Pulmonary Atresia With Intact Ventricular Septum
Pulmonary Atresia With Intact Ventricular Septum
Introduction
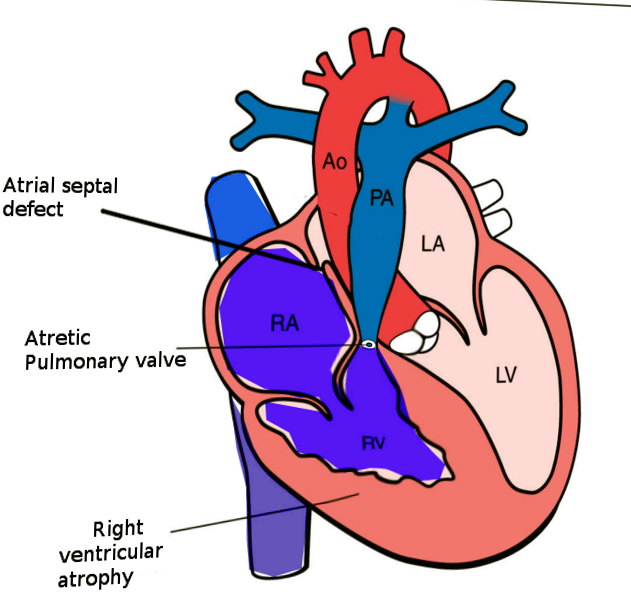
Pulmonary atresia with an intact ventricular septum (PA-IVS) is 1 of the rare forms of congenital heart disease (CHD), accounting for less than 1% of total heart defects (see Image. Pulmonary Atresia With Intact Ventricular Septum). Hunter first described this entity in 1783 and later by Peacock in 1869. As implied by the name, this disease is characterized by either membranous or long segment muscular atresia of the right ventricular outflow tract (RVOT) in the absence of communication at the level of ventricles. The spectrum of this disease ranges from simple membranous pulmonary atresia (PA) with normal-appearing right ventricle (RV) to hypoplastic RV with abnormal connections between the right ventricle and coronary arteries.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
An insult during the sensitive stages of embryological development is thought to cause this heart defect. However, the precise abnormality that leads to PA-IVS remains unclear. A few theories postulated to explain the pathogenesis of this disorder include the primary insult to the pulmonary valve leading to an atretic valve, abnormal venous valve limiting the flow of the flow through the tricuspid valve into the right ventricle, or abnormal coronary arterial development as a result.
Epidemiology
PA-IVS is the third most common form of cyanotic CHD, with incidence varying anywhere between 4 to 8 per 100000 live births. In the United Kingdom and Eire collaborative study published in 1998, the incidence of this disorder was reported to be close to 4 per 100000 live births.[1] In another study published in the United States in 1984, the incidence of PA-IVS was close to 8 per 100000 live births.[2]
With the widespread availability and use of fetal echocardiography, the number of patients with complex forms of CHD, including PA-IVS, being diagnosed during pregnancy across the world is increasing. This fact has not only helped the physicians with planning the care of these neonates ahead of time but also increased the rates of elective termination of pregnancies following the diagnosis of complex CHD. This elective abortion of pregnancies with complex CHD has led to an overall reduction in the incidence of various forms of CHD. When including these elective terminations, the incidence of PA-IVS is significantly higher than reported.
There is no predilection to either sex. Also, there has been no identified association with genetic disorders, even though De Stefano et al in 2008 reported PA-IVS in monozygotic twins.[3] Genetic evaluation of twins in that report was notable for 55 kb deletion at WFDC8 and WFDC9, and the clinical significance of this gene deletion is unknown. Similarly, in 1992, Chitayat et al, from Canada, reported the incidence of PA-IVS in 2 siblings with no other associated cardiac anomalies.[4]
Developmental Considerations and Anatomical Characteristics:
Regarding the timing of occurrence, in 1983, Kusche and Van Mierop suggested that the insult leading to PA-IVS happens later in gestation when compared to pulmonary atresia with ventricular septal defect (PA-VSD).[5] They suggested that the insult leading to PA-VSD occurs before the complete formation of the ventricular septum, whereas PA-IVS occurs following the completion of ventricular septal formation.
Atresia of the pulmonary valve is classified into membranous and muscular forms. It is essential to distinguish between these 2 types, as a membranous form of PA has a better long-term prognosis than muscular PA due to the higher incidence of abnormal connections (discussed in the latter part of this section) between the RV and coronary arteries. Due to high pressures in the RV, the tricuspid valve is generally abnormal. The tricuspid valve can be hypoplastic or dysplastic and has a malformed chordal apparatus.
Another characteristic feature, if present, of PA-IVS, is the abnormal connections between the RV and coronary arteries. The RV, especially in patients with a competent tricuspid valve, is hypertensive due to a lack of egress for the blood. Due to this, the RV develops these abnormal connections with the epicardial coronary arteries, which help to decompress the ventricle. When present over time, these abnormal connections lead to progressive stenosis of the coronary arteries related to high-velocity blood flowing through these abnormal connections. Due to progressive stenosis of the coronary arteries over time, some parts of the myocardium depend on the right ventricle for perfusion, known as right ventricular-dependent coronary circulation (RVDCC). Due to the progressive nature of RVDCC, it correlates with poor prognosis.
History and Physical
The most prevalent presenting signs and symptoms include cyanosis and desaturation. Neonates with PS-IVS become symptomatic following the closure of the patent ductus arteriosus, as their pulmonary circulation is dependent on it. They rarely present or have the symptoms of decreased cardiac output due to obligatory right to left shunting at the level of atrial septum through foramen ovale. The presence of low cardiac output syndrome should raise suspicion for myocardial ischemia, especially in patients with coronary fistulae. As noted with many other forms of cyanotic CHD, these patients do not have an improvement in their cyanosis/desaturations with 100% oxygen delivery or failed hyperoxia tests.
The examiner notes single first and second heart sounds on cardiac auscultation. If the tricuspid valve regurgitates, a pansystolic murmur is audible at the left lower sternal border. An additional murmur related to the flow across patent ductus arteriosus might be audible in patients with patent ductus, especially following the initiation of prostaglandin infusion to maintain the ductal patency. The peripheral pulses and capillary refill time are usually normal except in patients with severely restrictive right to left shunting at the level of atria.
Evaluation
2D echocardiography, an easy and readily available modality of cardiac evaluation, is used in diagnosing PA-IVS. Due to the widespread availability and use of fetal echocardiography in conjunction with prenatal screening for CHD, the majority of the patients with this disorder get diagnosed prenatally. In a recently published study on the utilization of fetal echocardiography, the overall rate of prenatal diagnosis for this specific heart defect was approximately 86%.[6]
Echocardiogram alone can diagnose PA-IVS, but additional information regarding coronary circulation, a significant predictor of outcomes, and type of repair cannot be discerned from this modality alone. Although echocardiography provides information regarding the anomalous connections between the RV and coronary arteries, it is not useful in the diagnosis of RVDCC. Therefore, cardiac catheterization with angiograms is often needed to arrive at a complete diagnosis, which includes the assessment for fistulous connections between the RV and coronary arteries and RVDCC.
Echocardiogram:
An apical four-chamber sweep can diagnose PA-IVS. The absence of an outflow tract from the RV with an intact ventricular septum is the diagnostic finding. When performing echocardiography in these patients, special attention should be paid to the anatomy of the interatrial septum, tricuspid valve, RV, and branch pulmonary arteries.
Interatrial septum:
These patients depend on obligatory right-to-left shunting at the level of the interatrial septum to maintain cardiac output and systemic perfusion. Therefore, it is prudent to evaluate for any obstruction across the interatrial septum. The subcostal views are generally optimal for imaging this part of the heart. A combination of 2D imaging, color Doppler imaging, and spectral Doppler imaging is required to assess the interatrial septum.
Tricuspid valve:
Assessing the anatomy of the tricuspid valve is crucial in these patients as the adequacy of the tricuspid valve is 1 of the major factors influencing the type of surgical repair. The assessment of the tricuspid valve should include the size of the tricuspid valve annulus, the morphology of the valve leaflets, and the functional status of the valve (atretic vs. patent, competent vs. regurgitate). Patients with regurgitant tricuspid valves have a lower incidence of coronary anomalies as regurgitation through the valve helps to decompress the RV.
Right ventricle:
The RV's morphological characteristics are another parameter requiring detailed assessment during the echocardiogram. Generally, the size of the RV is proportional to the size of the tricuspid valve. More than the absolute size of the right ventricle, it is important to evaluate the morphological characteristics of the right ventricle, which divides into 3 components: inflow, apical, and outflow. Suppose the RV is tripartite, with well-developed inflow, apical, and outflow components. In that case, the neonates can undergo biventricular repair even if it is hypoplastic, given that all the other characteristics favor the biventricular repair.
Cardiac catheterization:
Cardiac catheterization with angiocardiography is often used in evaluating patients with PA-IVS as it provides additional information regarding coronary circulation; this is particularly important in patients in whom decompression of the RV is a consideration. The primary goal of the cardiac catheterization is to assess for RVDCC. The diagnosis of RVDCC is made when a significant portion of the ventricular myocardium depends on RV for the blood supply. The angiographic criteria for diagnosing RVDCC include stenosis of 2 or more major coronary arteries or atresia of the coronary ostia and the myocardium distal to the obstruction receiving the blood supply via fistulous connections from the RV. Loomba et al proposed the aortic perfusion score based on the angiographic characteristics of coronary perfusion as a predictor of patient outcomes.[7]
Galindo et al described various angiographic techniques that help obtain adequate/optimal coronary imaging. The techniques described in that article include RV angiogram, aortogram, aortogram with balloon occlusion of the aorta, and selective coronary angiograms. The evidence of myocardial ischemia on the surface electrocardiogram following the placement of the catheter in the RV (decompression of the RV from catheter-related tricuspid valve regurgitation) is very suspicious for RVDCC.
Treatment / Management
Medical Management:
Immediately following the diagnosis of PA-IVS, an attempt should be made to initiate prostaglandin infusion to maintain the ductus arteriosus patency. This action is vital for the preoperative survival of these patients, as the ductus arteriosus is the sole source of pulmonary blood flow. Also, manipulating pulmonary vascular and systemic vascular resistance is crucial to achieving optimal pulmonary and systemic blood flow.
Procedural management:
Patients with PA-IVS ll need an intervention, either a catheter-based intervention or surgical procedure, as neonates. Because of this disorder's complexity and heterogeneity, no single procedure is effective for all patients. Even though biventricular repair is the ideal and preferred surgical approach, managing this disorder must be highly individualized. The preferred surgical or transcatheter intervention is influenced by several variables, which include tricuspid valve size and function, RV anatomy, coronary artery anatomy, and type of pulmonary atresia.
The currently available therapeutic algorithms are quite diverse. Chikkabyrappa et al published an article discussing the various therapeutic options for this disorder.[8] According to that article:
- Patients with adequate and functionally tripartite RV size, tricuspid valve z-score greater than 2.5, and normal coronary artery anatomy would benefit from biventricular repair, Radiofrequency perforation of the pulmonary valve for pulmonary atresia, and surgical right ventricular outflow tract reconstruction for long segment atresia.
- Patients with borderline bipartite hypoplastic RV and tricuspid valve z-scores between -2.5 and -4.5 might benefit from attempted biventricular repair ± systemic to pulmonary shunt. These patients require close surveillance for RV growth, in the absence of which, they benefit from one-and-a-half ventricular repairs.
- Single ventricular repair (bidirectional Glenn followed by Fontan) should be a consideration in patients with severe RV hypoplasia and patients with RVDCC.
- Primary heart transplantation might be a reasonable approach in patients with myocardial ischemia related to coronary artery abnormalities.
Clinicians should recall that the patients in categories 2, 3, and 4 need systemic to pulmonary artery shunt or stenting of the PDA in the immediate newborn period.
Differential Diagnosis
Since the major presenting symptoms are desaturations and cyanosis, exclude other respiratory and cardiac causes of these symptoms.
Prognosis
Several studies have shown a gradual improvement in the survival of patients with this disorder, thought to be due to the advancements in pediatric cardiology and cardiothoracic surgery. In a population-based study from the UK and Ireland published in 2005, the 1-year survival was about 71%, and 5-year survival was approximately 64%.[9] In the same article, the authors found that low birth weight and unipartite right ventricle were the independent risk factors for death. RVDCC, especially atresia of the coronary ostia, is an independent risk factor for poor outcomes.[10]
Complications
In the absence of extensive aortopulmonary collaterals, PA-IVS is not compatible with life without ductal patency.
Deterrence and Patient Education
Pulmonary atresia with the intact ventricular septum (PA-IVS) is a rare and complex form of congenital heart defect with a broad spectrum of presentation. An insult during sensitive stages of embryological development is proposed to lead to PA-IVS. This entity's presentation is similar to that of other complex cyanotic congenital heart defects with decreased pulmonary blood flow, like tetralogy of Fallot.
The echocardiogram plays a vital role in diagnosing PA-IVS. Cardiac catheterization provides additional information regarding the status of the coronary circulation.
The management of patients with this heart defect is complex. Many therapeutic algorithms guide the medical team in the management of these patients.
Enhancing Healthcare Team Outcomes
An interprofessional approach is needed to achieve optimal outcomes in these patients. The team most often includes a pediatric cardiologist, a cardiothoracic surgeon, an obstetrician, a maternal-fetal specialist (fetal diagnosis), a neonatal-perinatal specialist, neonatal and ICU nurses, and a cardiac intensivist. In patients diagnosed prenatally, it is imperative to have a management plan even before the baby is delivered. In individual cases, it is not unreasonable to consider delivering the neonate in a tertiary care center, where pediatric cardiothoracic surgical services are available. Every attempt should be made to prevent the delivery of the neonate during weekends and holidays when hospitals are not at full staffing.
Clinicians work with the family to help ensure optimal care. They can offer to counsel and answer family questions. Surgical nurses are invaluable during any delivery and subsequent neonatal surgery and participate in neonatal ICU care following procedures, reporting all status changes to the managing physicians. The mother and child can achieve the best possible outcome only with this interprofessional collaboration.
The patients should receive detailed counsel from the team of clinicians regarding heart defects and surgical strategies under consideration in managing this complex disorder. They should also understand the implications regarding the increased risk of heart defects with future pregnancies.
Media
(Click Image to Enlarge)
References
Daubeney PE, Sharland GK, Cook AC, Keeton BR, Anderson RH, Webber SA. Pulmonary atresia with intact ventricular septum: impact of fetal echocardiography on incidence at birth and postnatal outcome. UK and Eire Collaborative Study of Pulmonary Atresia with Intact Ventricular Septum. Circulation. 1998 Aug 11:98(6):562-6 [PubMed PMID: 9714114]
Ferencz C, Rubin JD, McCarter RJ, Brenner JI, Neill CA, Perry LW, Hepner SI, Downing JW. Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. American journal of epidemiology. 1985 Jan:121(1):31-6 [PubMed PMID: 3964990]
Level 2 (mid-level) evidenceDe Stefano D, Li P, Xiang B, Hui P, Zambrano E. Pulmonary atresia with intact ventricular septum (PA-IVS) in monozygotic twins. American journal of medical genetics. Part A. 2008 Feb 15:146A(4):525-8. doi: 10.1002/ajmg.a.32160. Epub [PubMed PMID: 18203206]
Level 3 (low-level) evidenceChitayat D, McIntosh N, Fouron JC. Pulmonary atresia with intact ventricular septum and hypoplastic right heart in sibs: a single gene disorder? American journal of medical genetics. 1992 Feb 1:42(3):304-6 [PubMed PMID: 1536166]
Level 3 (low-level) evidenceKutsche LM, Van Mierop LH. Pulmonary atresia with and without ventricular septal defect: a different etiology and pathogenesis for the atresia in the 2 types? The American journal of cardiology. 1983 Mar 15:51(6):932-5 [PubMed PMID: 6829467]
Gorla SR, Chakraborty A, Garg A, Gugol RA, Kardon RE, Swaminathan S. Emerging trends in the prenatal diagnosis of complex CHD and its influence on infant mortality in this cohort. Cardiology in the young. 2019 Mar:29(3):270-276. doi: 10.1017/S1047951118002147. Epub 2018 Dec 26 [PubMed PMID: 30585560]
Loomba RS, Pelech AN. Aortic perfusion score for pulmonary atresia with intact ventricular septum: An antegrade coronary perfusion scoring system that is predictive of need for transplant and mortality. Congenital heart disease. 2018 Jan:13(1):92-97. doi: 10.1111/chd.12510. Epub 2017 Jun 27 [PubMed PMID: 28653340]
Chikkabyrappa SM, Loomba RS, Tretter JT. Pulmonary Atresia With an Intact Ventricular Septum: Preoperative Physiology, Imaging, and Management. Seminars in cardiothoracic and vascular anesthesia. 2018 Sep:22(3):245-255. doi: 10.1177/1089253218756757. Epub 2018 Feb 7 [PubMed PMID: 29411679]
Daubeney PE, Wang D, Delany DJ, Keeton BR, Anderson RH, Slavik Z, Flather M, Webber SA, UK and Ireland Collaborative Study of Pulmonary Atresia with Intact Ventricular Septum. Pulmonary atresia with intact ventricular septum: predictors of early and medium-term outcome in a population-based study. The Journal of thoracic and cardiovascular surgery. 2005 Oct:130(4):1071 [PubMed PMID: 16214522]
Cheung EW, Richmond ME, Turner ME, Bacha EA, Torres AJ. Pulmonary atresia/intact ventricular septum: influence of coronary anatomy on single-ventricle outcome. The Annals of thoracic surgery. 2014 Oct:98(4):1371-7. doi: 10.1016/j.athoracsur.2014.06.039. Epub 2014 Aug 22 [PubMed PMID: 25152382]