Introduction
The sensory system is the portion of the nervous system responsible for processing input from the environment. Beginning with detection through the transfer of stimuli to the central nervous system, the peripheral nerves and their associated receptors rapidly relay information. The peripheral nervous system consists of the somatosensory nervous system and autonomic nervous system. The sensory pathway of the somatosensory system involves spinal nerves which transmit information about the external environment to the spinal cord. The autonomic nervous system has visceral sensory neurons which are responsible for monitoring the internal environment and eliciting appropriate changes in effector organs to maintain homeostasis. This article will address both somatic and visceral sensory neurons with an emphasis on the clinical significance of somatic sensory neuropathy.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The anatomy of peripheral nerves consists of nerve fibers, supporting connective tissue, and blood supply. Sensory neurons are the afferent limb of somatosensory neural pathways. The neuron consists of a cell body, axon, and dendrites. Dendrites are finger-like projections that receive sensory input and transmit the signal through the axon to the cell body. Unipolar cell bodies of sensory neurons are located within sensory ganglia which may be in the dorsal root of the spinal cord or along cranial nerves. The receptive field of the neurons limits the ability of the sensory system to relay environmental information. An individual neuron's receptive field is the space in which a stimulus can modify the electrical activity of the neuron. There are different types of receptors for differing stimuli: thermoreceptors, mechanoreceptors, nociceptors, photoreceptors, and chemoreceptors. The receptors within a specific field react to stimuli by generating electrical activity along the associated first-order neuron in the form of an action potential.
Sensory nerves have different types of nerves fibers depending on their associated receptors. Classification of sensory nerves includes the numerical or Erlanger and Gasser system.[1] Proprioceptors (position sensors) receive innervation via type Ia (A-alpha: muscle spindle), Ib (A-alpha: Golgi tendon organ), and II (A-beta: touch and pressure) sensory fibers. These fibers are large and myelinated with rapid conduction velocities. Mechanoreceptor innervation is by type II and III (A-delta: free nerve endings, cold) sensory fibers. Nociceptors (pain sensors) and thermoreceptor innervation by type III and IV (C: slow pain, heat) fibers. A-delta fibers are thinly myelinated and transmit information primarily related to acute pain to facilitate a withdrawal reflex upon synapse in the dorsal horn of the spinal cord. C fibers are smaller, unmyelinated fibers that require a higher threshold of stimulus than A-delta fibers. These are responsible for the slower onset of deeper pain after an initial insult relayed by the faster A-delta fibers.[2]
To summarize, in order of decreasing diameter and velocity:
- Proprioceptors: A-alpha, A-beta
- Mechanoreceptors: A-beta, A-delta
- Nociceptors and thermoreceptors: A-delta, C-fiber
The supportive structures of the nerve fibers include the mesoneurium, epineurium, perineurium, endoneurium, and myelin sheath.[3] The mesoneurium is the connective tissue sheath that suspends the nerve trunk within the soft tissue and is continuous with the underlying epineurium. The epineural sheath contains the extrinsic blood vessels, and further internal plexuses lie in the epineurium, perineurium, and endoneurium. The interfascicular epineurium is loose connective tissue composed of longitudinal collagen fibers that protect the nerve trunk against mechanical stress. The perineurium is the connective tissue layer covering individual fascicles or bundles of axons. The endoneurium is the fibrous tissue directly covering individual axons. Individual axons are insulated by myelin (except for C fibers) which is produced by Schwann cells in the peripheral nervous system.
Visceral sensory nerves transmit pain, stretch, temperature, and chemical change in visceral organs which gets interpreted as sensations like nausea, hunger, gas, cramping, etc.[4] General visceral afferent fibers are considered part of the autonomic nervous system, but unlike the efferent arm, GVA fibers do not classify as sympathetic or parasympathetic.[5] GVA run with general somatic afferent (GSA) fibers in the gray matter of the dorsal horn and can cause referred pain. Referred pain takes place in the dermatome of the corresponding spinal segment of the signal-producing internal organ. For example, myocardial ischemia can refer to the left shoulder; this is due to misinterpretation of the visceral signal as a somatic pain signal by the cortex since the fibers run together centrally. Cranial nerves with GVA fibers include the glossopharyngeal nerve and vagus nerve and explains "brain freezes" as thermoreceptors of the palate sense something very cold causing reflexive vasoconstriction mediated by cranial nerves IX and X resulting in engorged sinus capillaries causing a headache.
Embryology
The neural crest cells are the origin of the peripheral sensory nervous system. Neural crest cells are the detached cells of the neural plate as it separates from overlaying ectoderm. These cells give rise to the peripheral neurons with cell bodies and Schwann cells. A pair of dorsal root ganglia develop in a craniocaudal succession generating seven cervical, twelve thoracic, five lumbar, five sacral, and one pair of coccygeal dorsal root ganglia. Studies have shown that survival and differentiation of dorsal root ganglion cells rely on growth factors secreted by the neural tube including nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor (BDNF).[6][7] All peripheral nerves are initially bipolar, but the two polar processes then unite to form a single unipolar process with a peripheral extension that terminates in an afferent end with dendrites and a central extension that terminates in the spinal cord to synapse with a second-order neuron.
Blood Supply and Lymphatics
The endoneurial blood supply of peripheral nerves is the vasa nervorum. The vasa nervorum vessels are branches of adjacent vessels and are especially numerous near joints.[8] The tortuous nature of the vasa nervorum facilitates necessary translational movement of the peripheral nerves around joints. Also, these nutrient arteries form anastomoses throughout the course of the nerve and establish an extensive microvascular network that maintains the nutrition of a nerve even with the inevitable "watershed" areas that form due to variable intervals between supplying vessels.[9][10] This arterial supply contrasts with the blood supply to second-order neurons in the spinal cord because end-arteries supply the spinal cord.
Nerves
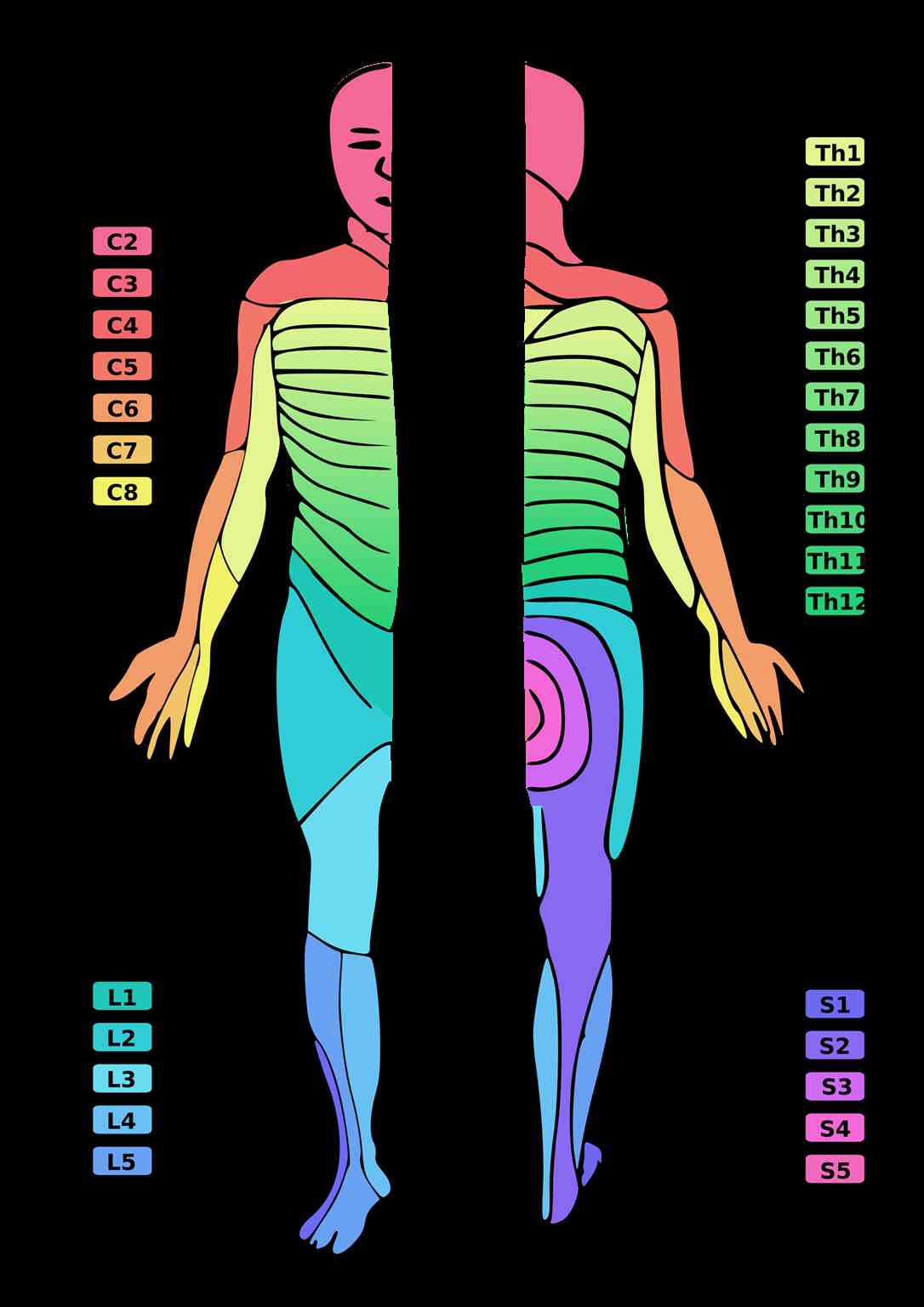
The receptive field of a peripheral sensory nerve (peripheral nerve field) crosses over different dermatomes. A dermatome is an area of skin supplied by a single spinal nerve. Therefore, the map of peripheral nerve fields over the body differs from the dermatomal distribution since individual peripheral nerves are composed of multiple nerve roots. Physicians use these known fields to map sensory deficits and localize lesions.
Physiologic Variants
Recognizing physiologic variations in sensory anatomy is clinically significant because accurate EMG or NCV studies rely on the correct mapping of nerves.[11] Knowledge of anatomic variations of sensory nerves is especially useful for the surgeon harvesting skin flaps that need an accessible donor sensory nerve.[12] Examples of variations recorded in the literature include the lateral antebrachial cutaneous nerve innervating the radial border of the dorsum of the hand instead of the superficial radial nerve.[13] The most extensive literature of sensory nerve variation is of the hand with median, ulnar, and radial nerves.[14][15][16]
Surgical Considerations
Peripheral nerve surgery includes repair of acute nerve injuries, entrapment neuropathies, and nerve sheath tumor resection. Acute nerve injury can result from stretching, compression, or laceration. An example of nerve stretching is brachial plexus injury during vaginal delivery of an infant with shoulder dystocia resulting in Erb palsy.[17] Nerve stretching is typically treated conservatively with surgical exploration reserved for severe cases where spontaneous recovery with physical therapy does not happen after several months, or if tearing of nerves is suspected.[18] Entrapment neuropathies occur when a nerve is externally compressed causing compromise of blood supply and local ischemia. Symptoms include paresthesia or muscle weakness. Examples of entrapment/compression neuropathy requiring surgical intervention include carpal or tarsal tunnel syndrome, herniated disc compressing nerve roots, or thoracic outlet syndrome.[19]
Peripheral nerve sheath tumors (PNST) can be benign or malignant. Benign tumors include neurofibromas or schwannomas, and malignant PNST are typically sarcomatous. Surgical consideration of the nerve fascicle that gave rise to the PNST involves intraoperative neurophysiologic monitoring to establish if the associated fascicle has a motor function because that determines whether to do a complete or partial resection. Malignant PNST involves wide local excision, sometimes consisting of proximal amputation. Studies have shown that pre-operative biopsy of PNST results in increased risk of postoperative neurologic deficit, suggesting that referral for surgery without biopsy is recommended.[20][21] Finally, there is the management of nerve laceration, usually due to trauma. Sharp transection, such as seen with a stab wound, has a better prognosis than a crush injury. Repair involves surgical approximation of the damaged epineurium in a tension-free manner. For defects great than 5cm, the gold standard is autologous nerve grafting.[22]
Clinical Significance
The morbidity of peripheral nerve injury is highest in trauma patients, specifically the upper extremity with the radial nerve the most commonly injured. Approximately 34% of discharged upper extremity trauma patients require support services, and 16% require rehabilitation according to a recent international survey of surgeons.[23] The wide variation in clinical outcomes after peripheral nerve injury makes it difficult to compare data, especially considering the high rate of co-morbidities in these trauma patients. The sensory exam is subjective, and so the examiner must be thorough when doing the sensory exam.
The best clinical tools for objective measurement of the extent of peripheral nerve injury include EMG and nerve conduction velocity (NCV). Characteristic findings of a compressive neuropathy on EMG include positive sharp waves representing fibrillations from denervation of muscle fibers. NCV in focal compression will show increased latency, decreased conduction velocity, reduced amplitude of motor action potential and sensory nerve action potential.[24]
The Seddon classification system of nerve injury can be used to predict the complexity of nerve regeneration.[25] This system includes neurapraxia, axonotmesis, and neurotmesis. Neurapraxia is nerve contusion due to focal nerve compression resulting in reversible local ischemia without Wallerian degeneration, in which the segment of axon distal to the injury degrades. An example would be meralgia paresthetica which is compression of the lateral femoral cutaneous nerve from tight clothing on an obese person that can often be easily be relieved by wearing looser clothing or losing weight. Axonotmesis is more severe resulting in Wallerian degeneration, but the endoneurium remains intact, and the damage is still reversible. This degeneration begins 24 to 36 hours after injury. A “stinger” is neurapraxia of the proximal upper trunk of the brachial plexus due to stretching. Spontaneous recovery occurs at a rate of 1mm per day and is usually complete as long as the fibers regenerate along their original endoneurial tubes. Nonoperative management with serial electromyography (EMG) is indicated for neurapraxia and axonotmesis.[26] Finally, neurotmesis is complete nerve division that is irreversible without surgical repair. As discussed previously, primary repair of the epineurium is crucial to ensure proper regeneration of the original fiber pattern with the best prognosis for recovery of function. About 2.5% of all peripheral nerve injuries today cannot be surgically repaired.[27]
The differential for non-traumatic peripheral neuropathy includes:
- Immune-mediated
- Metabolic
- Hereditary
- Toxic
- Infectious
- Entrapment
Sensory neuropathies can represent the neurologic manifestations of systemic disease.[27] Ascending, symmetric sensory loss in the context of recent infection with albuminocytologic dissociation on CSF analysis represents acute inflammatory demyelinating polyneuropathy (AIDP/GBS). Mononeuropathy multiplex, which is neuropathy of individual peripheral nerves in a multifocal distribution, can be seen in Churg-Strauss, polyarteritis nodosa, and Wegener granulomatosis. The most common metabolic neuropathy is seen in diabetes mellitus presenting as distal symmetric polyneuropathy. The A-delta and C fibers are the first involved since they are the smallest, so the insidious onset of symmetric loss of temperature and light touch sensation in a “stocking and glove” distribution is the common initial presentation. This neuropathy distribution is also characteristic of vitamin B12 deficiency.[28] Other vitamin deficiencies characterized by peripheral neuropathies include B1, B6, vitamin E, and niacin. Hereditary neuropathies include neurocristopathies such as neurofibromatosis and Charcot-Marie-Tooth.[29] Neurofibromatosis type I (NF1) is associated with cutaneous neurofibromas and NF2 involves bilateral acoustic schwannomas. Charcot-Marie-Tooth is a chronic demyelinating disease of the peripheral nerves associated with foot deformities like pes cavus, foot drop due to lower extremity weakness, and sensory deficits especially along the distribution of the peroneal nerve. Other hereditary diseases with peripheral neuropathy include the sphingolipidoses like Krabbe disease, metachromatic leukodystrophy, and Fabry disease. Neuropathies associated with infections include HIV distal sensory polyneuropathy due to neurotoxic side effects of anti-retroviral therapy and mononeuropathy associated with other infections like CMV. Lyme disease can cause facial neuropathy, unilateral or bilateral. Leprosy is the most common infectious cause of neuropathy in the world, presenting with painful mononeuropathies of cool areas like the nose, ears, and distal limbs.[30] Finally, entrapment and compressive neuropathies like carpal tunnel syndrome (median neuropathy) or peroneal neuropathy (meralgia paresthetica) can occur and be addressed surgically if sufficiently severe.
Media
(Click Image to Enlarge)
References
Watson JC, Dyck PJ. Peripheral Neuropathy: A Practical Approach to Diagnosis and Symptom Management. Mayo Clinic proceedings. 2015 Jul:90(7):940-51. doi: 10.1016/j.mayocp.2015.05.004. Epub [PubMed PMID: 26141332]
Beran R. Paraesthesia and peripheral neuropathy. Australian family physician. 2015 Mar:44(3):92-5 [PubMed PMID: 25770571]
Schraut NB, Walton S, Bou Monsef J, Shott S, Serici A, Soulii L, Amirouche F, Gonzalez MH, Kerns JM. What Protects Certain Nerves from Stretch Injury? Anatomical record (Hoboken, N.J. : 2007). 2016 Jan:299(1):111-7. doi: 10.1002/ar.23286. Epub 2015 Nov 25 [PubMed PMID: 26529568]
Gebhart GF, Bielefeldt K. Physiology of Visceral Pain. Comprehensive Physiology. 2016 Sep 15:6(4):1609-1633. doi: 10.1002/cphy.c150049. Epub 2016 Sep 15 [PubMed PMID: 27783853]
Spencer NJ, Zagorodnyuk V, Brookes SJ, Hibberd T. Spinal afferent nerve endings in visceral organs: recent advances. American journal of physiology. Gastrointestinal and liver physiology. 2016 Dec 1:311(6):G1056-G1063. doi: 10.1152/ajpgi.00319.2016. Epub 2016 Nov 17 [PubMed PMID: 27856418]
Level 3 (low-level) evidenceYuan Q, Sun L, Yu H, An C. Human microvascular endothelial cell promotes the development of dorsal root ganglion neurons via BDNF pathway in a co-culture system. Bioscience, biotechnology, and biochemistry. 2017 Jul:81(7):1335-1342. doi: 10.1080/09168451.2017.1313695. Epub 2017 Apr 10 [PubMed PMID: 28394221]
Chao YC, Xie F, Li X, Guo R, Yang N, Zhang C, Shi R, Guan Y, Yue Y, Wang Y. Demethylation regulation of BDNF gene expression in dorsal root ganglion neurons is implicated in opioid-induced pain hypersensitivity in rats. Neurochemistry international. 2016 Jul:97():91-8. doi: 10.1016/j.neuint.2016.03.007. Epub 2016 Mar 10 [PubMed PMID: 26970395]
Qureshi AI, Saleem MA, Ahrar A, Raja F. Imaging of the Vasa Nervorum Using Contrast-Enhanced Ultrasound. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2017 Nov:27(6):583-588. doi: 10.1111/jon.12429. Epub 2017 Feb 14 [PubMed PMID: 28195441]
Cellek S, Cameron NE, Cotter MA, Muneer A. Pathophysiology of diabetic erectile dysfunction: potential contribution of vasa nervorum and advanced glycation endproducts. International journal of impotence research. 2013 Jan:25(1):1-6. doi: 10.1038/ijir.2012.30. Epub 2012 Aug 23 [PubMed PMID: 22914567]
Ishibe K, Tamatsu Y, Miura M, Shimada K. Morphological study of the vasa nervorum in the peripheral branch of human facial nerve. Okajimas folia anatomica Japonica. 2011 Nov:88(3):111-9 [PubMed PMID: 22519070]
Jeon SK, Paik DJ, Hwang YI. Variations in sural nerve formation pattern and distribution on the dorsum of the foot. Clinical anatomy (New York, N.Y.). 2017 May:30(4):525-532. doi: 10.1002/ca.22873. Epub 2017 Apr 8 [PubMed PMID: 28281304]
Eid EM, Hegazy AM. Anatomical variations of the human sural nerve and its role in clinical and surgical procedures. Clinical anatomy (New York, N.Y.). 2011 Mar:24(2):237-45. doi: 10.1002/ca.21068. Epub 2010 Oct 14 [PubMed PMID: 20949489]
Davidovich ER, Nascimento OJ. Superficial radial nerve-lateral antebrachial cutaneous nerve anatomic variation. Brain and behavior. 2014 Jan:4(1):70-4. doi: 10.1002/brb3.195. Epub 2013 Dec 1 [PubMed PMID: 24653956]
Guru A, Kumar N, Ravindra Shanthakumar S, Patil J, Nayak Badagabettu S, Aithal Padur A, Nelluri VM. Anatomical Study of the Ulnar Nerve Variations at High Humeral Level and Their Possible Clinical and Diagnostic Implications. Anatomy research international. 2015:2015():378063. doi: 10.1155/2015/378063. Epub 2015 Jul 12 [PubMed PMID: 26246909]
Falconer D, Spinner M. Anatomic variations in the motor and sensory supply of the thumb. Clinical orthopaedics and related research. 1985 May:(195):83-96 [PubMed PMID: 3978968]
Bas H, Kleinert JM. Anatomic variations in sensory innervation of the hand and digits. The Journal of hand surgery. 1999 Nov:24(6):1171-84 [PubMed PMID: 10584938]
Raducha JE, Cohen B, Blood T, Katarincic J. A Review of Brachial Plexus Birth Palsy: Injury and Rehabilitation. Rhode Island medical journal (2013). 2017 Nov 1:100(11):17-21 [PubMed PMID: 29088569]
Kubiak CA, Kung TA, Brown DL, Cederna PS, Kemp SWP. State-of-the-Art Techniques in Treating Peripheral Nerve Injury. Plastic and reconstructive surgery. 2018 Mar:141(3):702-710. doi: 10.1097/PRS.0000000000004121. Epub [PubMed PMID: 29140901]
Middleton SD, Anakwe RE. Carpal tunnel syndrome. BMJ (Clinical research ed.). 2014 Nov 6:349():g6437. doi: 10.1136/bmj.g6437. Epub 2014 Nov 6 [PubMed PMID: 25378457]
Levi AD, Ross AL, Cuartas E, Qadir R, Temple HT. The surgical management of symptomatic peripheral nerve sheath tumors. Neurosurgery. 2010 Apr:66(4):833-40. doi: 10.1227/01.NEU.0000367636.91555.70. Epub [PubMed PMID: 20190660]
Level 2 (mid-level) evidenceStratton JA, Assinck P, Sinha S, Kumar R, Moulson A, Patrick N, Raharjo E, Chan JA, Midha R, Tetzlaff W, Biernaskie J. Factors Within the Endoneurial Microenvironment Act to Suppress Tumorigenesis of MPNST. Frontiers in cellular neuroscience. 2018:12():356. doi: 10.3389/fncel.2018.00356. Epub 2018 Oct 11 [PubMed PMID: 30364248]
Muir D. The potentiation of peripheral nerve sheaths in regeneration and repair. Experimental neurology. 2010 May:223(1):102-11. doi: 10.1016/j.expneurol.2009.05.038. Epub 2009 Jun 6 [PubMed PMID: 19505459]
Level 3 (low-level) evidenceScholz T, Krichevsky A, Sumarto A, Jaffurs D, Wirth GA, Paydar K, Evans GR. Peripheral nerve injuries: an international survey of current treatments and future perspectives. Journal of reconstructive microsurgery. 2009 Jul:25(6):339-44. doi: 10.1055/s-0029-1215529. Epub 2009 Mar 19 [PubMed PMID: 19301234]
Level 3 (low-level) evidenceRaducha JE, Gil JA, DeFroda SF, Wawrzynski J, Weiss AC. An Evidence-Based Approach to the Differentiation of Compressive Neuropathy from Polysensory Neuropathy in the Upper Extremity. JBJS reviews. 2017 Oct:5(10):e9. doi: 10.2106/JBJS.RVW.17.00028. Epub [PubMed PMID: 29087965]
Kaya Y, Sarikcioglu L. Sir Herbert Seddon (1903-1977) and his classification scheme for peripheral nerve injury. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2015 Feb:31(2):177-80. doi: 10.1007/s00381-014-2560-y. Epub 2014 Oct 1 [PubMed PMID: 25269543]
Torg JS. Cervical spinal stenosis with cord neurapraxia and transient quadriplegia. Sports medicine (Auckland, N.Z.). 1995 Dec:20(6):429-34 [PubMed PMID: 8614762]
Wang E, Inaba K, Byerly S, Escamilla D, Cho J, Carey J, Stevanovic M, Ghiassi A, Demetriades D. Optimal timing for repair of peripheral nerve injuries. The journal of trauma and acute care surgery. 2017 Nov:83(5):875-881. doi: 10.1097/TA.0000000000001570. Epub [PubMed PMID: 28590354]
Schloss J, Colosimo M. B Vitamin Complex and Chemotherapy-Induced Peripheral Neuropathy. Current oncology reports. 2017 Oct 5:19(12):76. doi: 10.1007/s11912-017-0636-z. Epub 2017 Oct 5 [PubMed PMID: 28983799]
Ramchandren S. Charcot-Marie-Tooth Disease and Other Genetic Polyneuropathies. Continuum (Minneapolis, Minn.). 2017 Oct:23(5, Peripheral Nerve and Motor Neuron Disorders):1360-1377. doi: 10.1212/CON.0000000000000529. Epub [PubMed PMID: 28968366]
Santos DFD, Mendonça MR, Antunes DE, Sabino EFP, Pereira RC, Goulart LR, Goulart IMB. Revisiting primary neural leprosy: Clinical, serological, molecular, and neurophysiological aspects. PLoS neglected tropical diseases. 2017 Nov:11(11):e0006086. doi: 10.1371/journal.pntd.0006086. Epub 2017 Nov 27 [PubMed PMID: 29176796]