Introduction
Pneumonia has been defined as an infection of the lung parenchyma. Rather than looking at it as a single disease, health care professionals must remember that pneumonia is an umbrella term for a group of syndromes caused by a variety of organisms resulting in varied manifestations and sequelae.[1]
There have been many attempts to classify pneumonia based on the etiology, clinical setting in which the patent acquired the infection, and the pattern of involvement of lung parenchyma, among other classifications. This article reviews pneumonia based on the classification followed by the American Thoracic Society.
Community-Acquired Pneumonia (CAP)
Any pneumonia acquired outside of a hospital in a community setting.[2]
Hospital-Acquired Pneumonia (HAP)
Any pneumonia acquired 48 hours after being admitted in an inpatient setting such as a hospital and not incubating at the time of admission is considered as HAP. This classification helps clear the confusion surrounding the terms healthcare-associated and hospital-acquired pneumonia. Now all pneumonia acquired in the setting of assisted-living facilities, rehabilitation facilities, and other healthcare facilities have been included under community-acquired pneumonia, and a hospital setting is necessary for classifying pneumonia as HAP.[3]
Ventilator Associated Pneumonia (VAP)
Any pneumonia acquired 48 hours after endotracheal intubation is considered as VAP.[4]
These categories have helped establish the common organisms responsible for each type of pneumonia and helped to formulate treatment guidelines for the efficient management in both in-patient and out-patient setting.
Depending on the pattern of involvement, pneumonia has historically also been studied as:
- Focal non-segmental or lobar pneumonia: involvement of a single lobe of the lung.
- Multifocal bronchopneumonia or lobular pneumonia
- Focal or diffuse interstitial pneumonia[5]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
While identifying an etiologic agent for pneumonia is essential for effective treatment as well as epidemiological record keeping, this is seldom seen in clinical practice. Widespread reviews have shown that a single cause of pneumonia has often been identified in less than 10% of patients presenting to the emergency department.[6] Nonetheless, the most common organisms causing pneumonia can be studied under the headings mentioned earlier.
Community-Acquired Pneumonia
Bacterial causes
They have been classically studied under the subheadings "typical" and "atypical" organisms in terms of ease of culture positivity. Common typical organisms include Pneumococcus, Haemophilus influenzae, Moraxella catarrhalis, Group A Streptococcus, and other aerobic and anaerobic gram-negative organisms. Atypical organisms commonly seen in clinical practice include Legionella, Mycoplasma, Chlamydia, among others.[7] In the United States, the most common bacterial causes of CAP include Streptococcus pneumoniae, Staphylococcus aureus, Mycoplasma pneumoniae, and gram-negative enteric bacilli.[8]
Viral causes
It is often observed that viral species colonize nasopharynx of patients with CAP. Whether they are the primary cause or contribute to the pathogenesis by secondary bacterial causes is still being investigated. However, some of the most frequent viral agents implicated in CAP in the United States include influenza virus followed by respiratory syncytial virus, parainfluenza virus, and adenoviruses. [8]
Fungal causes
Fungal infections are usually implicated in patients with certain predisposing immunocompromised states like HIV and organ transplant recipients, among others. However, often overlooked, some fungal species can cause pneumonia in immunocompetent individuals which results in a delay in diagnosis and leads to unfavorable outcomes. The 3 commonest ones in North America include Histoplasma, Blastomyces, and Coccidioides. [9]
Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia
There is considerable overlap in the etiologic agents in non-ventilated hospitalized patients and ventilated patients with pneumonia, and it is, therefore, appropriate to consider them together. These include:
- Gram-negative bacilli like Escherichia coli, Pseudomonas Aerugenosa, Acinetobacter, and Enterobacter among others
- Gram-positive cocci like Staphylococcus aureus; both Methicillin-sensitive and resistant, although the latter is more prevalent[10][11]
- Other viruses and fungi that are more prevalent in immunocompromised and severely ill patients
Epidemiology
Pneumonia is a fairly prevalent disease and carries a heavy burden in all populations. A study carried out by the US Centers for Disease Control and Prevention (CDC) aimed at estimating its burden in North America found that CAP accounted for the eighth leading cause of mortality in the United States and the seventh leading cause of mortality in Canda after adjusting for various gender and age differences.[8] One of the largest studies over a period of 2 years in a Louisville population of 587,499 adults from 2014 to 2016 found that the annual age-adjusted incidence of CAP was 649 patients hospitalized per 100,000 adults (95% confidence interval, 628.2 to 669.8), corresponding to 1,591,825 annual adult CAP hospitalizations in the United States.[12] Moreover, the study found that the mortality during hospitalization was 6.5%, corresponding to 102,821 annual deaths in the United States. Mortality at 30 days, 6 months, and 1 year was 13.0%, 23.4%, and 30.6%, respectively. These indices were higher in economically weaker sections and in populations that were predominantly Hispanic or African-American.[12] The Community-Acquired Pneumonia Organization (CAPO) database formulated based on incidence in 16 countries clustered in 3 distinct areas, namely the United States/Canada, Europe, and Latin America found that the mortality rates in these regions were 7.3%, 9.1%, and 13.3% respectively.[13]
Data regarding incidence and prevalence of HAP and VAP are not extensive, largely because of the confounding factors related to patient comorbidities. Various estimates have proposed the incidence of VAP is about 2 to 16 episodes per 1000 ventilator days with an attributable mortality of 3% to 17%.[14] The major concern in treating HAP and VAP resides in the high prevalence of multi-drug resistance in the implicated organisms isolated from such patients. The major risk factors to take into consideration while estimating the risk for drug resistance include patient comorbidities, recent receipt of antibiotics, functional status, and severity of illness.[15]
Pathophysiology
There is an intricate balance between the organisms residing in the lower respiratory tract and the local and systemic defense mechanisms (both innate and acquired) which when disturbed gives rise to inflammation of the lung parenchyma, i.e., pneumonia. Common defense mechanisms that are compromised in the pathogenesis of pneumonia include:
- Systemic defense mechanisms like humoral and complement-mediated immunity that is compromised in diseases like common variable immunodeficiency (CVID), X-linked agammaglobulinemia (inherited), and functional asplenia (acquired). Impaired cell-mediated immunity predisposes individuals to infection by intracellular organisms like viruses and organisms of low virulence like Pneumocystis pneumonia (PJP), fungal causes, among others
- The mucociliary clearance that is often impaired in cigarette smokers, post-viral state, Kartergerner syndrome, and other related conditions
- Impaired cough reflex seen in comatose patients, certain substances of abuse
- Accumulation of secretions as seen in cystic fibrosis or bronchial obstruction
The resident macrophages serve to protect the lung from foreign pathogens. Ironically, the inflammatory reaction triggered by these very macrophages is what is responsible for the histopathological and clinical findings seen in pneumonia. The macrophages engulf these pathogens and trigger signal molecules or cytokines like TNF-a, IL-8, and IL-1 that recruit inflammatory cells like neutrophils to the site of infection. They also serve to present these antigens to the T cells that trigger both cellular and humoral defense mechanisms, activate complement and form antibodies against these organisms. This, in turn, causes inflammation of the lung parenchyma and makes the lining capillaries "leaky," which leads to exudative congestion and underlines the pathogenesis of pneumonia.
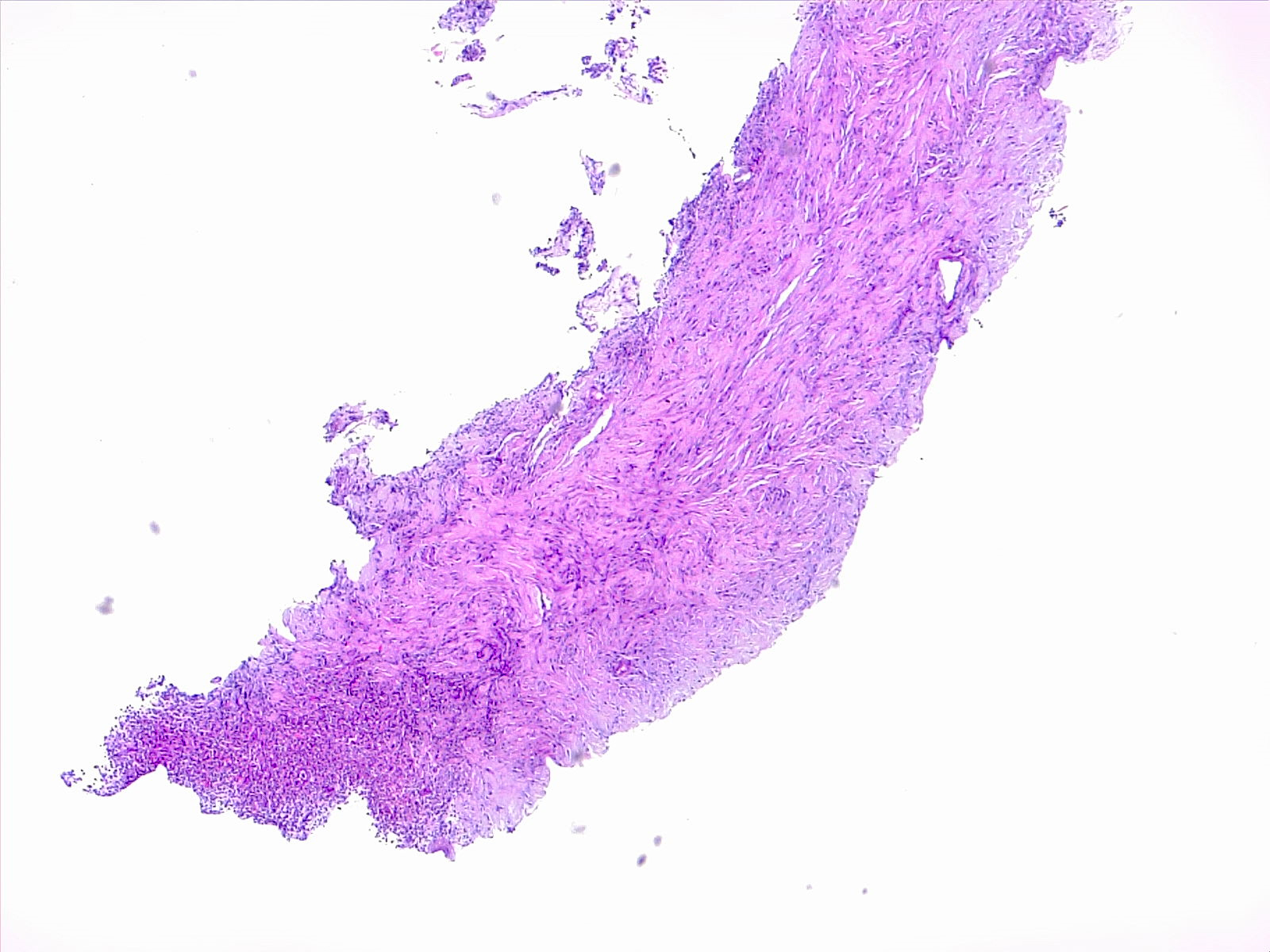
Histopathology
The histopathology in pneumonia can be broadly studied under 2 main headings: bronchopneumonia/lobular pneumonia or lobar pneumonia.
Lobar Pneumonia
Lobar pneumonia is diffuse consolidation involving the entire lobe of the lung. Its evolvement can be broken down into 4 stages as follows:
- Congestion: This stage is characterized by grossly heavy and boggy appearing lung tissue, diffuse congestion, vascular engorgement, and the accumulation of alveolar fluid rich in infective organisms. There are few red blood cells (RBC) and neutrophils at this stage.
- Red hepatization: Marked infiltration of red blood cells, neutrophils, and fibrin into the alveolar fluid is seen. Grossly, the lungs appear red and firm akin to a liver, hence the term hepatization.
- Gray hepatization: The RBC break down and is associated with fibrinopurulent exudates causing a red to gray color transformation.
- Resolution: Characterized by clearing of the exudates by resident macrophages with or without residual scar tissue formation.
Bronchopneumonia
Bronchopneumonia is characterized by suppurative inflammation localized in patches around bronchi which may or may not be localized to a single lobe of the lung.
Very rarely, severe forms of pneumonia may result in the formation of lung abscess, a complete breakdown of tissue and formation of pus-filled pockets in focal areas of the lung. Also, the infection may spread to the pleural space forming a fibrinopurulent exudate filling this space- known as empyema.
History and Physical
Historically, the chief complaints in case of pneumonia include systemic signs like fever with chills, malaise, loss of appetite, and myalgias. These findings are more common in viral pneumonia as compared to bacterial pneumonia. A small fraction of patients may have an altered mental status, abdominal pain, chest pain, and other systemic findings. Pulmonary findings include cough with or without sputum production. Bacterial pneumonia is associated with purulent or rarely blood-tinged sputum. Viral pneumonia is associated with watery or occasionally mucopurulent sputum production. There may be an associated pleuritic chest pain with the concomitant involvement of the pleura. Dyspnea and a diffuse heaviness of the chest are also seen occasionally.
Common findings on physical examination include:
- Tachypnea
- Tachycardia
- Fever with or without chills
- Decreased or bronchial breath sounds
- Egophony and tactile fremitus, both suggestive of a consolidative process
- Crackles on auscultation of the affected regions of the lung
- Dullness on percussion
Evaluation
Evaluation of CAP and HAP involves:
Clinical Evaluation
Involves performing a thorough history and physical examination as summarized in the section above.
Radiological Evaluation
According to the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guidelines, a demonstratable infiltrate by chest x-ray is necessary and is considered the best method (with supportive clinical findings) for the diagnosis of pneumonia.[2] Findings may vary from lobar to interstitial infiltrate, to occasionally cavitary lesions with air-fluid levels suggestive of a more severe disease process.
Laboratory Evaluation
These include a series of tests like blood culture, sputum culture and microscopy, routine blood counts, and lymphocyte count. Special tests such as urinary antigen testing, bronchial aspirate, or induced sputum may be used for certain pathogens. Two tests, procalcitonin and C-reactive protein help differentiate viral from bacterial causes when clinical and radiological findings may not be obvious. It is also noteworthy that empiric antibiotic treatment may be initiated in all typical cases of pneumonia, and the entire battery of tests is seldom needed.[2]
Evaluation of VAP, on the other hand, is a bit different from that of CAP. It requires radiological and microbiological evidence prior to initiation of antimicrobial therapy. VAP should be suspected in ventilated patients who have new onset dyspnea, fall in oxygen saturation on the same ventilator settings, fevers with chills or new onset lung infiltrates. All suspected patients require a chest x-ray (or a CT scan if x-ray findings are inconclusive). This must be followed by invasive sampling techniques like mini broncho-alveolar lavage (BAL) or bronchoscopic BAL or even protected specimen brush (PSB) to identify causal organisms. Once the diagnosis is confirmed, the appropriate antimicrobial therapy can be initiated.[16]
Treatment / Management
Management of CAP involves initial risk stratification of the patient and to decide whether to manage the patient on an outpatient basis, in a general medicine ward, or in an intensive care unit (ICU) setting. The "CURB-65" scale has been used extensively for this purpose. The components of this scale include confusion, uremia (BUN greater than 20 mg/dl), a respiratory rate greater than 30 per minute, blood pressure less than 90 mm Hg systolic or less than 60 mm Hg diastolic, and age greater than 65. One point is awarded for every positive criterion that the patient meets. Patient disposition is decided as follows.
- A score of 0 to 1: Outpatient management. These patients are treated empirically using Fluoroquinolones or Beta-lactams+ Macrolides if adverse comorbidities are present and with Macrolides or Doxycycline if no comorbidities are present.
- A score of 2 to 3 indicates admission and management in a general medicine ward. The first line of treatment is a choice between fluoroquinolones or macrolides plus beta-lactams.
- A score of 4 or more warrants management in an ICU. The empiric regimen, in this case, is a choice between a combination of a beta-lactam plus fluoroquinolones or beta-lactams plus macrolides.[17][2] (B2)
Management of VAP and HaP is in accordance with the ATS/IDSA guidelines. It is much more prolonged, complicated, and involves the use of broad-spectrum antibiotics as compared to the management of CAP. It involves early identification of signs of pneumonia and thorough evaluation as discussed above, before starting empiric therapy. Empiric therapy is guided by resistance patterns prevalent in that region as well as patient risk factors for multi-drug resistant organisms. Generally, regimes coving S. aureus, Pseudomonas, and gram-negative bacilli are designed for patients of HAP and VAP. For patients without MDR risk factors, the regimen generally followed is piperacillin/tazobactam plus cefepime plus levofloxacin. For patients with MDR risk factors, the preferred regime involves a combination of an Aminoglycoside along with one of imipenem, meropenem, aztreonam, piperacillin/tazobactam, ceftazidime, or cefepime.[3](A1)
Differential Diagnosis
Differential diagnosis of pneumonia includes asthma, chronic obstructive pulmonary disease (COPD), pulmonary edema, malignancies, non-infective consolidative processes of the lung, pleuritis, pulmonary embolism, aspiration of a foreign body, bronchiectasis, bronchiolitis, and others just to name a few. In case a differentiation becomes difficult, parameters like C-reactive protein, erythrocyte sedimentation rate, procalcitonin levels, leucocyte count, and temperature may be used to establish a diagnosis.[18]
Complications
Complications of untreated or under-treated pneumonia include respiratory failure, sepsis, metastatic infections, empyema, lung abscess, and multi-organ dysfunction.[7]
Enhancing Healthcare Team Outcomes
Pneumonia is a common cause of mortality and morbidity. It can have a myriad of clinical presentations and can pose a diagnostic dilemma especially in the setting of severely ill patients with several comorbidities and underlying lung pathologies. It is vital to have a strong interplay among physicians, the ICU team, nursing staff, pharmacists, and radiologists, functioning as an interprofessional team, to improve patient outcomes. The nursing staff plays a pivotal role in recording temperature variations and other vitals that the physician needs before arriving at a diagnosis. The nursing staff has an important role to play especially in the ICU setting by maintaining clean and hygienic ventilator settings and preventing aspiration among the patients. The pharmacist has to provide the right dose and the right drugs as prescribed by the clinician. Also, the pharmacy has a special role to play in the dosing of special antibiotics like vancomycin; the clinician may want to consult with a board-certified infectious disease pharmacist and review the latest antibiogram data to decide on which antimicrobial to use. The radiologists also take center stage because of the radiological findings in the different types of pneumonia vary considerably and require expert interpretation. The current guidelines for the management of CAP, VAP, and HAP are laid down by the American Thoracic Society in conjunction with the Infectious Diseases Society of America and are reviewed periodically.[2][3] [Level 1] With an interprofessional paradigm pneumonia outcomes for patients will be optimized, leading to a quicker recovery. [Level 5]
Media
(Click Image to Enlarge)
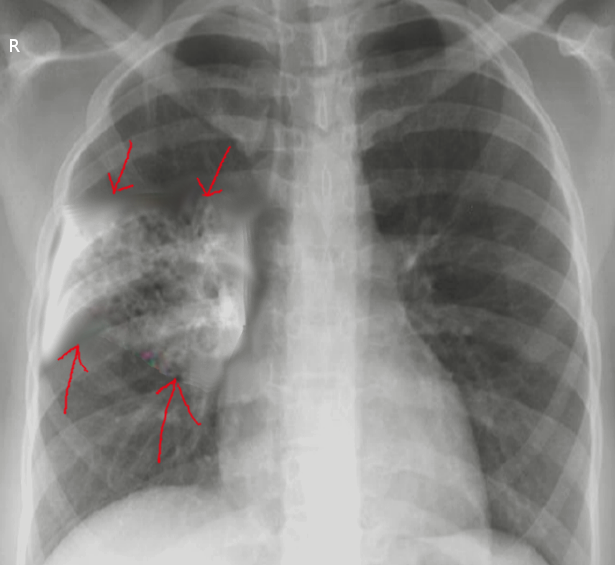
Ventilator-Associated Aspiration Pneumonia. Chest radiograph showing ventilator-associated aspiration pneumonia.
Melvil, Public Domain, via Wikimedia Commons.
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Mackenzie G. The definition and classification of pneumonia. Pneumonia (Nathan Qld.). 2016:8():14. doi: 10.1186/s41479-016-0012-z. Epub 2016 Aug 22 [PubMed PMID: 28702293]
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG, Infectious Diseases Society of America, American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007 Mar 1:44 Suppl 2(Suppl 2):S27-72 [PubMed PMID: 17278083]
Level 3 (low-level) evidenceKalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. Executive Summary: Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Sep 1:63(5):575-82. doi: 10.1093/cid/ciw504. Epub [PubMed PMID: 27521441]
Level 1 (high-level) evidenceCanadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. The New England journal of medicine. 2006 Dec 21:355(25):2619-30 [PubMed PMID: 17182987]
Level 1 (high-level) evidenceGharib AM, Stern EJ. Radiology of pneumonia. The Medical clinics of North America. 2001 Nov:85(6):1461-91, x [PubMed PMID: 11680112]
Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 May:52 Suppl 4():S296-304. doi: 10.1093/cid/cir045. Epub [PubMed PMID: 21460288]
Sattar SBA, Sharma S. Bacterial Pneumonia. StatPearls. 2023 Jan:(): [PubMed PMID: 30020693]
Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, Chappell JD, Qi C, Hart EM, Carroll F, Trabue C, Donnelly HK, Williams DJ, Zhu Y, Arnold SR, Ampofo K, Waterer GW, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, McCullers JA, Pavia AT, Edwards KM, Finelli L, CDC EPIC Study Team. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. The New England journal of medicine. 2015 Jul 30:373(5):415-27. doi: 10.1056/NEJMoa1500245. Epub 2015 Jul 14 [PubMed PMID: 26172429]
Level 2 (mid-level) evidenceHage CA, Knox KS, Wheat LJ. Endemic mycoses: overlooked causes of community acquired pneumonia. Respiratory medicine. 2012 Jun:106(6):769-76. doi: 10.1016/j.rmed.2012.02.004. Epub 2012 Mar 3 [PubMed PMID: 22386326]
Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infection control and hospital epidemiology. 2016 Nov:37(11):1288-1301 [PubMed PMID: 27573805]
Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010 Aug 1:51 Suppl 1():S81-7. doi: 10.1086/653053. Epub [PubMed PMID: 20597676]
Ramirez JA, Wiemken TL, Peyrani P, Arnold FW, Kelley R, Mattingly WA, Nakamatsu R, Pena S, Guinn BE, Furmanek SP, Persaud AK, Raghuram A, Fernandez F, Beavin L, Bosson R, Fernandez-Botran R, Cavallazzi R, Bordon J, Valdivieso C, Schulte J, Carrico RM, University of Louisville Pneumonia Study Group. Adults Hospitalized With Pneumonia in the United States: Incidence, Epidemiology, and Mortality. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Nov 13:65(11):1806-1812. doi: 10.1093/cid/cix647. Epub [PubMed PMID: 29020164]
Arnold FW, Wiemken TL, Peyrani P, Ramirez JA, Brock GN, CAPO authors. Mortality differences among hospitalized patients with community-acquired pneumonia in three world regions: results from the Community-Acquired Pneumonia Organization (CAPO) International Cohort Study. Respiratory medicine. 2013 Jul:107(7):1101-11. doi: 10.1016/j.rmed.2013.04.003. Epub 2013 May 6 [PubMed PMID: 23660396]
Level 2 (mid-level) evidenceBarbier F, Andremont A, Wolff M, Bouadma L. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Current opinion in pulmonary medicine. 2013 May:19(3):216-28. doi: 10.1097/MCP.0b013e32835f27be. Epub [PubMed PMID: 23524477]
Level 3 (low-level) evidenceShorr AF, Zilberberg MD, Reichley R, Kan J, Hoban A, Hoffman J, Micek ST, Kollef MH. Validation of a clinical score for assessing the risk of resistant pathogens in patients with pneumonia presenting to the emergency department. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 Jan 15:54(2):193-8. doi: 10.1093/cid/cir813. Epub 2011 Nov 21 [PubMed PMID: 22109951]
Level 2 (mid-level) evidenceTorres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, Li Bassi G, Luna CM, Martin-Loeches I, Paiva JA, Read RC, Rigau D, Timsit JF, Welte T, Wunderink R. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). The European respiratory journal. 2017 Sep:50(3):. pii: 1700582. doi: 10.1183/13993003.00582-2017. Epub 2017 Sep 10 [PubMed PMID: 28890434]
Mbata GC, Chukwuka CJ, Onyedum CC, Onwubere BJ. The CURB-65 scoring system in severity assessment of Eastern Nigerian patients with community-acquired pneumonia: a prospective observational study. Primary care respiratory journal : journal of the General Practice Airways Group. 2013 Jun:22(2):175-80. doi: 10.4104/pcrj.2013.00034. Epub [PubMed PMID: 23633130]
Level 2 (mid-level) evidenceCastro-Guardiola A, Armengou-Arxé A, Viejo-Rodríguez A, Peñarroja-Matutano G, Garcia-Bragado F. Differential diagnosis between community-acquired pneumonia and non-pneumonia diseases of the chest in the emergency ward. European journal of internal medicine. 2000 Dec 20:11(6):334-339 [PubMed PMID: 11113658]