Introduction
The retina is a layer of photoreceptors cells and glial cells within the eye that captures incoming photons and transmits them along neuronal pathways as both electrical and chemical signals for the brain to perceive a visual picture. The retina is located in the posterior segment and forms the innermost boundary among the other major layers of the eye that include the vascular choroid and the fibrous sclera. Disease manifestations can occur in the retina at different stages of life, many of which severely compromise visual ability and consequently the quality of life.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The following topics relating to the structure and function of the retina appear below:
- Photoreceptor cells
- Layers of the retina
- Macula
Photoreceptor Cells
Photoreceptor cells include rods and cones and are uniquely located towards the posterior aspect of the retinal sublayers, further away from the pupil where light enters the eye. Rods are more sensitive in dim light (scotopic vision) and reside in the periphery of the retina. Cones are more sensitive in daylight (photopic vision) and capture wavelengths of colored light. Cones localize in the center of the retina at the fovea. There are approximately 6 million cones and often more than 100 million rods within the retina.[1] There exist three types of cones including tritans, deutrans, and protans, named for detecting short, medium, and long wavelengths, respectively. In terms of sensing colored light, each type of cone cell can respectively characterize as detecting blue, green, and red wavelengths. The overlap of detectable wavelength spectrums between the three types of cones results in the visible light spectrum perceived by humans. Rod cells contain rhodopsin, which is a light-sensitive pigment made of retinal that allows for the absorption of photons. Retinal is vitamin A aldehyde, making vitamin A an essential dietary component for the facilitation of the phototransduction pathway. Vitamin A deficiency is a significant risk factor for blindness in young children and remains prominent in under-developed regions, including South Asia and sub-Saharan Africa.[2]
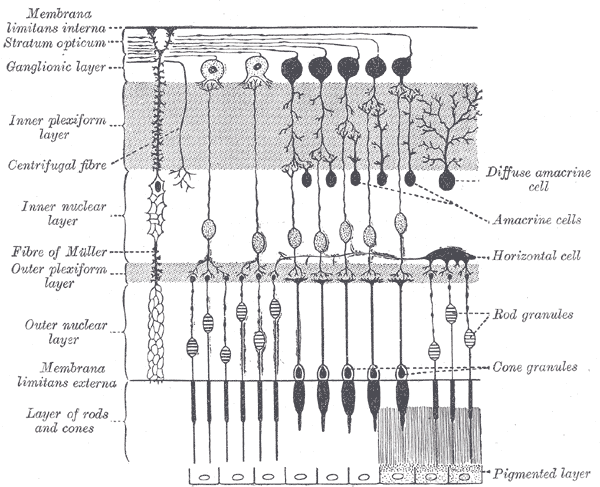
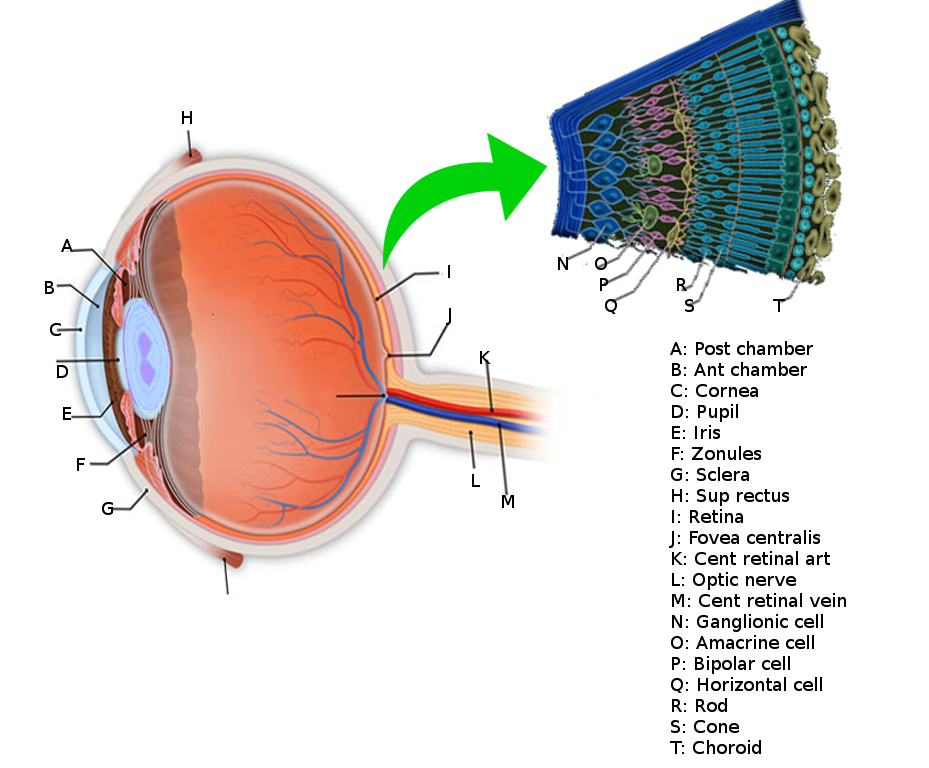
Layers of the Retina
The retina, more specifically, subdivides into ten distinct layers that are described in order from the innermost layers closer to the pupil to the layers further towards the posterior and periphery of the eyeball:
- Inner Limiting Membrane – the innermost layer of the retina that forms a smooth boundary against the vitreous humor which fills the vitreous chamber of the eye. The peripheral boundary of this layer consists of Müller glial cells, which function to maintain retinal homeostasis by upholding retinal lamination and by supporting other cells.[3]
- Retinal Nerve Fiber Layer – the layer composed of retinal ganglion cell axons mixed with astrocytes and the processes of the Muller cells. The inner limiting membrane serves as the basal lamina for the cells of the retinal nerve fiber layer.[4]
- Ganglion Cell Layer – the layer of ganglion cell bodies that project their axons, eventually to form the optic nerve.
- Inner Plexiform Layer – this layer is where the axons of bipolar cells synapse onto the ganglion cells. The dendrites of amacrine cells also synapse at this zone and function in modulating the electrical conduction between the bipolar cells and ganglion cells, preventing lateral potentiation.[5][6]
- Inner Nuclear Layer – the layer composed of the cell bodies of bipolar cells, horizontal cells, and amacrine cells. Bipolar cells function as channels that transmit and encode various synaptic inputs from photoreceptor cells onto ganglion cells.[7] Horizontal cells provide feedback modulation onto rod and cone cells.[8]
- Outer Plexiform Layer – the region where projections from photoreceptor cells synapse with the dendrites of the cells residing in the inner nuclear layer.
- Outer Nuclear Layer – the layer containing the cell bodies of both rods and cones.
- External Limiting Membrane – the region that is composed of gap-junctions between photoreceptor cells and Muller cells; it separates the cell bodies of the rods and cones from their inner segments and outer segments.
- Photoreceptor Layer – the region consisting of the inner segments and outer segments of rods and cones. The outer photoreceptor segments consist of membrane-bound discs that contain the light-sensitive pigments such as rhodopsin that are necessary for phototransduction. The inner segments house the abundance of mitochondria needed to meet the high metabolic demands of the photoreceptor cells.[9]
- Retinal Pigment Epithelium – the outermost retinal layer that spans a width of a single cell located between the neural retina and the Bruch membrane, adjacent to the highly-vascularized choroid layer. The retinal pigment epithelium (RPE) contributes to the blood-retinal barrier in conjunction with the endothelium of the retinal vessels and has many functions including ion and water transport and secretion of growth factors and cytokines.[10] The RPE cells intermingle with the outer segments of the rods and cones. This proximity allows for the recycling of all-trans-retinal back into 11-cis-retinal and its delivery back to the cones and rods to be used again for phototransduction.[11] RPE cells are crucial in the support and maintenance of both photoreceptor cells and the underlying capillary endothelium.
Macula
The macula, also called the macula lutea for its yellowish pigmented appearance, makes up the most sensitive area of the retina, offering the highest visual acuity. It is found temporally from the optic disc upon fundoscopic examination. Lutein and zeaxanthin are carotenoids that make up the macular pigments and produce the yellow coloring. These macular pigments are known to have anti-inflammatory and blue-light filtering properties.[12] Dietary supplementation of lutein and zeaxanthin has been shown to increase pigment density and is associated with reduced risk of diabetic retinopathy in adults and retinopathy of prematurity in infants born pre-term.[13] In the center of the macula is an avascular depression called the fovea, which contains a high concentration of cones.
The macula further subdivides into the following sequentially-smaller concentric zones that characteristically show decreasing rod density and fewer layers of cells covering the photoreceptor cells:
- Perifovea
- Parafovea
- Fovea
- Foveal avascular zone
- Foveola
- Umbo
Embryology
The retina arises from the diencephalon of the embryo as the optic vesicle during the first month and forms an invagination called the optic sulcus.[14] This process of invagination results in the formation of the neural layers and the pigmented layer of the retina. During this time, mesenchymal tissue will surround the optic vesicle, which will eventually develop into the uvea.[15] The neural retina originates from the inner layer, and the pigmented retina originates from the peripheral layer. Axons from the ganglion cells travel through the optic stalk that bridges the optic cup to the embryo and eventually form the optic nerve.[3] Ventrally, another invagination called the optic fissure engulfs a temporary blood vessel called the hyaloid artery which ultimately develops into the central retinal artery.
Blood Supply and Lymphatics
Blood vessels and the choroid vascularizes the retina. The choroid supplies the outer layers of the retina while branches of significant blood vessels supply the inner retinal layers. Descriptions of the individual vessels that contribute to the vascularization of the retina appear below.
Central Retinal Artery – the major vessel that supplies the inner layers of the retina; it travels inside of the optic nerve sheath and similarly penetrates the eye at the optic disc. The central retinal artery divides into superior and inferior arcades that will form the blood-retina barrier.[16] It originates as a major branch of the ophthalmic artery.
Central Retinal Vein – the main drainage pathway of the retina and travels alongside the central retinal artery within the sheath of the optic nerve.
Long Posterior Ciliary Arteries – these two vessels branch from the ophthalmic artery and pierce the sclera on the posterior of the eye near the entry zone of the optic nerve. The long posterior ciliary arteries supply the choroid in the medial and lateral horizontal planes and, eventually, the anterior structures of the eye.
Short Posterior Ciliary Arteries – these vessels arise as a few branches from the ophthalmic artery and subsequently branch into 10 to 20 smaller vessels that penetrate the posterior sclera in a ring around the optic nerve. These branched vessels anastomose to form the circle of Zinn that encircles and supplies the area of the optic cup at the level of the choroid. Perpendicular terminal arterioles from the short posterior ciliary arteries also supply the Bruch membrane and the outer retina.[16]
Choroid – the second major layer, or tunic, of the eye that vascularizes the outer layers of the retina.[15] The Bruch membrane sits between the retinal pigment epithelial layer and the choriocapillaris, forming the basement membrane of the choroid. The choriocapillaris, also known as the capillary lamina of the choroid, is the thickest behind the fovea (10 micrometers) and thins out towards the periphery (7 micrometers).[15]
The retina does not contain lymphatic vessels.[17]
Nerves
The optic nerve is the primary sensory tract for visual information collected by the photoreceptor cells of the retina to travel to the brain. The axons of the ganglion cells that form the optic nerve converge and exit from the globe at the optic disc where there is an absence of rods and cones, resulting in a deficit within the visual field called the blind spot.[18]
Physiologic Variants
Myopia is a common refractive disorder where the focal convergence of light that passes through the cornea and the lens falls short of the fovea, resulting in nearsightedness. One cause of myopia is the axial elongation of the globe, which results in the straining of the retina. High myopia, which is approximately an error of refraction between -6.0 to -8.0 diopters, is a risk factor for retinal detachment and is common in Eastern Asia.[19] In severe cases of myopia, individuals may develop pathological myopia which is distinct from high myopia in that not only is there an axial elongation of the globe but also characteristic deformations of the posterior segment, such as posterior staphyloma, that often result in retinal lesions.[20] Posterior staphyloma is an abnormally smaller curvature radius of the posterior pole that includes the retina and often the uvea. It results from the bulging and thinning of the sclera from axial elongation. The collection of pathologies of the posterior pole that specifically affect the macula are known as myopic maculopathies and are among the leading causes of low vision and legal blindness in the world.[20]
Ocular albinism is an X-linked disorder where there is hypopigmentation of the fundus that often presents with congenital nystagmus, iris translucency, severely reduced visual acuity and strabismus.[21][22] Ocular albinism occurs from a mutation in a gene that codes for the G protein-coupled receptor 143 that is thought to be a melanosome transmembrane protein, resulting in a defect with the transportation of melanin into the retinal pigment epithelium and the iris.[23]
Surgical Considerations
Round hole retinal detachment is often surgically repaired by laser demarcation which promotes adhesion between the neural retinal layers and the retinal pigment epithelium.[24] Laser photocoagulation is commonly used for small detachments since the procedure only limits the progression of the subretinal fluid, acting as a barrier.
In cases of vitreous cloudings, such as steroid hyalosis or vitreoretinal traction where the degradation of the vitreous pulls on the retina, a vitrectomy is a commonly performed procedure. Vitrectomy involves vitreous removal, done through the pars plana of the sclera.
Clinical Significance
The following are pathologies relating to the retina:
- Retinal detachment
- Retinal artery occlusion
- Age-related macular degeneration
- Glaucoma
- Diabetic retinopathy
- Cytomegalovirus retinitis
- Retinopathy of prematurity
- Retinitis pigmentosa
- Retinoblastoma
- Central serous chorioretinopathy
- Color vision deficiency
Retinal Detachment – the disconnection of the retinal layers occurring between the retinal pigment epithelium and the inner neural layers of the retina resulting in ischemia and subsequent photoreceptor degeneration. Permanent vision loss can be prevented with early detection and treatment, as retinal detachment is usually an ocular emergency. Prominent risk factors for retinal detachment include myopia, trauma, cataract surgery, diabetic retinopathy, and old age.[25] There are three types of retinal detachments, including rhegmatogenous, tractional, and exudative. Rhegmatogenous retinal detachment is the most common of the three; it a tear in the retina that allows liquified vitreous to seep under the retinal layers.[26] Posterior vitreous detachment is a common cause of rhegmatogenous retinal detachment where the collagenous fibers of the vitreous fail to separate from the inner limiting membrane of the retina during the natural condensation of the vitreous, resulting in a retinal tear as it pulls away. Tractional retinal detachment occurs less frequently and results from retinal scarring often seen in diabetic retinopathy.[26] Physical disruption of the subretinal space from the fluid collection and blood-retinal barrier breakdown without retinal tears or holes is known as exudative retinal detachment and results from diseases such as intraocular tumors or age-related macular degeneration.[27]
Retinal Artery Occlusion – the blockage of either the central retinal artery or the branch retinal arteries resulting in retinal ischemia and severe vision loss. Central retinal artery occlusion (CRAO) is an ophthalmic emergency that is often caused by atherosclerosis or an embolism originating in the carotid arteries.[28] Infarction leads to ischemia in the inner retinal layers contributing to the loss of vision and sometimes retinal hemorrhages. The common sign present with CRAO upon fundoscopic examination is the appearance of a cherry-red spot on the macula with a pale clearance around its periphery due to ischemia. Branch retinal artery occlusions (BRAO) commonly occur temporally at branching points between smaller vessels of the central retinal artery and account for approximately 38% of all acute retinal occlusions.[29] BRAO has better visual prognosis than CRAO.[29][30]
Age-Related Macular Degeneration – a neurodegenerative disease that compromises the junction between the neural retinal layers and the retinal pigment epithelium (RPE) and results in severe central vision loss. Age-related macular degeneration (AMD) categorizes into two types that include the non-neovascular atrophic type known as “dry” macular degeneration and the neovascular type that is known as “wet” macular degeneration. Dry AMD demonstrates characteristic lesions of the RPE as a result of the build-up of cellular debris called lipofuscin, which, in turn, causes the formation of the pathognomonic yellowish, amorphic deposits called drusen between the RPE and the underlying the Bruch membrane.[31] Wet AMD results from abnormal neovascularization that extends from the choroid and penetrates the Bruch membrane. These fragile vessels can result in spontaneous hemorrhage and subsequent leakage of plasma, causing macular edema. The non-neovascular atrophic type (dry) is more common of the two types and is seen in 90% of all cases while the other 10% is in the form of neovascular AMD.[31] Increased age is a prominent risk factor for AMD, which is the leading cause of blindness among both males and females aged 55 years and older.[32]
Glaucoma – the gradual degeneration of ganglion cells and their axons resulting in the loss of peripheral and, eventually, central vision.[33] This loss in ganglion cells results in optic cupping where the optic nerve cup becomes larger in comparison to the optic disc as the neuroretinal rim of the optic disc thins from degeneration.[34] Optic cupping may be present in patients without glaucoma, but an increased size ratio of the optic cup to the optic disc along with particular margins of the optic cup can be suggestive of glaucoma.[35] Glaucoma categorizes as either closed-angle or open-angle, which describes the iridocorneal angle. In closed-angle glaucoma, the iridocorneal angle closes, such that the iris is pressed against the cornea and obstructs the flow of aqueous humor from being collected by the trabecular meshwork with a resulting increase in intraocular pressure that causes the described optic neuropathy. In the case of open-angle glaucoma where the iridocorneal angle is not obstructing the flow of aqueous humor, the etiology is not well understood and may or may not present with increased intraocular pressure.[33]
Diabetic Retinopathy – retinal microvascular pathology that results from elevated blood sugar and presents with both type I diabetes and type II diabetes. It is the most common complication within diabetic eye disease which is the leading cause of blindness in adults under 75 years of age in developed countries.[36] Diabetic retinopathy can generally be classified into two stages that include the non-proliferative stage, which often presents early among patients with diabetes, and the more advanced proliferative stage. Non-proliferative diabetic retinopathy is characterized by the degeneration of the retinal microvasculature that often results in microaneurysms and the leakage of plasma through the compromised blood-retinal barrier. This leakage of fluid can potentially accumulate and result in macular edema, which can lead to the loss of visual acuity.[37] In proliferative diabetic retinopathy, neovascularization occurs in reaction to artery occlusion and ischemia. These fragile vessels are prone to rupturing which results in vitreous hemorrhage where leaked blood in the vitreous can block light from hitting the fovea and also cause scarring which can result in tractional retinal detachment.[36]
Cytomegalovirus Retinitis – an opportunistic viral infection of the retina caused by cytomegalovirus (CMV) which is a member of the herpes virus family. CMV retinitis is characterized by progressive retinal necrosis and scarring that can lead to retinal detachment, macular edema, and vision loss.[38] Populations that are considered high risk for CMV retinitis are characterized by the following: having HIV/AIDS, immunosuppression, or organ transplantation. CMV retinitis is the most common intraocular complication in patients with HIV and is the leading cause of blindness in patients who have AIDS.[38] Treatments often include antiviral medications that can be administered orally, intravenously, or through ocular injection.[39]
Retinopathy of Prematurity – the abnormal proliferation of blood vessels and subsequent incomplete vascularization of the retina in the developing newborn as a result of premature birth. The pathological growth of the retinal blood vessels is due to hypoxia and in extensive cases can lead to a retinal detachment which may result in blindness if left untreated.[40] Retinopathy of prematurity is classified by five stages that describe the severity of the disease and hallmark pathological formations.[41]
Retinitis Pigmentosa – a group of inherited rod-cone dystrophies characterized by the gradual loss of rods from the periphery followed by the loss of cones that are more centrally located in the retina. This progressive degeneration often results in tunnel vision and, in extensive cases, complete vision loss. Retinitis pigmentosa is also known as hereditary retinal dystrophy and is the most common inherited retinal disease.[42] The mode of inheritance can be autosomal dominant, autosomal recessive, or X-linked recessive. Night blindness, or nyctalopia, is a common symptom seen during early stages of retinitis pigmentosa, while retinal atrophy with bone-spicule shaped pigment deposits present during middle and late stages upon fundus examination.[43]
Retinoblastoma – a malignant tumor of the developing neural retina of young children due to a loss-of-function mutation in the Rb1 tumor suppressor gene.[44] Retinoblastoma is the most common childhood intraocular neoplasm and accounts for approximately 3% of all pediatric cancers.[45] Major clinical features of retinoblastoma include leukocoria and strabismus.
Central Serous Chorioretinopathy – a macular disease where the retinal pigment epithelium is compromised, resulting in fluid leakage from the capillary lamina of the choroid into the subretinal area. The resulting macular edema causes central vision loss where 30 to 40% of cases have shown disease recurrence while most other cases are self-limiting.[46] Central serous chorioretinopathy is more prevalent in middle-aged males and is associated with exogenous glucocorticoid usage.[47]
Color Vision Deficiency – abnormal color perception and reduced color contrast sensitivity as a result of photopigment defects within the cone cells of the retina. The most common variation is red-green color blindness, which results from a deficit or abnormality in either the red (protans) or the green (deutrans) cone cells. All color vision disorders that contribute to red-green colorblindness are passed between generations in an X-linked recessive mode of inheritance, affecting males more than females.[48] The dysfunctionalities of red and green cone cells are known as protanopia and deuteranopia, respectively. Protanomaly and deuteranomaly characteristically demonstrate abnormal red and green photopigments resulting in a milder color vision deficiency. Blue-yellow color blindness is rarer than red-green color blindness and results from dysfunctional blue cone cells (tritanopia) or abnormal blue photopigments (tritanomaly). The modes of inheritance for tritanopia and tritanomaly are autosomal dominant and autosomal recessive, affecting males and females equally.[48][49] Total color vision deficiency is the most severe form of color blindness and results from rod monochromatism and cone monochromatism. Rod monochromatism, also known as achromatopsia, is a complete deficit in any functionality of cones cells resulting in complete grayscale vision solely from rod cells. Cone monochromatism is characterized as a deficit in two out of the three types of cone cells, preventing the ability to compare color vision stimuli, which is a requirement for the brain to perceive color. Individuals with achromatopsia or cone monochromatism often have poor visual acuity and often present with nystagmus.[50][51]
Other Issues
Drug Delivery Routes
With age-related macular degeneration and diabetic retinopathy becoming highly prevalent, there is increasing interest in improving drug delivery methods to the retina. The current standard is the use of intravitreal injections, which offers efficient bioavailability but is highly invasive and often leads to ocular complications with repeated use. Nanomedicine is a relatively new method of drug delivery to the retina, which involves the use of bioengineered nanoparticles such as hydrogels, liposomes, and synthetic polymers to carry and deliver drugs to target tissues.[52][53] These nanoparticles have engineered speculations of size and membrane composition to bypass selective structures such as the blood-retinal barrier and blood-aqueous barriers to specifically target the retina rather than all tissues in the proximity.
Media
(Click Image to Enlarge)
References
Rehman I, Hazhirkarzar B, Patel BC. Anatomy, Head and Neck, Eye. StatPearls. 2023 Jan:(): [PubMed PMID: 29494035]
Stevens GA, Bennett JE, Hennocq Q, Lu Y, De-Regil LM, Rogers L, Danaei G, Li G, White RA, Flaxman SR, Oehrle SP, Finucane MM, Guerrero R, Bhutta ZA, Then-Paulino A, Fawzi W, Black RE, Ezzati M. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. The Lancet. Global health. 2015 Sep:3(9):e528-36. doi: 10.1016/S2214-109X(15)00039-X. Epub [PubMed PMID: 26275329]
Level 3 (low-level) evidenceHeavner W, Pevny L. Eye development and retinogenesis. Cold Spring Harbor perspectives in biology. 2012 Dec 1:4(12):. doi: 10.1101/cshperspect.a008391. Epub 2012 Dec 1 [PubMed PMID: 23071378]
Level 3 (low-level) evidenceJonas JB, Dichtl A. Evaluation of the retinal nerve fiber layer. Survey of ophthalmology. 1996 Mar-Apr:40(5):369-78 [PubMed PMID: 8779083]
Level 3 (low-level) evidenceHartveit E, Veruki ML. Electrical synapses between AII amacrine cells in the retina: Function and modulation. Brain research. 2012 Dec 3:1487():160-72. doi: 10.1016/j.brainres.2012.05.060. Epub 2012 Jul 7 [PubMed PMID: 22776293]
Level 3 (low-level) evidenceTanaka M, Tachibana M. Independent control of reciprocal and lateral inhibition at the axon terminal of retinal bipolar cells. The Journal of physiology. 2013 Aug 15:591(16):3833-51. doi: 10.1113/jphysiol.2013.253179. Epub 2013 May 20 [PubMed PMID: 23690563]
Level 3 (low-level) evidenceEuler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: elementary building blocks of vision. Nature reviews. Neuroscience. 2014 Aug:15(8):507-19 [PubMed PMID: 25158357]
Level 3 (low-level) evidenceMasland RH. The neuronal organization of the retina. Neuron. 2012 Oct 18:76(2):266-80. doi: 10.1016/j.neuron.2012.10.002. Epub 2012 Oct 17 [PubMed PMID: 23083731]
Level 3 (low-level) evidenceNarayan DS, Chidlow G, Wood JP, Casson RJ. Glucose metabolism in mammalian photoreceptor inner and outer segments. Clinical & experimental ophthalmology. 2017 Sep:45(7):730-741. doi: 10.1111/ceo.12952. Epub 2017 May 17 [PubMed PMID: 28334493]
Spencer C, Abend S, McHugh KJ, Saint-Geniez M. Identification of a synergistic interaction between endothelial cells and retinal pigment epithelium. Journal of cellular and molecular medicine. 2017 Oct:21(10):2542-2552. doi: 10.1111/jcmm.13175. Epub 2017 Apr 12 [PubMed PMID: 28402065]
Kolb H, Fernandez E, Nelson R, Strauss O. The Retinal Pigment Epithelium. Webvision: The Organization of the Retina and Visual System. 1995:(): [PubMed PMID: 21563333]
Jia YP, Sun L, Yu HS, Liang LP, Li W, Ding H, Song XB, Zhang LJ. The Pharmacological Effects of Lutein and Zeaxanthin on Visual Disorders and Cognition Diseases. Molecules (Basel, Switzerland). 2017 Apr 20:22(4):. doi: 10.3390/molecules22040610. Epub 2017 Apr 20 [PubMed PMID: 28425969]
Gong X, Rubin LP. Role of macular xanthophylls in prevention of common neovascular retinopathies: retinopathy of prematurity and diabetic retinopathy. Archives of biochemistry and biophysics. 2015 Apr 15:572():40-48. doi: 10.1016/j.abb.2015.02.004. Epub 2015 Feb 18 [PubMed PMID: 25701588]
Level 3 (low-level) evidenceBales TR, Lopez MJ, Clark J. Embryology, Eye. StatPearls. 2023 Jan:(): [PubMed PMID: 30860715]
Nickla DL, Wallman J. The multifunctional choroid. Progress in retinal and eye research. 2010 Mar:29(2):144-68. doi: 10.1016/j.preteyeres.2009.12.002. Epub 2009 Dec 29 [PubMed PMID: 20044062]
Level 3 (low-level) evidenceGupta N,Singh G, Anatomy, Head and Neck, Eye Arteries 2019 Jan; [PubMed PMID: 30725748]
Chen L. Ocular lymphatics: state-of-the-art review. Lymphology. 2009 Jun:42(2):66-76 [PubMed PMID: 19725271]
Smith AM, Czyz CN. Neuroanatomy, Cranial Nerve 2 (Optic). StatPearls. 2023 Jan:(): [PubMed PMID: 29939684]
Ohno-Matsui K. WHAT IS THE FUNDAMENTAL NATURE OF PATHOLOGIC MYOPIA? Retina (Philadelphia, Pa.). 2017 Jun:37(6):1043-1048. doi: 10.1097/IAE.0000000000001348. Epub [PubMed PMID: 27755375]
Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CM, Saw SM, Verhoeven VJ, Klaver CC, Moriyama M, Shinohara K, Kawasaki Y, Yamazaki M, Meuer S, Ishibashi T, Yasuda M, Yamashita H, Sugano A, Wang JJ, Mitchell P, Wong TY, META-analysis for Pathologic Myopia (META-PM) Study Group. International photographic classification and grading system for myopic maculopathy. American journal of ophthalmology. 2015 May:159(5):877-83.e7. doi: 10.1016/j.ajo.2015.01.022. Epub 2015 Jan 26 [PubMed PMID: 25634530]
Level 2 (mid-level) evidenceKubasch AS, Meurer M. [Oculocutaneous and ocular albinism]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete. 2017 Nov:68(11):867-875. doi: 10.1007/s00105-017-4061-x. Epub [PubMed PMID: 29018889]
Preising M, Op de Laak JP, Lorenz B. Deletion in the OA1 gene in a family with congenital X linked nystagmus. The British journal of ophthalmology. 2001 Sep:85(9):1098-103 [PubMed PMID: 11520764]
Level 3 (low-level) evidenceJia X, Yuan J, Jia X, Ling S, Li S, Guo X. GPR143 mutations in Chinese patients with ocular albinism type 1. Molecular medicine reports. 2017 May:15(5):3069-3075. doi: 10.3892/mmr.2017.6366. Epub 2017 Mar 23 [PubMed PMID: 28339057]
Greenberg PB, Baumal CR. Laser therapy for rhegmatogenous retinal detachment. Current opinion in ophthalmology. 2001 Jun:12(3):171-4 [PubMed PMID: 11389341]
Level 3 (low-level) evidencePokhrel PK, Loftus SA. Ocular emergencies. American family physician. 2007 Sep 15:76(6):829-36 [PubMed PMID: 17910297]
Feltgen N, Walter P. Rhegmatogenous retinal detachment--an ophthalmologic emergency. Deutsches Arzteblatt international. 2014 Jan 6:111(1-2):12-21; quiz 22. doi: 10.3238/arztebl.2014.0012. Epub [PubMed PMID: 24565273]
Amer R,Nalcı H,Yalçındağ N, Exudative retinal detachment. Survey of ophthalmology. 2017 Nov - Dec; [PubMed PMID: 28506603]
Level 3 (low-level) evidenceCugati S, Varma DD, Chen CS, Lee AW. Treatment options for central retinal artery occlusion. Current treatment options in neurology. 2013 Feb:15(1):63-77. doi: 10.1007/s11940-012-0202-9. Epub [PubMed PMID: 23070637]
Mason JO 3rd, Shah AA, Vail RS, Nixon PA, Ready EL, Kimble JA. Branch retinal artery occlusion: visual prognosis. American journal of ophthalmology. 2008 Sep:146(3):455-7. doi: 10.1016/j.ajo.2008.05.009. Epub 2008 Jul 2 [PubMed PMID: 18599018]
Level 2 (mid-level) evidenceHayreh SS, Podhajsky PA, Zimmerman MB. Branch retinal artery occlusion: natural history of visual outcome. Ophthalmology. 2009 Jun:116(6):1188-94.e1-4. doi: 10.1016/j.ophtha.2009.01.015. Epub 2009 Apr 18 [PubMed PMID: 19376586]
Level 2 (mid-level) evidenceZając-Pytrus HM, Pilecka A, Turno-Kręcicka A, Adamiec-Mroczek J, Misiuk-Hojło M. The Dry Form of Age-Related Macular Degeneration (AMD): The Current Concepts of Pathogenesis and Prospects for Treatment. Advances in clinical and experimental medicine : official organ Wroclaw Medical University. 2015 Nov-Dec:24(6):1099-104. doi: 10.17219/acem/27093. Epub [PubMed PMID: 26771984]
Level 3 (low-level) evidenceChakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, Buggage R, Pleil A, Mitchell P. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC ophthalmology. 2010 Dec 13:10():31. doi: 10.1186/1471-2415-10-31. Epub 2010 Dec 13 [PubMed PMID: 21144031]
Level 1 (high-level) evidenceDietze J, Blair K, Havens SJ. Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 30855805]
Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. The New England journal of medicine. 2009 Mar 12:360(11):1113-24. doi: 10.1056/NEJMra0804630. Epub [PubMed PMID: 19279343]
Kirsch RE, Anderson DR. Clinical Recognition of Glaucomatous Cupping. American journal of ophthalmology. 2018 Sep:193():xxviii-xxxviii. doi: 10.1016/j.ajo.2018.06.008. Epub [PubMed PMID: 30144901]
Williams R, Airey M, Baxter H, Forrester J, Kennedy-Martin T, Girach A. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye (London, England). 2004 Oct:18(10):963-83 [PubMed PMID: 15232600]
Level 1 (high-level) evidenceCOGAN DG, TOUSSAINT D, KUWABARA T. Retinal vascular patterns. IV. Diabetic retinopathy. Archives of ophthalmology (Chicago, Ill. : 1960). 1961 Sep:66():366-78 [PubMed PMID: 13694291]
Vadlapudi AD, Vadlapatla RK, Mitra AK. Current and emerging antivirals for the treatment of cytomegalovirus (CMV) retinitis: an update on recent patents. Recent patents on anti-infective drug discovery. 2012 Apr:7(1):8-18 [PubMed PMID: 22044356]
Level 3 (low-level) evidenceNewman H, Gooding C. Viral ocular manifestations: a broad overview. Reviews in medical virology. 2013 Sep:23(5):281-94. doi: 10.1002/rmv.1749. Epub 2013 Jun 25 [PubMed PMID: 23797960]
Level 3 (low-level) evidenceFagerholm R, Vesti E. Retinopathy of prematurity - from recognition of risk factors to treatment recommendations. Duodecim; laaketieteellinen aikakauskirja. 2017:133(4):337-44 [PubMed PMID: 29205980]
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of ophthalmology (Chicago, Ill. : 1960). 2005 Jul:123(7):991-9 [PubMed PMID: 16009843]
O'Neal TB, Luther EE. Retinitis Pigmentosa. StatPearls. 2023 Jan:(): [PubMed PMID: 30137803]
Ali MU, Rahman MSU, Cao J, Yuan PX. Genetic characterization and disease mechanism of retinitis pigmentosa; current scenario. 3 Biotech. 2017 Aug:7(4):251. doi: 10.1007/s13205-017-0878-3. Epub 2017 Jul 18 [PubMed PMID: 28721681]
Mendoza PR, Grossniklaus HE. The Biology of Retinoblastoma. Progress in molecular biology and translational science. 2015:134():503-16. doi: 10.1016/bs.pmbts.2015.06.012. Epub 2015 Jul 14 [PubMed PMID: 26310174]
Rao R, Honavar SG. Retinoblastoma. Indian journal of pediatrics. 2017 Dec:84(12):937-944. doi: 10.1007/s12098-017-2395-0. Epub 2017 Jun 16 [PubMed PMID: 28620731]
Nicholson BP, Atchison E, Idris AA, Bakri SJ. Central serous chorioretinopathy and glucocorticoids: an update on evidence for association. Survey of ophthalmology. 2018 Jan-Feb:63(1):1-8. doi: 10.1016/j.survophthal.2017.06.008. Epub 2017 Jun 30 [PubMed PMID: 28673727]
Level 3 (low-level) evidenceLiew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clinical & experimental ophthalmology. 2013 Mar:41(2):201-14. doi: 10.1111/j.1442-9071.2012.02848.x. Epub 2012 Sep 21 [PubMed PMID: 22788735]
Cole BL. Assessment of inherited colour vision defects in clinical practice. Clinical & experimental optometry. 2007 May:90(3):157-75 [PubMed PMID: 17425762]
HENRY GH, COLE BL, NATHAN J. THE INHERITANCE OF CONGENITAL TRITANOPIA WITH THE REPORT OF AN EXTENSIVE PEDIGREE. Annals of human genetics. 1964 Mar:27():219-31 [PubMed PMID: 14128207]
Tsang SH, Sharma T. Rod Monochromatism (Achromatopsia). Advances in experimental medicine and biology. 2018:1085():119-123. doi: 10.1007/978-3-319-95046-4_24. Epub [PubMed PMID: 30578497]
Level 3 (low-level) evidenceAboshiha J, Dubis AM, Carroll J, Hardcastle AJ, Michaelides M. The cone dysfunction syndromes. The British journal of ophthalmology. 2016 Jan:100(1):115-21. doi: 10.1136/bjophthalmol-2014-306505. Epub 2015 Mar 13 [PubMed PMID: 25770143]
Jiang S, Franco YL, Zhou Y, Chen J. Nanotechnology in retinal drug delivery. International journal of ophthalmology. 2018:11(6):1038-1044. doi: 10.18240/ijo.2018.06.23. Epub 2018 Jun 18 [PubMed PMID: 29977820]
Bozzuto G, Molinari A. Liposomes as nanomedical devices. International journal of nanomedicine. 2015:10():975-99. doi: 10.2147/IJN.S68861. Epub 2015 Feb 2 [PubMed PMID: 25678787]