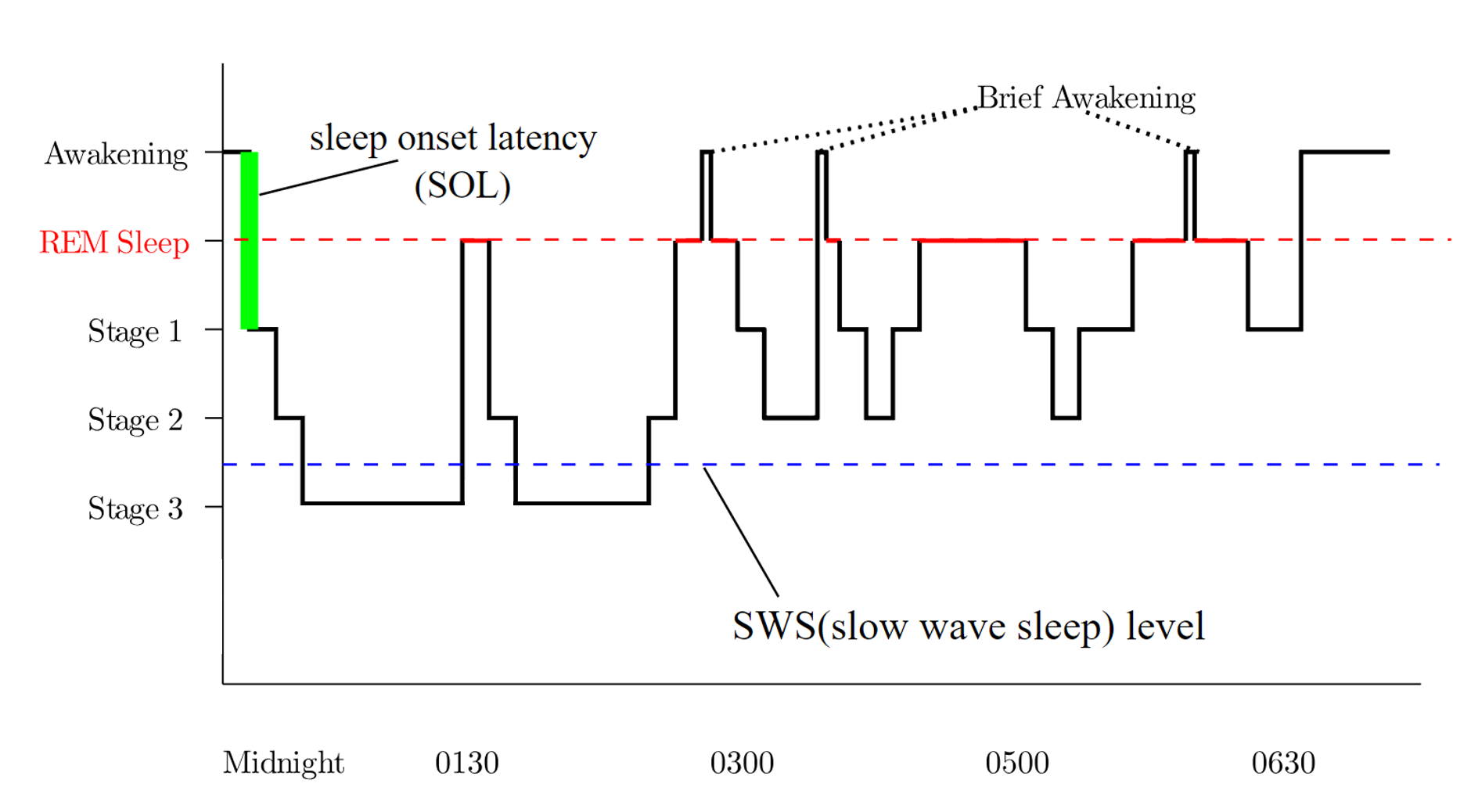
Introduction
Rapid eye movement (REM), or the REM rebound effect, is a compensatory response in which a person temporarily receives more REM sleep than normal. During REM rebound, heightened frequency, greater depth, and increased intensity of REM sleep following episodes of sleep deprivation, significant stress, or the consumption or withdrawal of specific medications or recreational drugs.[1] This phenomenon arises from the neurophysiological and hormonal processes that play a crucial role in maintaining the balance of normal sleep patterns and homeostasis. Maintaining a healthy sleep structure is essential for promoting and safeguarding physical and mental well-being. Sleep offers numerous health benefits, including conserving energy, facilitating physical recuperation, enhancing brain plasticity, consolidating memories, processing emotions, and fostering cognitive integration. Disrupted sleep triggers the body's innate mechanisms to restore a harmonious sleep cycle.
Sleep is a complex condition characterized by distinct phases that involve a temporary disconnection from the external environment, reduced consciousness, muscular atonia, and alterations in metabolism.[2] Sleep is categorized into 2 stages—rapid eye movement (REM) and non-REM (NREM) sleep. Under normal circumstances, individuals progress through a predictable sequence of stages, commencing with wakefulness, transitioning through various NREM sleep stages, and ultimately entering REM sleep. The average sleep cycle occurs over approximately 90 minutes, with REM sleep gradually extending throughout the night, totaling 4 to 6 cycles.[3]
REM sleep is distinguished by ocular saccadic movements, muscular atonia, and fast-wave electroencephalography (EEG) patterns that align with dream-like activity and resemble the waking state. NREM sleep consists of 3 stages—N1, N2, and N3—each displaying distinctive EEG wave patterns. Experimental studies utilizing EEG and hormonal assays demonstrate that human and animal subjects experiencing sleep deprivation or significant stressors exhibit an amplified frequency and intensity of REM sleep as a compensatory mechanism.[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Researchers hypothesize that REM sleep might offer an ideal opportunity for recontextualizing negative experiences and modulating emotional reactivity when the typical stress response is subdued. In one study, human participants demonstrated reduced adverse reactions to images of fearful faces and enhanced positive responses to happy faces after a 90-minute nap.[5]
Both REM sleep deprivation and stress exposure can induce alterations in hormone release through the hypothalamic-pituitary-adrenal (HPA) axis and impact neurotransmitter levels.[6] These changes in signaling mechanisms contribute to a decline in sleep duration and quality while extending periods of wakefulness, ultimately leading to insomnia.[1]
Research using rat models exposed to various forms of stress, such as footshock and immobilization, illustrates a disruption in the HPA axis and sympathetic response systems, particularly within the locus coeruleus and adrenal medulla. Although rat models are insightful in exploring the effects of physical stress, the most impactful stressors for humans seem to be social, encompassing familial and work-related challenges, as well as interpersonal relationships.[1]
In addition to sleep deprivation and stress, REM rebound may occur in individuals discontinuing REM-inhibiting drugs and those with obstructive sleep apnea (OSA) initiating continuous positive airway pressure (CPAP) therapy. Patients who discontinue using benzodiazepines, barbiturates, certain antidepressants, some antipsychotic medications, and cannabis may experience REM rebound. Alcohol suppresses REM sleep during the initial part of the night, leading to a REM rebound in the later portion of the night. Cocaine is also known for its REM sleep-suppressing effects.
Considering all the suggested potential benefits associated with engaging in REM sleep, the occurrence of REM rebound could potentially serve as a beneficial adaptive strategy. An inability to activate this process could lead to potentially significant adverse consequences.[1]
Epidemiology
Given the widespread occurrence of sleep deprivation and stress, REM sleep rebound is prevalent in the United States (US) and worldwide. According to data from the Centers for Disease Control and Prevention (CDC), more than one-third of the US population struggles with sleep deprivation, which is defined as the condition of getting less than 7 hours of sleep per night.[7]
Notably, adequate sleep promotes physical health and overall well-being, although the precise extent of chronic sleep deficiency within the population remains to be accurately quantified.[8] A recent study determined that the average nightly sleep duration has decreased by approximately 1 hour globally over the past century, with the most significant reductions noted in highly industrialized regions such as Europe, North America, and Asia.[9]
Inadequate sleep is associated with the onset of numerous health conditions. The conditions and their associated comorbidities that give rise to national and global concerns about the repercussions of inadequate sleep [7] include obesity, metabolic disorders, hypertension, coronary artery disease and stroke, depression and anxiety, motor vehicle and workplace accidents, immunosuppression, renal function decline, medical errors, diminished quality of life, and reduced work productivity.[1][7]
Pathophysiology
Circadian and Homeostatic Rhythms
The interaction between neurotransmitters and neuropeptide systems, primarily located in the hypothalamus and brain stem, governs sleep regulation. These systems function through 2 primary mechanisms—homeostatic and circadian. Homeostatic drive, also known as "process S," pertains to the physiological urge for sleep, commonly called sleep pressure. In contrast, circadian rhythms, also known as "process C," regulate sleep onset by synchronizing with the natural day-night cycle. They serve as a timing mechanism that controls the fluctuation of alertness. The interaction between these mechanisms initiates sleep when there is a significant accumulation of sleep pressure due to increasing adenosine levels throughout the day. The suprachiasmatic nucleus collaborates with the pineal gland to release melatonin, monitoring the light-dark cycle simultaneously. This coordinated process is responsible for inducing sleep.[6]
Although typical sleep architecture is influenced by the interplay of homeostatic and circadian systems, sleep homeostasis depends explicitly on the duration and quality of the preceding wakefulness period.[6] The severity of sleep deprivation correlates with increasing compensatory changes in sleep architecture. For instance, shorter periods of sleep deprivation, lasting up to 6 hours, are associated with increased non-REM sleep. In contrast, more prolonged sleep deprivation, lasting 12 to 24 hours, increases non-REM and REM sleep. Groups subjected to approximately 96 hours of sleep deprivation primarily display a notable increase in REM sleep.[6]
The HPA Axis and Sleep
Hormones such as cortisol, adrenocorticotropic hormone (ACTH), corticotropin-releasing hormone (CRH), growth hormone-releasing hormone, and melatonin play a crucial role in influencing sleep duration, onset, and the distribution of sleep stages throughout the night. During the first half of the night, cortisol activity significantly decreases, a phenomenon attributed to the inhibitory phase of the HPA axis. As the night advances and REM sleep becomes more predominant, the inhibitory effects of the HPA axis become less potent, leading to a gradual increase in the adrenal hormone cortisol. In the latter part of the night, there is an increase in cortisol, CRH, and ACTH secretion, accompanied by a simultaneous rise in sympathetic nervous system (SNS) activity. Heightened cortisol levels and an extended duration of REM sleep mark the final sleep cycle of the night.
Patients with Addison disease, acquired primary adrenal insufficiency, typically exhibit elevated non-REM sleep and reduced REM sleep, which improve with glucocorticoid replacement therapy. Conversely, individuals with Cushing's syndrome, marked by excess cortisol, tend to experience reduced NREM sleep, heightened REM sleep, and fragmented sleep cycles.[6] CRH triggers REM rebound and non-REM sleep while simultaneously promoting wakefulness when administered intravenously, ultimately reducing the capacity to recuperate from sleep deprivation.[1][10] ACTH acts as a promoter of wakefulness during both the light and dark phases of the circadian rhythm.[1] Corticotropin-like intermediate peptide (CLIP), derived from proopiomelanocortin (POMC), is associated with the induction of prolonged REM sleep episodes.[6]
Prolactin
Prolactin, an essential neurotrophic hormone secreted by the pituitary gland, is vital in facilitating REM rebound and averting stress-induced reductions in neurogenesis. Several studies have elucidated the role of prolactin in inducing REM sleep and regulating the phenomenon of stress-induced REM sleep rebound. In these experiments, prolactin microinfusions into the dorsal raphe nucleus (DRN) demonstrated the hormone's ability to induce REM sleep. Furthermore, anti-prolactin antibodies have been shown to inhibit REM sleep in rats, providing additional evidence of the connection between prolactin and REM sleep.[4][6]
Serotonin
Serotonin, also known as 5-hydroxytryptamine (5-HT), is central in regulating various facets of sleep, particularly influencing REM sleep and wakefulness, inhibiting REM sleep, and maintaining alertness. Specific neurons in the brainstem that produce serotonin and noradrenaline achieve this regulatory function. These neurotransmitters exert inhibitory control over cholinergic neurons located in the pons, which are crucial for initiating REM sleep by activating the glutamatergic sublaterodorsal nucleus.
An abnormal increase in serotonin activity, as induced by selective serotonin reuptake inhibitors (SSRIs), can result in the loss of muscle atonia during REM sleep, a condition known as REM sleep without atonia (RSWA).[11] Abrupt discontinuation of serotonergic antidepressant medication can result in REM rebound, frequently characterized by the occurrence of vivid dreams.[12] Furthermore, empirical evidence suggests a correlation between the duration of REM sleep rebound, elevated plasma prolactin levels, and serotonin levels in the hypothalamus. Serotonin stimulates prolactin release, and subsequently, prolactin activates cholinergic neurons within the mesopontine tegmental region of the brain, which is a critical area for initiating REM sleep.[1]
History and Physical
A meticulous clinical assessment is instrumental in identifying sleep disturbances and related pathologies that might lead to REM rebound. A comprehensive sleep history, including sleep duration, onset, difficulties waking up, interruptions in sleep maintenance, and sleep quality, should be obtained. A significant indicator in the patient's history suggesting REM rebound is the experience of extended vivid dreams without any specific causative pharmacological agents. Patients may also report waking up with feelings of disorientation, confusion, and headaches.
Furthermore, healthcare providers should focus on relevant factors such as the patient's past medical history, existing medical conditions, medication usage, and social history that might impact sleep patterns. This information is vital for identifying potential causes for sleep disturbances and evaluating whether additional medical comorbidities need further assessment. A comprehensive physical exam, actigraphy sleep movement evaluation, and maintaining a sleep diary can improve differential diagnosis precision and determine whether polysomnography is necessary for further evaluation.
Evaluation
In routine clinical settings, an evaluation for isolated REM rebound is unnecessary. Additional evaluation is warranted if the patient's history or physical examination raises concerns for other sleep disorders. Experiencing REM rebound does not necessarily indicate an underlying sleep disorder. However, individuals with underlying sleep disorders frequently encounter disruptions in their sleep patterns. REM rebound is commonly observed in patients with parasomnias, narcolepsy, and OSA.
Patients with REM sleep behavior disorder (RBD) require additional assessment. Individuals with RBD seem to act out their dreams due to the absence of muscle atonia during REM sleep. Dream reenactment may occur generally during heightened emotional distress or withdrawal from alcohol or medications. However, it is noteworthy that most patients with RBD will later go on to develop Parkinson disease or dementia with Lewy bodies. Distinguishing RBD from typical sleep behaviors is often aided by symptoms persisting for at least 6 months or longer.
Treatment / Management
Managing REM rebound typically involves adhering to the treatment plan established for any diagnosed sleep disorder in the patient. Evidence indicates that addressing sleep disturbances can improve REM sleep, provided high-quality sleep adequately relieves REM pressure. Although implementing sleep hygiene techniques might potentially enhance sleep quality, current studies indicate limited evidence supporting their effectiveness in addressing REM rebound, particularly in the absence of insomnia.[13]
Differential Diagnosis
REM rebound is a clinical phenomenon associated with various sleep disorders that span a broad spectrum of clinical conditions, including insomnia, psychosocial stress, psychiatric or neuropsychiatric disorders, parasomnias, narcolepsy, OSA, medication withdrawal, alcohol use, inadequate sleep hygiene, or shift work.
Toxicity and Adverse Effect Management
Various psychotropic medications can significantly impact sleep architecture, either increasing or decreasing the likelihood of experiencing REM rebound. For instance, benzodiazepines usually suppress REM sleep, which can result in REM rebound when these medications are discontinued or during withdrawal.[14] Similar effects have been noted with barbiturates and alcohol. In contrast, newer hypnotic sleep aids, such as zolpidem, do not lead to REM rebound upon discontinuation. After the sudden discontinuation of serotonergic antidepressants, numerous patients report experiencing more prolonged and intense dreams, along with symptoms associated with REM rebound.[15]
A meta-analysis of several drugs of abuse has revealed that withdrawal from substances such as tetrahydrocannabinol (THC), cocaine, heroin, and stimulants leads to significant REM rebound. This indicates a compensatory adjustment toward sleep homeostasis following the discontinuation of these substances.[16]
Prognosis
The prognosis for resolving REM rebound is generally favorable, although it is contingent on effectively addressing and resolving any underlying sleep disorders while restoring a normal sleep architecture. Effectively addressing the factors contributing to sleep disturbances aids in gradually resolving the REM rebound phenomenon over time.
The timeline for resolution can vary significantly depending on the individual and the specifics of their disordered sleep. Maintaining consistent, high-quality sleep hygiene and seeking medical or psychological interventions, whenever necessary, can significantly support the recovery process. If underlying issues are not effectively addressed, they can intermittently trigger REM rebound and its associated symptoms, potentially resulting in recurrent cycles of poor sleep.
Complications
Numerous studies demonstrate the significant adverse effects of poor sleep quality and sleep deprivation on mental health. Insufficient sleep can have detrimental impacts on mood and emotional regulation. Research has shown that sleep-deprived individuals often exhibit elevated levels of irritability, anxiety, and depression.[1] Furthermore, they may exhibit increased emotional reactivity to negative stimuli and a decreased ability to derive pleasure from positive experiences.
Chronic sleep problems are often associated with mental health disorders. For instance, insomnia is highly prevalent among individuals with major depressive disorder, generalized anxiety disorder, and post-traumatic stress disorder (PTSD). A recent study demonstrated a negative correlation between the duration of REM sleep episodes and the development of PTSD after exposure to traumatic events. Increased REM sleep appears to provide a protective shield against the adverse effects of stress.[17]
The relationship between sleep disturbances and these disorders is often bidirectional, implying that each can exacerbate the other, forming a vicious cycle that can be challenging to disrupt. Sleep plays a critical role in memory consolidation—a process in which the brain stabilizes and integrates memories for long-term storage. As REM sleep is likely involved in consolidating procedural and spatial memory, sleep deprivation can result in challenges in remembering new information.
Inadequate sleep contributes to various physical health issues, placing affected individuals at higher risk for heart disease, diabetes, obesity, and compromised immune function. Maintaining good sleep hygiene and seeking appropriate treatment for sleep disorders is essential for overall health and well-being.[1] Nearly all antidepressants decrease REM sleep by approximately 30%, whereas specific antipsychotic medications, such as phenelzine, can almost completely suppress REM sleep. This has raised inquiries regarding the intricate relationship among psychiatric symptoms, REM sleep deprivation, and medication-induced weight gain.[18] According to specific research findings, regular REM sleep may reduce the risk of obesity. In addition, the absence of the final REM period (fREMP) could potentially enhance appetite, leading to excessive calorie intake and subsequent weight gain in individuals experiencing chronic sleep deprivation.[18]
Deterrence and Patient Education
REM and NREM sleep are the 2 primary phases during a typical night of sleep, and they follow a predictable sleep cycle. These phases are described below with their unique characteristics and functions in the sleep cycle.
NREM sleep: This phase includes 3 stages—N1, N2, and N3—marking the initial part of the sleep cycle. A distinct brainwave pattern distinguishes each stage. NREM sleep is characterized by bodily relaxation and the facilitation of crucial physiological repair processes.
REM sleep: This phase is characterized by ocular (eye) saccadic movements, temporary muscle atonia (paralysis), and rapid brainwave patterns resembling wakefulness. This stage of sleep is known for vivid dream experiences.
In a normal sleep cycle, the sequence initiates with wakefulness and proceeds through NREM sleep stages before transitioning into REM sleep. This sleep cycle repeats approximately every 90 minutes, with REM sleep becoming increasingly prominent as the night progresses. Most individuals undergo 4 to 6 of these cycles during a typical night of sleep.
Maintaining a proper sleep architecture, which involves organizing these sleep phases, is vital for preserving physical and mental well-being. Sleep offers numerous benefits, including conserving energy, aiding in bodily repair, enhancing brain plasticity (the brain's adaptability), consolidating memories, processing emotions, and facilitating learning and cognitive functions.
Occasionally, sleep disturbances occur due to factors such as sleep deprivation or substantial stress. In such cases, the body attempts to restore balance in the sleep cycle, and this mechanism is referred to as REM rebound. Withdrawal from medications such as antidepressants and benzodiazepines, cannabis, alcohol use, or the initial use of CPAP with OSA can all trigger REM rebound.
Increased frequency, depth, and intensity of REM sleep characterize REM rebound. Patients typically encounter a more frequent occurrence of vivid dreams, potential disorientation upon waking, confusion, and reports of headaches.
Pearls and Other Issues
Sleep deprivation and stress alter neurotransmitter levels and induce changes in hormone release through the HPA. REM rebound occurs as the body compensates for these periods by undergoing a surge in the frequency, depth, and intensity of REM sleep. The most influential stressors for humans typically revolve around social factors, including challenges related to family, work, and interpersonal relationships. As per the CDC, over one-third of the US population experiences sleep deprivation, defined as receiving less than 7 hours of sleep per night.
Sleep deprivation increases the risk of various conditions, including obesity, metabolic disorders, high blood pressure, coronary artery disease, stroke, psychiatric conditions, and motor vehicle and workplace accidents. Sleep deprivation also contributes to medical errors and reduced work productivity, making it a concern on a national and global scale. Managing REM rebound involves addressing the identified sleep disorder in the patient. Additionally, certain recreational drugs and medications, including benzodiazepines, alcohol, barbiturates, antidepressants, certain antipsychotics, cannabis, heroin, cocaine, and other stimulants, are recognized for suppressing REM sleep and inducing REM rebound upon cessation. This phenomenon typically resolves over time.
Enhancing Healthcare Team Outcomes
Various healthcare specialties address health issues caused by poor sleep quality in individuals. Underlying sleep disorders can potentially cause REM rebound. OSA is a prevalent medical condition that significantly deteriorates the sleep quality in patients. The diagnostic gold standard for OSA is overnight polysomnography, and the most effective treatment is overnight CPAP.[19][20] A meta-analysis comparing treatments for OSA reveals no significant difference in outcomes between CPAP and bilevel-positive airway pressure (BiPAP). Nevertheless, treatment compliance remains a significant challenge for all OSA treatments except for surgical intervention.[20] Notably, a study by Koo et al demonstrated that the onset of REM sleep rebound after initial OSA treatment correlated with improved sleep quality and elevated mood.[21]
A multidisciplinary approach is essential to navigate the intricacies of OSA and other parasomnia treatments effectively. This approach utilizes the unique skills and strategies of various healthcare professionals tailored to the underlying diagnosis. Establishing an interprofessional team not only enhances care coordination and fosters patient-centered care but also raises the chances of improving treatment adherence and overall health outcomes. Furthermore, educating patients about the advantages of sleep hygiene as an integral component of their comprehensive care plan improves overall outcomes.
Media
(Click Image to Enlarge)
References
Suchecki D, Tiba PA, Machado RB. REM Sleep Rebound as an Adaptive Response to Stressful Situations. Frontiers in neurology. 2012:3():41. doi: 10.3389/fneur.2012.00041. Epub 2012 Apr 2 [PubMed PMID: 22485105]
Feriante J, Araujo JF. Physiology, REM Sleep. StatPearls. 2023 Jan:(): [PubMed PMID: 30285349]
Henry M, Thomas KGF, Ross IL. Sleep, Cognition and Cortisol in Addison's Disease: A Mechanistic Relationship. Frontiers in endocrinology. 2021:12():694046. doi: 10.3389/fendo.2021.694046. Epub 2021 Aug 27 [PubMed PMID: 34512546]
Machado RB, Rocha MR, Suchecki D. Brain prolactin is involved in stress-induced REM sleep rebound. Hormones and behavior. 2017 Mar:89():38-47. doi: 10.1016/j.yhbeh.2016.12.004. Epub 2016 Dec 23 [PubMed PMID: 28017595]
Gujar N, McDonald SA, Nishida M, Walker MP. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cerebral cortex (New York, N.Y. : 1991). 2011 Jan:21(1):115-23. doi: 10.1093/cercor/bhq064. Epub 2010 Apr 26 [PubMed PMID: 20421251]
Machado RB, Suchecki D. Neuroendocrine and Peptidergic Regulation of Stress-Induced REM Sleep Rebound. Frontiers in endocrinology. 2016:7():163. doi: 10.3389/fendo.2016.00163. Epub 2016 Dec 23 [PubMed PMID: 28066328]
Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of Healthy Sleep Duration among Adults--United States, 2014. MMWR. Morbidity and mortality weekly report. 2016 Feb 19:65(6):137-41. doi: 10.15585/mmwr.mm6506a1. Epub 2016 Feb 19 [PubMed PMID: 26890214]
Stephenson R, Caron AM, Famina S. Behavioral sleep-wake homeostasis and EEG delta power are decoupled by chronic sleep restriction in the rat. Sleep. 2015 May 1:38(5):685-97. doi: 10.5665/sleep.4656. Epub 2015 May 1 [PubMed PMID: 25669184]
Level 3 (low-level) evidenceMatricciani L, Olds T, Petkov J. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep medicine reviews. 2012 Jun:16(3):203-11. doi: 10.1016/j.smrv.2011.03.005. Epub 2011 May 25 [PubMed PMID: 21612957]
Level 1 (high-level) evidenceSteiger A. Neurochemical regulation of sleep. Journal of psychiatric research. 2007 Oct:41(7):537-52 [PubMed PMID: 16777143]
Arnaldi D, Famà F, De Carli F, Morbelli S, Ferrara M, Picco A, Accardo J, Primavera A, Sambuceti G, Nobili F. The Role of the Serotonergic System in REM Sleep Behavior Disorder. Sleep. 2015 Sep 1:38(9):1505-9. doi: 10.5665/sleep.5000. Epub 2015 Sep 1 [PubMed PMID: 25845692]
Henssler J, Heinz A, Brandt L, Bschor T. Antidepressant Withdrawal and Rebound Phenomena. Deutsches Arzteblatt international. 2019 May 17:116(20):355-361. doi: 10.3238/arztebl.2019.0355. Epub [PubMed PMID: 31288917]
Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep medicine reviews. 2015 Aug:22():23-36. doi: 10.1016/j.smrv.2014.10.001. Epub 2014 Oct 16 [PubMed PMID: 25454674]
Pagel JF, Parnes BL. Medications for the Treatment of Sleep Disorders: An Overview. Primary care companion to the Journal of clinical psychiatry. 2001 Jun:3(3):118-125 [PubMed PMID: 15014609]
Level 3 (low-level) evidenceCosta e Silva JA. Sleep disorders in psychiatry. Metabolism: clinical and experimental. 2006 Oct:55(10 Suppl 2):S40-4 [PubMed PMID: 16979426]
Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana. Sleep medicine reviews. 2008 Oct:12(5):381-9. doi: 10.1016/j.smrv.2007.12.004. Epub 2008 Mar 3 [PubMed PMID: 18313952]
Level 3 (low-level) evidenceMellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. The American journal of psychiatry. 2002 Oct:159(10):1696-701 [PubMed PMID: 12359675]
Horne J. REM sleep vs exploratory wakefulness: Alternatives within adult 'sleep debt'? Sleep medicine reviews. 2020 Apr:50():101252. doi: 10.1016/j.smrv.2019.101252. Epub 2019 Dec 23 [PubMed PMID: 31955131]
Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of Adult Obstructive Sleep Apnea With Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2019 Feb 15:15(2):301-334. doi: 10.5664/jcsm.7638. Epub 2019 Feb 15 [PubMed PMID: 30736888]
Level 1 (high-level) evidenceSlowik JM, Sankari A, Collen JF. Obstructive Sleep Apnea. StatPearls. 2023 Jan:(): [PubMed PMID: 29083619]
Koo BB, Wiggins R, Molina C. REM rebound and CPAP compliance. Sleep medicine. 2012 Aug:13(7):864-8. doi: 10.1016/j.sleep.2012.03.019. Epub 2012 Jun 15 [PubMed PMID: 22705243]