 30387697]
Level 1 (high-level) evidencePeigne V, Perez P, Resche Rigon M, Mariotte E, Canet E, Mira JP, Coppo P, Veyradier A, Azoulay E. Causes and risk factors of death in patients with thrombotic microangiopathies. Intensive care medicine. 2012 Nov:38(11):1810-7. doi: 10.1007/s00134-012-2638-5. Epub 2012 Jul 14 [PubMed PMID: 22797353]
Level 2 (mid-level) evidenceGoel R, Ness PM, Takemoto CM, Krishnamurti L, King KE, Tobian AA. Platelet transfusions in platelet consumptive disorders are associated with arterial thrombosis and in-hospital mortality. Blood. 2015 Feb 26:125(9):1470-6. doi: 10.1182/blood-2014-10-605493. Epub 2015 Jan 14 [PubMed PMID: 25588677]
Watson H, Davidson S, Keeling D, Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. British journal of haematology. 2012 Dec:159(5):528-40. doi: 10.1111/bjh.12059. Epub 2012 Oct 9 [PubMed PMID: 23043677]
Kiefel V. Reactions Induced by Platelet Transfusions. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2008:35(5):354-358 [PubMed PMID: 21512624]
Geiger TL, Howard SC. Acetaminophen and diphenhydramine premedication for allergic and febrile nonhemolytic transfusion reactions: good prophylaxis or bad practice? Transfusion medicine reviews. 2007 Jan:21(1):1-12 [PubMed PMID: 17174216]
Cohen R, Escorcia A, Tasmin F, Lima A, Lin Y, Lieberman L, Pendergrast J, Callum J, Cserti-Gazdewich C. Feeling the burn: the significant burden of febrile nonhemolytic transfusion reactions. Transfusion. 2017 Jul:57(7):1674-1683. doi: 10.1111/trf.14099. Epub 2017 Mar 28 [PubMed PMID: 28369916]
Goel R, Tobian AAR, Shaz BH. Noninfectious transfusion-associated adverse events and their mitigation strategies. Blood. 2019 Apr 25:133(17):1831-1839. doi: 10.1182/blood-2018-10-833988. Epub 2019 Feb 26 [PubMed PMID: 30808635]
Cognasse F, Duchez AC, Audoux E, Ebermeyer T, Arthaud CA, Prier A, Eyraud MA, Mismetti P, Garraud O, Bertoletti L, Hamzeh-Cognasse H. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Frontiers in immunology. 2022:13():825892. doi: 10.3389/fimmu.2022.825892. Epub 2022 Feb 3 [PubMed PMID: 35185916]
Addas-Carvalho M, Salles TS, Saad ST. The association of cytokine gene polymorphisms with febrile non-hemolytic transfusion reaction in multitransfused patients. Transfusion medicine (Oxford, England). 2006 Jun:16(3):184-91 [PubMed PMID: 16764597]
King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004 Jan:44(1):25-9 [PubMed PMID: 14692963]
van Hout FMA, van der Meer PF, Wiersum-Osselton JC, Middelburg RA, Schipperus MR, van der Bom JG, Kerkhoffs JL. Transfusion reactions after transfusion of platelets stored in PAS-B, PAS-C, or plasma: a nationwide comparison. Transfusion. 2018 Apr:58(4):1021-1027. doi: 10.1111/trf.14509. Epub 2018 Feb 6 [PubMed PMID: 29405304]
Ning S, Solh Z, Arnold DM, Morin PA. Premedication for the prevention of nonhemolytic transfusion reactions: a systematic review and meta-analysis. Transfusion. 2019 Dec:59(12):3609-3616. doi: 10.1111/trf.15566. Epub 2019 Oct 31 [PubMed PMID: 31670424]
Level 1 (high-level) evidenceTobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011 Aug:51(8):1676-83. doi: 10.1111/j.1537-2995.2010.03008.x. Epub 2011 Jan 7 [PubMed PMID: 21214585]
Vassallo RR. Review: IgA anaphylactic transfusion reactions. Part I. Laboratory diagnosis, incidence, and supply of IgA-deficient products. Immunohematology. 2004:20(4):226-33 [PubMed PMID: 15679454]
Manduzio P. Transfusion-associated graft-versus-host disease: A concise review. Hematology reports. 2018 Nov 6:10(4):7724. doi: 10.4081/hr.2018.7724. Epub 2018 Nov 6 [PubMed PMID: 30542528]
Semple JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. 2019 Apr 25:133(17):1840-1853. doi: 10.1182/blood-2018-10-860809. Epub 2019 Feb 26 [PubMed PMID: 30808638]
Tung JP, Chiaretti S, Dean MM, Sultana AJ, Reade MC, Fung YL. Transfusion-related acute lung injury (TRALI): Potential pathways of development, strategies for prevention and treatment, and future research directions. Blood reviews. 2022 May:53():100926. doi: 10.1016/j.blre.2021.100926. Epub 2022 Jan 5 [PubMed PMID: 35065815]
Pavenski K, Webert KE, Goldman M. Consequences of transfusion of platelet antibody: a case report and literature review. Transfusion. 2008 Sep:48(9):1981-9. doi: 10.1111/j.1537-2995.2008.01796.x. Epub 2008 Jun 28 [PubMed PMID: 18564398]
Level 3 (low-level) evidenceHawkins J, Aster RH, Curtis BR. Post-Transfusion Purpura: Current Perspectives. Journal of blood medicine. 2019:10():405-415. doi: 10.2147/JBM.S189176. Epub 2019 Dec 9 [PubMed PMID: 31849555]
Level 3 (low-level) evidenceVamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood reviews. 2007 Nov:21(6):327-48 [PubMed PMID: 17804128]
Alam A, Lin Y, Lima A, Hansen M, Callum JL. The prevention of transfusion-associated circulatory overload. Transfusion medicine reviews. 2013 Apr:27(2):105-12. doi: 10.1016/j.tmrv.2013.02.001. Epub 2013 Mar 1 [PubMed PMID: 23465703]
Haass KA, Sapiano MRP, Savinkina A, Kuehnert MJ, Basavaraju SV. Transfusion-Transmitted Infections Reported to the National Healthcare Safety Network Hemovigilance Module. Transfusion medicine reviews. 2019 Apr:33(2):84-91. doi: 10.1016/j.tmrv.2019.01.001. Epub 2019 Jan 25 [PubMed PMID: 30930009]
Levy JH, Neal MD, Herman JH. Bacterial contamination of platelets for transfusion: strategies for prevention. Critical care (London, England). 2018 Oct 27:22(1):271. doi: 10.1186/s13054-018-2212-9. Epub 2018 Oct 27 [PubMed PMID: 30367640]
Barrett BB, Andersen JW, Anderson KC. Strategies for the avoidance of bacterial contamination of blood components. Transfusion. 1993 Mar:33(3):228-33 [PubMed PMID: 8438224]
Mair B, Leparc GF. Hypotensive reactions associated with platelet transfusions and angiotensin-converting enzyme inhibitors. Vox sanguinis. 1998:74(1):27-30 [PubMed PMID: 9481857]