Introduction
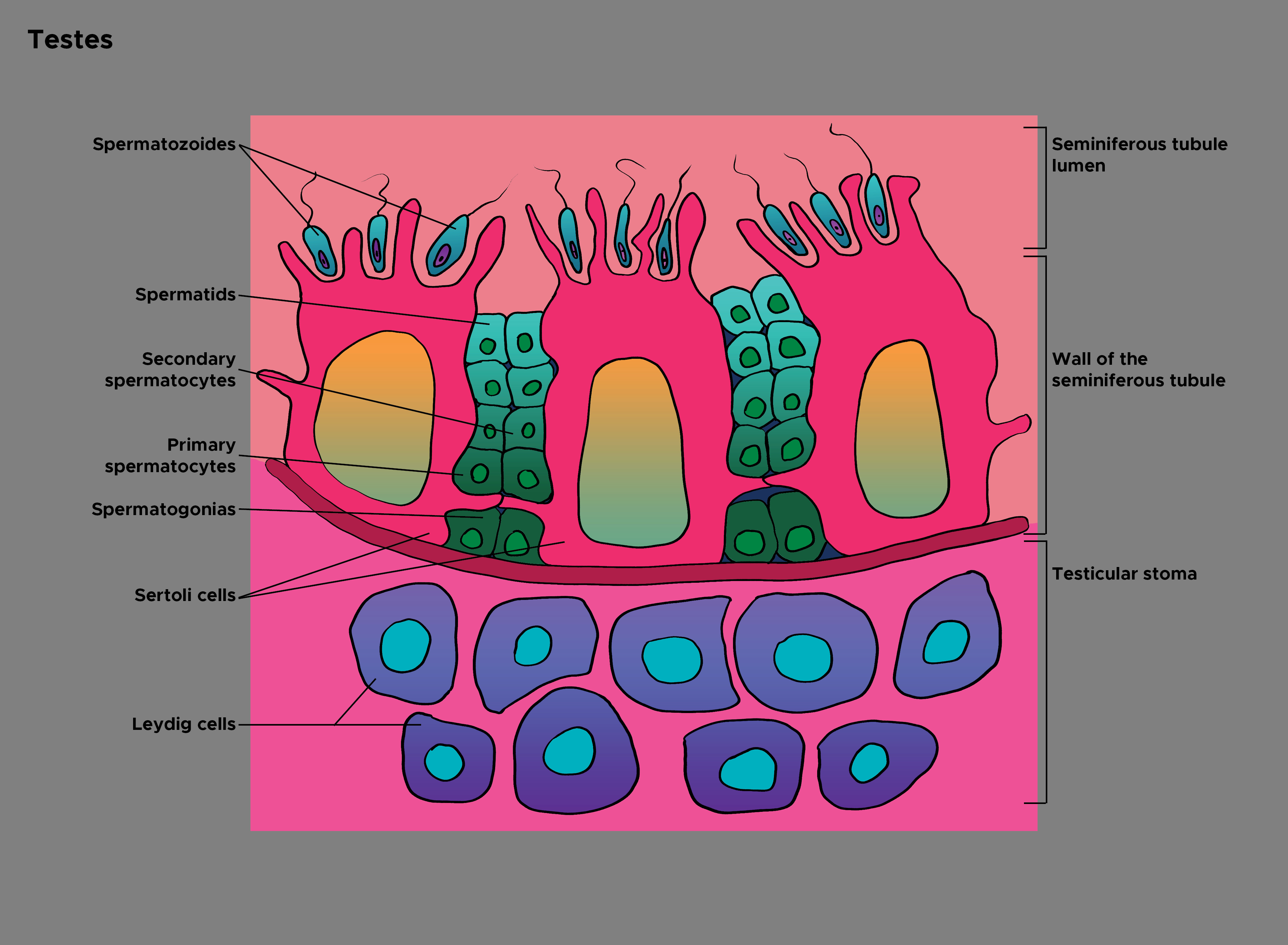
Sertoli cells are present in the seminiferous tubules of the male gonads, the testes. They were first observed in 1865 by a young Italian physician Enrico Sertoli, and are named after him.[1] Sertoli cells comprise one of the 2 types of cells in the germinal epithelium, the other being of the spermatogonia lineage. Sertoli cells are one of the most important cells necessary for sperm production in men. They are often identifiable as big, tightly linked cells near the basolateral portion of the seminiferous tubule. They are also known as sustentacular cells of Sertoli and are the nursemaid cells of the primary spermatogonia.[2] See Image. Histology of Testes.
Sertoli cells help to facilitate the process of spermiogenesis and, thus, the production of viable sperm. Sertoli cells also secrete a myriad of vital molecules, including androgen binding protein (ABP), inhibin B, and activin. These secretions facilitate spermatogenesis directly or indirectly via a hormonal negative feedback system. Sertoli cells also respond to pituitary hormones such as follicle-stimulating hormone (FSH) to begin the process of spermatogenesis, supplementing the adjacent spermatogonia.[3][4][5] Sertoli cells are so important that their mere absence in the testes can lead to infertility in adult men even though the production of sperm is normal.[6]
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
During the development and differentiation of Sertoli cells from their supporting cell precursors in the genital ridge, an autosomal gene known as the SOX9 (SRY-box transcription factor 9) is essential. Apart from the sex-determining region of the Y chromosome (SRY), the steroidogenic factor 1 (NR5A1) also helps differentiate the Sertoli cells. The absence of these factors will result in the formation of gonads and ovaries. Upon reaching the essential levels of SRY and SOX9, the gonads start demonstrating morphological changes to a true testis. These changes include the epithelization of the Sertoli cells, differentiation of Leydig and myoid cells, testicular cords formation, and mitotic arrest of germ cells.[7]
Structure
Sertoli cells are the biggest, non-uniformly shaped cells of the germinal epithelium in the seminiferous tubules. They are most reliably identified by always being the largest columnar cell that is still attached to the basal lamina of the basement membrane and span to the apical lumen of the cross-sectioned seminiferous tubule. They are often irregular or pyramidal-shaped but will always be towards the basolateral side, as opposed to the secondary spermatogonia and spermatids towards the lumen. The Sertoli cells can be differentiated from the nearby primary spermatogonia. Primary spermatogonia often have darker distinct nuclei in a small circular uniform cell, as opposed to Sertoli cells having paler nuclei in a larger, irregularly shaped cell.[3][8]
It is important to note that the Sertoli cell structure forms tight junctions and connective adhesion molecules with neighboring Sertoli cells so that there can be local sequestering of testosterone. This junction is the basis of the blood-testes-barrier, which provides many necessary conditions for appropriate spermatogenesis, including ion regulation, testosterone concentration, immune system evasion, and barrier protection. The Sertoli cells form the inner perimeter of the cross-sectioned seminiferous tubule, with the germ cell epithelium being the only other exterior cellular structures, resulting in mature spermatogonia and spermatids being interior to the Sertoli cell blood-testes-barrier.[8][9]
Function
Sertoli cells play many roles and have many functions within the seminiferous tubules. One of the most important features is the secretion of a compound called Mullerian inhibiting factor, which helps to prevent the development of female sex organs following the determining of the testes embryologically. Sertoli cells also secrete Inhibin B, which helps to regulate FSH by acting on the anterior pituitary. Sertoli cells aid in concentrating the testosterone available by secreting a substance called androgen-binding protein. Sertoli cells also maintain optimal health conditions for the primary spermatogonia and spermatogenesis process by regulating the ions and amino acids—and ultimately “nursing” the spermatogonia. Overall, the Sertoli cell is paramount to the spermatogenesis process and regulates this process via FSH receptor stimulation from the anterior pituitary. In the final process of spermiogenesis, which is the last step of spermatid maturation, the Sertoli cells function to degrade residual cytoplasm after it is shed from the spermatid.[9][10][11][12]
Structurally, the Sertoli cell has another outstanding job, which is to maintain the blood-testes-barrier. This barrier forms from tight junctions between adjacent Sertoli cells, which all sit on the basement membrane of the seminiferous tubules. This arrangement creates two useful layers of the seminiferous tubules. Interior to the tight junctions are the primary and secondary spermatogonia, as well as spermiogenesis. Exterior to the tight junction are germinal epithelial cells and primitive spermatogonia. This barrier is also essential to allow for conditions to be capable of sequestering testosterone and increasing local concentrations necessary for spermatogenesis. The blood-testes-barrier also serves to help evade auto-immune diseases or any surveillance by the body’s immune system.[10][13][14]
A summarization of Sertoli cell function is as follows:
- Help in supporting, protecting, and providing nutrition to spermatogenic cells [15][16]
- Play a role in the paracrine and endocrine control of spermatogenesis [17]
- Sertoli cells regulate cholesterol metabolism at the time of spermatogenesis [7]
- Help in the movement of spermatozoa by secreting fluids [18]
- Phagocytose apoptotic cells and foreign bodies [19]
- Secrete the androgen-binding protein under the influence of follicular stimulating hormone (FSH)[7]
- Secrete inhibin B hormone that suppresses the release of FSH [20]
- Formation of a blood-testes barrier by tight junctions between them [7]
Histochemistry and Cytochemistry
In histochemistry, Sertoli cells stand out when stained for the androgen receptors. The androgen receptor is only on the nucleus of the Sertoli cell. Because of this, the androgen receptor can be reliably identified with the Sertoli cell. There are, however, other histochemistry and cytochemistry markers for which the Sertoli cell would also stain positively. These markers include SOX9, WT1, the GATA binding proteins, and many more. It is important to note that the SOX9 marker represents the SRY-coded gene, and the WT1 gene represents a Wilms tumor.[21][9]
Microscopy, Light
Sertoli cell identification first starts by looking towards the basolateral aspect of the seminiferous tubules. One can observe that Sertoli cells are large irregularly shaped cells that form an inner perimeter, along with smaller more-uniformly-shaped-circular cells. These are the primary spermatogonia that the Sertoli cells will provide sustenance, too. The Sertoli cell can also be identified by looking for the paler nucleus’ in comparison to its neighboring cell, the primary spermatogonia, which has much darker, rounder nuclei. There are also primitive spermatids resting over the apical end of the Sertoli cell, which will undergo spermiogenesis and bud off into the lumen of the seminiferous tubules.[21][9][22]
Microscopy, Electron
Under microscopy electron observation of Sertoli cells, one can observe that the nucleus is indented, which is often the identifying factor when scanning the basolateral aspect of the seminiferous tubules. Sertoli cells are the biggest and most asymmetrically shaped cells in the basolateral aspect. They can be further identified by their prominent larger nucleus, in comparison to the large volume of the cytoplasm of the Sertoli cell. The nucleus is oval-shaped and basal, with a prominent nucleolus. The developing spermatogenic cells in different stages indent all over the Sertoli cells. Up to 40 germ cells in different stages of differentiation are in contact with each Sertoli cell.[19]
The most primitive cells, like spermatogonia and spermatocytes, are indented near the basement membrane. The more mature cells, such as spermatids, indent near the apex of the Sertoli cells, towards the center of the lumen of the seminiferous tubules. Researchers have noted that Sertoli cells provide a phagocytic function to help remove cytoplasmic waste and debris from the seminiferous tubules that would be in vacuoles of the Sertoli cell. There are also often many layers of smooth endoplasmic reticulum surrounding the nucleus of the Sertoli cell, where the SER functions in lipid removal and processing. There can be many mitochondria observed as well, as the Sertoli cell needs adenosine triphosphate to be able to secrete and synthesize compounds such as inhibin B, aminoethoxydiphenyl borate, and activin. There are 2 prominent deoxyribonucleic acid chromocenter satellites present as well, as they form connections with primitive spermatids and draw the spermatids closer to the basolateral crypts of the Sertoli cell.[9][21][23]
Clinical Significance
Sertoli cell-only syndrome is a condition in which there is a complete absence of germ cells within the testes, resulting in male infertility. It is seen mostly with Klinefelter syndrome and Yq microdeletions patients.[24] In Klinefelter syndrome, a primary hypogonadotropic hypogonadism disorder is characterized by trisomy 47 XXY, and patients will present with very typical characteristics. These include gynecomastia, tall, long extremities, a feminized body type, and lastly, dysfunction of the gonadal processes. Because there are no functional Leydig or Sertoli cells, there will be low testosterone levels due to the lack of Leydig cells, and there will be no inhibin released by the Sertoli cells. Because there is no inhibin to shut down GnRH release, GnRH levels will typically be high, and this subsequently leads to high LH and FSH levels. It is important to note that testicular histology will often show fibrosis and connective tissue damage of the interstitial space and the seminiferous tubules.[25][26][27]
Sertoli cell pathology can also occur in Sertoli cell tumors or Sertoli-Leydig cell tumors. These are sex-cord-stromal cancers and can occur in men and women. However, Sertoli-Leydig cell tumors most commonly occur in the older women demographic. It is also essential to note that in patients with Sertoli-Leydig cell tumors, there will often be elevated hormone levels, such as increased androgens in Sertoli-Leydig tumors. This condition would present clinically as a significant painless mass in the ovaries or testes and cause possible virilization, hirsutism, clitoromegaly, increased lean muscle mass, etc.[28][29][30] A disruption in the normal development of the Sertoli cell results in a condition known as testicular dysgenesis syndrome. It is a condition in which the patient will have hypospadias, cryptorchidism, poor quality of semen, and going into testicular germ cell cancer.[31]
Media
(Click Image to Enlarge)
References
Griswold MD. 50 years of spermatogenesis: Sertoli cells and their interactions with germ cells. Biology of reproduction. 2018 Jul 1:99(1):87-100. doi: 10.1093/biolre/ioy027. Epub [PubMed PMID: 29462262]
Duan P, Hu C, Quan C, Yu T, Huang W, Chen W, Tang S, Shi Y, Martin FL, Yang K. 4-Nonylphenol induces autophagy and attenuates mTOR-p70S6K/4EBP1 signaling by modulating AMPK activation in Sertoli cells. Toxicology letters. 2017 Feb 5:267():21-31. doi: 10.1016/j.toxlet.2016.12.015. Epub 2016 Dec 29 [PubMed PMID: 28041982]
Level 3 (low-level) evidenceGriswold MD. The central role of Sertoli cells in spermatogenesis. Seminars in cell & developmental biology. 1998 Aug:9(4):411-6 [PubMed PMID: 9813187]
Level 3 (low-level) evidenceIshizaka K,Oshima H, [Organization and regulation of spermatogenesis]. Nihon rinsho. Japanese journal of clinical medicine. 1997 Nov; [PubMed PMID: 9396270]
Level 3 (low-level) evidenceRavel C, Jaillard S. [The Sertoli cell]. Morphologie : bulletin de l'Association des anatomistes. 2011 Dec:95(311):151-8. doi: 10.1016/j.morpho.2011.07.118. Epub 2011 Nov 16 [PubMed PMID: 22094070]
Chojnacka K, Zarzycka M, Mruk DD. Biology of the Sertoli Cell in the Fetal, Pubertal, and Adult Mammalian Testis. Results and problems in cell differentiation. 2016:58():225-51. doi: 10.1007/978-3-319-31973-5_9. Epub [PubMed PMID: 27300181]
Titi-Lartey OA, Khan YS. Embryology, Testicle. StatPearls. 2024 Jan:(): [PubMed PMID: 32491695]
Suede SH,Malik A,Sapra A, Histology, Spermatogenesis 2020 Jan; [PubMed PMID: 31985935]
Nistal M, Abaurrea MA, Paniagua R. Morphological and histometric study on the human Sertoli cell from birth to the onset of puberty. Journal of anatomy. 1982 Mar:134(Pt 2):351-63 [PubMed PMID: 7076559]
Nishimura H, L'Hernault SW. Spermatogenesis. Current biology : CB. 2017 Sep 25:27(18):R988-R994. doi: 10.1016/j.cub.2017.07.067. Epub [PubMed PMID: 28950090]
Johnson L, Thompson DL Jr, Varner DD. Role of Sertoli cell number and function on regulation of spermatogenesis. Animal reproduction science. 2008 Apr:105(1-2):23-51. doi: 10.1016/j.anireprosci.2007.11.029. Epub 2007 Dec 15 [PubMed PMID: 18242891]
Level 3 (low-level) evidenceTaketo T, Saeed J, Manganaro T, Takahashi M, Donahoe PK. Müllerian inhibiting substance production associated with loss of oocytes and testicular differentiation in the transplanted mouse XX gonadal primordium. Biology of reproduction. 1993 Jul:49(1):13-23 [PubMed PMID: 8353178]
Level 3 (low-level) evidenceSiu MK, Cheng CY. Extracellular matrix: recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis. Biology of reproduction. 2004 Aug:71(2):375-91 [PubMed PMID: 15115723]
Level 3 (low-level) evidenceVerhoeven G, Cailleau J. Testicular peritubular cells secrete a protein under androgen control that inhibits induction of aromatase activity in Sertoli cells. Endocrinology. 1988 Oct:123(4):2100-10 [PubMed PMID: 2458250]
Level 3 (low-level) evidenceNi FD, Hao SL, Yang WX. Multiple signaling pathways in Sertoli cells: recent findings in spermatogenesis. Cell death & disease. 2019 Jul 17:10(8):541. doi: 10.1038/s41419-019-1782-z. Epub 2019 Jul 17 [PubMed PMID: 31316051]
Khan YS,Farhana A, Histology, Cell . 2020 Jan [PubMed PMID: 32119269]
Shi JF, Li YK, Ren K, Xie YJ, Yin WD, Mo ZC. Characterization of cholesterol metabolism in Sertoli cells and spermatogenesis (Review). Molecular medicine reports. 2018 Jan:17(1):705-713. doi: 10.3892/mmr.2017.8000. Epub 2017 Nov 7 [PubMed PMID: 29115523]
Rato L, Socorro S, Cavaco JE, Oliveira PF. Tubular fluid secretion in the seminiferous epithelium: ion transporters and aquaporins in Sertoli cells. The Journal of membrane biology. 2010 Jul:236(2):215-24. doi: 10.1007/s00232-010-9294-x. Epub 2010 Aug 10 [PubMed PMID: 20697886]
Level 3 (low-level) evidenceArandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nature immunology. 2015 Sep:16(9):907-17. doi: 10.1038/ni.3253. Epub [PubMed PMID: 26287597]
Carlsen E, Olsson C, Petersen JH, Andersson AM, Skakkebaek NE. Diurnal rhythm in serum levels of inhibin B in normal men: relation to testicular steroids and gonadotropins. The Journal of clinical endocrinology and metabolism. 1999 May:84(5):1664-9 [PubMed PMID: 10323397]
França LR, Hess RA, Dufour JM, Hofmann MC, Griswold MD. The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology. 2016 Mar:4(2):189-212. doi: 10.1111/andr.12165. Epub 2016 Feb 4 [PubMed PMID: 26846984]
Nistal M, Paniagua R. The postnatal development of the human sertoli cells. Zeitschrift fur mikroskopisch-anatomische Forschung. 1983:97(5):739-52 [PubMed PMID: 6673386]
Carr I, Clegg EJ, Meek GA. Sertoli cells as phagocytes: an electron microscopic study. Journal of anatomy. 1968 Mar:102(Pt 3):501-9 [PubMed PMID: 5656139]
Level 3 (low-level) evidenceStouffs K, Gheldof A, Tournaye H, Vandermaelen D, Bonduelle M, Lissens W, Seneca S. Sertoli Cell-Only Syndrome: Behind the Genetic Scenes. BioMed research international. 2016:2016():6191307. doi: 10.1155/2016/6191307. Epub 2016 Jan 26 [PubMed PMID: 26925412]
Høst C, Skakkebæk A, Groth KA, Bojesen A. The role of hypogonadism in Klinefelter syndrome. Asian journal of andrology. 2014 Mar-Apr:16(2):185-91. doi: 10.4103/1008-682X.122201. Epub [PubMed PMID: 24407186]
Wikström AM, Dunkel L. Klinefelter syndrome. Best practice & research. Clinical endocrinology & metabolism. 2011 Apr:25(2):239-50. doi: 10.1016/j.beem.2010.09.006. Epub [PubMed PMID: 21397196]
Aksglaede L, Wikström AM, Rajpert-De Meyts E, Dunkel L, Skakkebaek NE, Juul A. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Human reproduction update. 2006 Jan-Feb:12(1):39-48 [PubMed PMID: 16172111]
Level 3 (low-level) evidenceUlbright TM. Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005 Feb:18 Suppl 2():S61-79 [PubMed PMID: 15761467]
Chen FY, Sheu BC, Lin MC, Chow SN, Lin HH. Sertoli-Leydig cell tumor of the ovary. Journal of the Formosan Medical Association = Taiwan yi zhi. 2004 May:103(5):388-91 [PubMed PMID: 15216408]
Level 3 (low-level) evidenceSachdeva P, Arora R, Dubey C, Sukhija A, Daga M, Singh DK. Sertoli-Leydig cell tumor: a rare ovarian neoplasm. Case report and review of literature. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2008 Apr:24(4):230-4. doi: 10.1080/09513590801953465. Epub [PubMed PMID: 18382911]
Level 3 (low-level) evidenceChen GR, Dong L, Ge RS, Hardy MP. [Relationship between phthalates and testicular dysgenesis syndrome]. Zhonghua nan ke xue = National journal of andrology. 2007 Mar:13(3):195-200 [PubMed PMID: 17393778]