Introduction
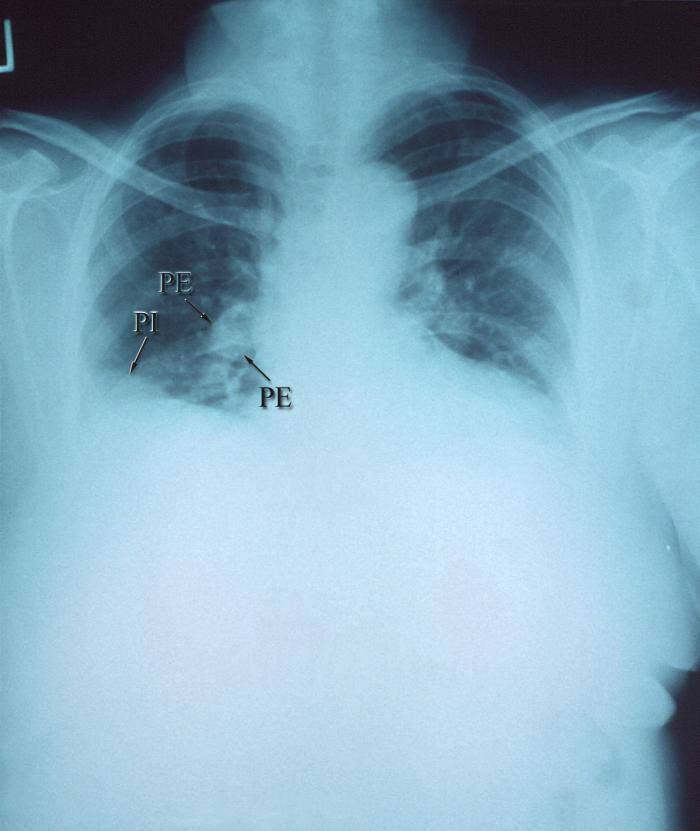
Pulmonary infarction results from an occlusion of a distal pulmonary artery. This results in ischemia and possible hemorrhage or tissue necrosis of the pulmonary tissue distal to the occlusion. Pulmonary infarction is caused by another primary disease state, most commonly pulmonary embolism. See Image. Right Pulmonary Arterial Embolism. Understanding the broad differential diagnosis associated with pulmonary infarction is important, as associated signs and symptoms have limited specificity, and pulmonary infarction may be the first indication of significant underlying pathology.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Several prospective and retrospective studies have investigated the prevalence of pulmonary infarction associated with pulmonary embolism, the most common etiology for developing pulmonary infarction.[1][2][3][4] The reported incidence of pulmonary infarction in the setting of pulmonary embolism is around 30%.[3][5] Other disease states that can lead to pulmonary infarction include infection,[6] malignancy,[7] surgical iatrogenesis,[4][8] amyloidosis, sickle cell disease,[9] vasculitis,[10] among others. Smoking is a risk factor for all causes of pulmonary infarction, including those associated with PE.[4][11] Paradoxically, younger age (peaking at 40) and increased height are associated with an increased likelihood of developing a pulmonary infarction from a pulmonary embolism. In contrast, obesity is associated with a reduced likelihood.[1][2] The early literature suggested that patients with underlying cardiac disease are at the greatest risk for developing a pulmonary infarction, as the hypothesis was thought to be due to poor collateral circulation, which, in combination with pulmonary thromboembolism, resulted in infarction.[12][13] However, recent literature suggests the opposite is true. Specifically, younger patients without cardiopulmonary disease were found to be more likely to suffer a pulmonary infarction secondary to a pulmonary embolism. Experts hypothesized that longstanding local tissue hypoxia from chronic cardiopulmonary disease states led to more robust bronchial vascular collateralization, protecting parenchyma from infarction.[2]
Epidemiology
The incidence of pulmonary infarctions from all causes is not well studied as it is more studied in the subpopulation of those with pulmonary embolisms. The overall incidence of those with pulmonary embolism is approximately 1 in 1000 are complicated by pulmonary infarction at a rate of 16% to 31%.[1][2][14] The mortality rates have varied over the recent decades, given the changes in treatment options in both the short and long term. A study from 2017 found the survival to discharge rate of those suffering from a pulmonary infarction to be high at around 97%.[3] In one study, the 30-day mortality for patients found to have a pulmonary embolism was 31%, and the mortality ratio in the first decade after developing a pulmonary embolism was 41%.[15] Studies evaluating hospital length-of-stay and mortality suggest similar prognoses for pulmonary embolism with infarction and pulmonary embolism without radiographic evidence of infarction.[2][3]
Pathophysiology
A unique thing about the lung is that it receives blood supply from more than just arterial circulation. This gives the lung some resiliency in an insult such as a pulmonary embolism. The lung parenchyma receives its oxygen supply from 3 non-redundant sources: deoxygenated blood from pulmonary arteries, oxygenated blood from the bronchial circulation, and direct oxygen diffusion from alveoli.[13] A sufficient impedance from one of these sources can cause infarction and subsequent tissue necrosis. Inflammatory mediators from ischemic parenchyma can further limit gas exchange following the resultant vasoconstriction and bronchoconstriction.[16] When ischemia of lung tissue is not reversed promptly, infarction ensues. A unilateral infarct occurs in 77% to 87% of pulmonary infarctions, with the strongest predilection for the right lower lobe. Multiple studies show a stark predominance of pulmonary infarction in the lower lobes relative to the upper lobes.[1][2][17] This has been hypothesized to be due to gravity’s influence on the unique relationship between alveolar, pulmonary, and bronchial arterial pressure.[2] In patients with sickle cell disease, acute chest syndrome was caused by vascular infarction by direct occlusion of sickle-type hemoglobin in 16% of cases.[18] Vasculitides may result in a pulmonary infarction through antibody deposition, the formation of microaneurysms, or macrophage activation, leading to vessel wall inflammation.[19] The common end pathway is ischemic lung tissue regardless of the primary etiology.
Histopathology
The description of lung infarction of a thromboembolic etiology was first described by Virchow in 1856.[3] It was later noted that following infarction, hemorrhage and then edema ensued into the alveoli distally. Histologically, it is characterized by organized thrombi and hemorrhage surrounding a core of necrotic alveoli with adjacent structures (see Image. Pulmonary Embolism).[7] In some cases, this is reversible, whereby the intra-alveolar blood is resorbed in 2-4 days. If the process is not reversed, hemosiderin is formed from the intra-alveolar erythrocytes, and infarction of the surrounding lung parenchyma is seen within 1-2 days. If a neutrophilic reaction is combined with a microbiological culture growing a specific organism, this would indicate an infectious etiology as a possible culprit.[4]
History and Physical
Given pulmonary embolism serves as the etiology of pulmonary infarction in most cases, it is important to understand the clinical picture representing both conditions. The presenting features of a pulmonary infarction overlap those of a pulmonary embolism with a few important distinctions. In patients with concurrent pulmonary embolism and pulmonary infarction, the following features are present: dyspnea (69% to 78%), chest pain (49% to 70%), swelling or pain in a unilateral lower extremity (27% to 31%), fever (5% to 11%), and hemoptysis (4% to 19%).[1][3][12] Presenting features in all patients with the diagnosis of pulmonary embolism, regardless of the presence of pulmonary infarction, included dyspnea (72% to 75%), chest pain (36% to 38%), signs of DVT (22% to 33%), hemoptysis (4% to 8%).[1][20] Thus, pleuritic chest pain and hemoptysis were both more common in patients with pulmonary embolism and pulmonary infarction versus those with pulmonary embolism and no pulmonary infarction. Symptoms noted to have no statistical difference between the 2 groups include cough, syncope, sudden onset dyspnea, signs of DVT, fever, and right ventricular overload. Among patients incidentally found to have a pulmonary infarction after a biopsy of a radiographically discovered lung nodule, 65% of patients had no respiratory complaints, 26% had dyspnea, 7% had chest pain, and 5% had hemoptysis.[4] This data highlights the difficulty of diagnosing a pulmonary infarction and emphasizes the importance of considering a broad differential diagnosis for patients who present with symptoms that could be secondary to a pulmonary infarction.
Evaluation
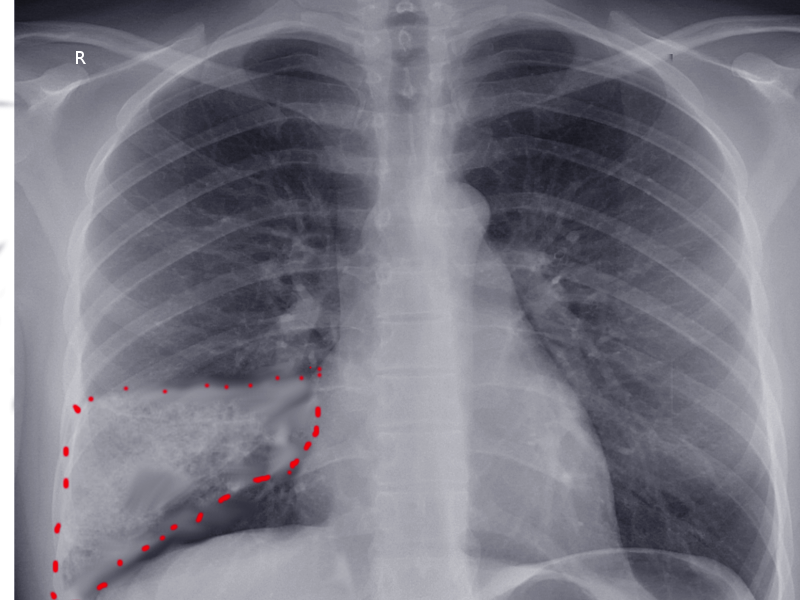
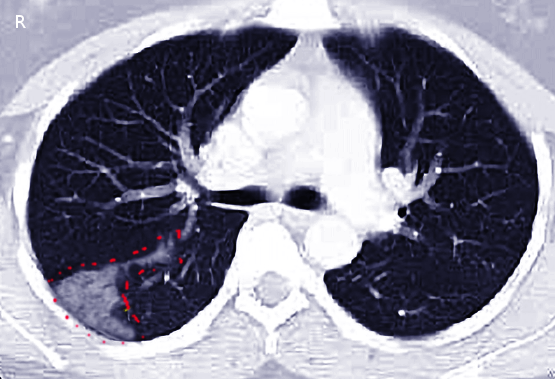
Computed tomography (CT) is the most commonly used imaging technique to diagnose pulmonary infarction in combination with appropriate clinical context. CT findings associated with pulmonary infarction include a feeding vessel or "vessel sign," central lucency, and a semicircular shape. The finding of air bronchograms made a pulmonary infarction less likely.[2] If a vessel sign with a central lucency and no air bronchogram is present on CT, the specificity for detecting pulmonary infarction is 99%.[21] An X-ray image may be the first clue toward diagnosing pulmonary infarction (see image. Wedge-Shaped Pulmonary Infarction). A "Hampton’s hump" (wedge-shaped consolidation at the lung periphery), Westermark’s sign (radiographic oligemia or increased lucency), and Fleischer sign (prominent pulmonary artery) are specific findings but lack sensitivity to be diagnostically sufficient. It has been quoted that a Hamptom hump had a sensitivity and specificity of 22% and 82%, respectively.[22] Other features, such as atelectasis or focal consolidation, may be present but are neither sensitive nor specific.[23] In patients suspected of having infective endocarditis, consider that a pulmonary embolism or pulmonary infarction is a minor criterion in the Duke Criteria for Infective Carditis, with a reported incidence ranging from 13% to 49%.[24] Abnormalities seen in diagnostic studies, such as electrocardiograms, DD-dimers, ventilation/perfusion scans, and echocardiograms, play a role in predicting pulmonary infarction and pulmonary embolism.
Treatment / Management
Given the fact that pulmonary infarction can be fatal, signs of respiratory distress or hemodynamic collapse should be addressed immediately with supportive care measures. Patients with pulmonary infarction may develop obstructive shock associated with a pulmonary embolism or cardiorespiratory collapse secondary to hemodynamic collapse or persistent hypoxia. In addition to supportive management, treatment is guided by the underlying condition that has led to the pulmonary infarction. Pulmonary embolism initially requires anticoagulation. In patients requiring admission, heparin or low-molecular-weight heparin is started to transition to some of the newer direct oral anticoagulation medications like apixaban or rivaroxaban or some older medications like coumadin for continued outpatient management.[25] In patients with hemodynamic instability due to a moderate or high-risk pulmonary embolism (formerly submissive and massive pulmonary embolism), catheter-based and systemic fibrinolytics, or surgical interventions like embolectomies are institution-dependent treatment options.[26] Additionally, there is growing evidence that the lowest-risk patients with pulmonary embolism may be discharged directly from the emergency department on new or direct oral anticoagulants.[27] Treatment for pulmonary infarction not caused by pulmonary embolism is varied and based on the etiology. The differential diagnoses are listed below.
The average resolution time for pulmonary infarction has not been well studied. However, one retrospective review found that of 32 patients evaluated by CT scanning after pulmonary infarctions with intervals varying from 1 to 69 weeks after initial diagnosis, 10 were found to have continued evidence of pulmonary infarction at an average interval of 10 weeks after the initial diagnosis.[3] Pulmonary infarction in patients with pulmonary embolism did not alter long-term perfusion deficits determined by lung scintigraphy.[1] Thus, there is no standardized recommendation for repeat CT scanning to evaluate for resolution of pulmonary infarction. Such decisions should be based on the clinical status of the patient being evaluated. Current radiographic technology in the setting of the appropriate clinical picture makes it such that biopsy is rarely used to diagnose pulmonary infarction. A lung biopsy is typically reserved for investigating a pulmonary nodule, suspected mass, interstitial lung disease or collecting a sample for culture.[4][10][28][29](B2)
Differential Diagnosis
The differential diagnosis is broad and includes the following:
- Pulmonary embolism
- Acute chest syndrome (sickle cell anemia)
- Infective endocarditis
- Malignancy
- Heatstroke
- AV malformation
- Cocaine toxicity
- Diffuse intravascular coagulation
- Diffuse alveolar hemorrhage
- Iatrogenic instrumentation, for example, bronchial artery embolization, IV catheter ablation
- Amyloidosis
- Pulmonary infection like pneumonia or aspergillosis
- Vasculitides
Enhancing Healthcare Team Outcomes
While pulmonary embolism is the most likely cause of pulmonary infarction, there is a myriad of other diagnoses that must be considered. One must consider the variability in clinical presentations and similarities in radiographic results of disease states which mimic pulmonary infarction such as solitary lung nodules. Evidence of a pulmonary infarction on X-ray or CT imaging may be the first indicator the patient has a pulmonary embolism. If warranted, patients require education on tobacco cessation as this further increases the risk of pulmonary infarction.[4] Anticipate an interprofessional approach for patients with a pulmonary infarction: internal medicine versus critical care for initial management; surgical specialties or pathology for further diagnostics or resection; and sub-specialists such as hematology or cardiology, depending on the etiology of the pulmonary infarction.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of Pulmonary Infarction. Medicine. 2015 Oct:94(41):e1488. doi: 10.1097/MD.0000000000001488. Epub [PubMed PMID: 26469892]
Islam M, Filopei J, Frank M, Ramesh N, Verzosa S, Ehrlich M, Bondarsky E, Miller A, Steiger D. Pulmonary infarction secondary to pulmonary embolism: An evolving paradigm. Respirology (Carlton, Vic.). 2018 Mar 25:():. doi: 10.1111/resp.13299. Epub 2018 Mar 25 [PubMed PMID: 29577524]
Chengsupanimit T, Sundaram B, Lau WB, Keith SW, Kane GC. Clinical characteristics of patients with pulmonary infarction - A retrospective review. Respiratory medicine. 2018 Jun:139():13-18. doi: 10.1016/j.rmed.2018.04.008. Epub 2018 Apr 17 [PubMed PMID: 29857996]
Level 2 (mid-level) evidenceParambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest. 2005 Apr:127(4):1178-83 [PubMed PMID: 15821192]
Level 3 (low-level) evidenceKaptein FHJ, Kroft LJM, Hammerschlag G, Ninaber MK, Bauer MP, Huisman MV, Klok FA. Pulmonary infarction in acute pulmonary embolism. Thrombosis research. 2021 Jun:202():162-169. doi: 10.1016/j.thromres.2021.03.022. Epub 2021 Apr 1 [PubMed PMID: 33862471]
Morgenthaler TI, Ryu JH, Utz JP. Cavitary pulmonary infarct in immunocompromised hosts. Mayo Clinic proceedings. 1995 Jan:70(1):66-8 [PubMed PMID: 7808055]
Level 3 (low-level) evidenceGeorge CJ, Tazelaar HD, Swensen SJ, Ryu JH. Clinicoradiological features of pulmonary infarctions mimicking lung cancer. Mayo Clinic proceedings. 2004 Jul:79(7):895-8 [PubMed PMID: 15244386]
Terry PB, Buescher PC. Pulmonary Infarction: In the Beginning: The Natural History of Pulmonary Infarction. Chest. 2017 Dec:152(6):1135-1139. doi: 10.1016/j.chest.2017.07.005. Epub 2017 Jul 14 [PubMed PMID: 28716646]
Knight J, Murphy TM, Browning I. The lung in sickle cell disease. Pediatric pulmonology. 1999 Sep:28(3):205-16 [PubMed PMID: 10495338]
Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2009 May:22(5):679-85. doi: 10.1038/modpathol.2009.20. Epub 2009 Mar 13 [PubMed PMID: 19287460]
Miniati M. Pulmonary Infarction: An Often Unrecognized Clinical Entity. Seminars in thrombosis and hemostasis. 2016 Nov:42(8):865-869 [PubMed PMID: 27743556]
Dalen JE, Haffajee CI, Alpert JS 3rd, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. The New England journal of medicine. 1977 Jun 23:296(25):1431-5 [PubMed PMID: 865513]
Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. The American journal of medicine. 1982 Apr:72(4):599-606 [PubMed PMID: 6462058]
Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Archives of internal medicine. 2011 May 9:171(9):831-7. doi: 10.1001/archinternmed.2011.178. Epub [PubMed PMID: 21555660]
Søgaard KK, Schmidt M, Pedersen L, Horváth-Puhó E, Sørensen HT. 30-year mortality after venous thromboembolism: a population-based cohort study. Circulation. 2014 Sep 2:130(10):829-36. doi: 10.1161/CIRCULATIONAHA.114.009107. Epub 2014 Jun 26 [PubMed PMID: 24970783]
Level 2 (mid-level) evidenceKroegel C, Reissig A. Principle mechanisms underlying venous thromboembolism: epidemiology, risk factors, pathophysiology and pathogenesis. Respiration; international review of thoracic diseases. 2003 Jan-Feb:70(1):7-30 [PubMed PMID: 12584387]
Choi SH, Cha SI, Shin KM, Lim JK, Yoo SS, Lee SY, Lee J, Kim CH, Park JY, Lee DH. Clinical Relevance of Pleural Effusion in Patients with Pulmonary Embolism. Respiration; international review of thoracic diseases. 2017:93(4):271-278. doi: 10.1159/000457132. Epub 2017 Feb 15 [PubMed PMID: 28196360]
Vichinsky EP, Neumayr LD, Earles AN, Williams R, Lennette ET, Dean D, Nickerson B, Orringer E, McKie V, Bellevue R, Daeschner C, Manci EA. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. The New England journal of medicine. 2000 Jun 22:342(25):1855-65 [PubMed PMID: 10861320]
Emmi G, Silvestri E, Squatrito D, Amedei A, Niccolai E, D'Elios MM, Della Bella C, Grassi A, Becatti M, Fiorillo C, Emmi L, Vaglio A, Prisco D. Thrombosis in vasculitis: from pathogenesis to treatment. Thrombosis journal. 2015:13():15. doi: 10.1186/s12959-015-0047-z. Epub 2015 Apr 16 [PubMed PMID: 25883536]
Bajaj N, Bozarth AL, Guillot J, Kojokittah J, Appalaneni SR, Cestero C, Amankona RK, Pippim JA. Clinical features in patients with pulmonary embolism at a community hospital: analysis of 4 years of data. Journal of thrombosis and thrombolysis. 2014 Apr:37(3):287-92. doi: 10.1007/s11239-013-0942-8. Epub [PubMed PMID: 23681675]
Level 2 (mid-level) evidenceRevel MP, Triki R, Chatellier G, Couchon S, Haddad N, Hernigou A, Danel C, Frija G. Is It possible to recognize pulmonary infarction on multisection CT images? Radiology. 2007 Sep:244(3):875-82 [PubMed PMID: 17709834]
Level 2 (mid-level) evidenceHogg K, Brown G, Dunning J, Wright J, Carley S, Foex B, Mackway-Jones K. Diagnosis of pulmonary embolism with CT pulmonary angiography: a systematic review. Emergency medicine journal : EMJ. 2006 Mar:23(3):172-8 [PubMed PMID: 16498151]
Level 1 (high-level) evidenceWorsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE. Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED Study. Radiology. 1993 Oct:189(1):133-6 [PubMed PMID: 8372182]
Habib G. Embolic risk in subacute bacterial endocarditis: determinants and role of transesophageal echocardiography. Current cardiology reports. 2003 Mar:5(2):129-36 [PubMed PMID: 12583856]
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016 Feb:149(2):315-352. doi: 10.1016/j.chest.2015.11.026. Epub 2016 Jan 7 [PubMed PMID: 26867832]
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK, American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, American Heart Association Council on Peripheral Vascular Disease, American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011 Apr 26:123(16):1788-830. doi: 10.1161/CIR.0b013e318214914f. Epub 2011 Mar 21 [PubMed PMID: 21422387]
Frank Peacock W, Coleman CI, Diercks DB, Francis S, Kabrhel C, Keay C, Kline JA, Manteuffel J, Wildgoose P, Xiang J, Singer AJ. Emergency Department Discharge of Pulmonary Embolus Patients. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2018 Sep:25(9):995-1003. doi: 10.1111/acem.13451. Epub 2018 Jun 11 [PubMed PMID: 29757489]
Raj R, Raparia K, Lynch DA, Brown KK. Surgical Lung Biopsy for Interstitial Lung Diseases. Chest. 2017 May:151(5):1131-1140. doi: 10.1016/j.chest.2016.06.019. Epub 2016 Jul 26 [PubMed PMID: 27471113]
Winokur RS, Pua BB, Sullivan BW, Madoff DC. Percutaneous lung biopsy: technique, efficacy, and complications. Seminars in interventional radiology. 2013 Jun:30(2):121-7. doi: 10.1055/s-0033-1342952. Epub [PubMed PMID: 24436527]