Definition/Introduction
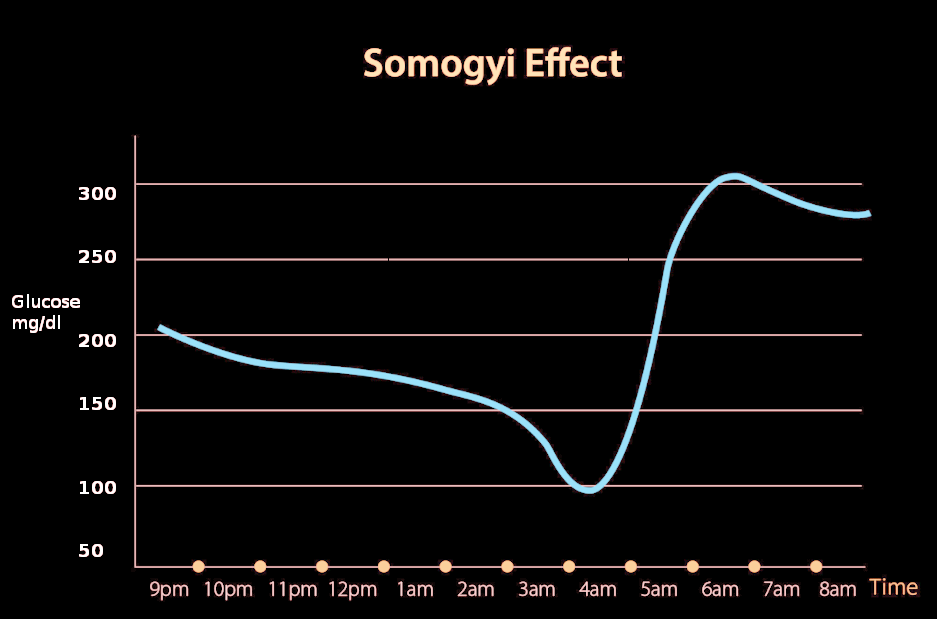
The Somogyi effect, also known as the "chronic Somogyi rebound" or "posthypoglycemic hyperglycemia," was a theory proposed in the 1930s by Dr. Michael Somogyi, a Hungarian-born professor at Washington University, St. Louis, MO, United States.[1] He described the paradoxical tendency of the body to react to hypoglycemia by producing hyperglycemia. Somogyi proposed that when blood glucose levels drop too low during the late evening, activation of counterregulatory hormones such as adrenaline, corticosteroids, growth hormone, and glucagon may be observed, leading to activation of gluconeogenesis and resultant hyperglycemia in the early morning.[2]
However, more recent studies involving continuous glucose monitoring (CGM) have disputed this theory. Also, clinicians have observed that patients with early morning hyperglycemia tend to have high blood glucose measurements at night rather than low.[1] As a result, the debate continues in the scientific community regarding Somogyi's theory. Moreover, recently proposed mechanisms of morning hyperglycemia include nocturnal growth hormone secretion, hypoinsulinemia, and insulin resistance associated with metabolic syndrome.[3]
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Somogyi Phenomenon vs. Dawn Phenomenon
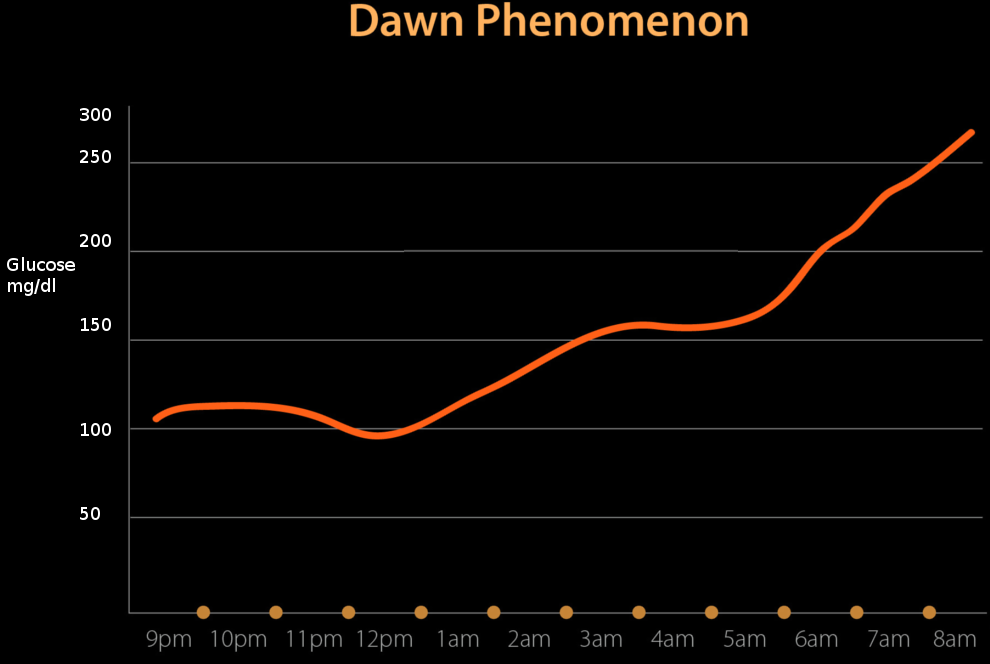
A phenomenon known as the dawn phenomenon was introduced by Dr. Schimdt in the 1980s, stating that morning hyperglycemia is due to the decreased levels of endogenous insulin secreted at night.[1] The dawn phenomenon also contributes to morning hyperglycemia to increased concentrations of insulin-antagonist hormones. The dawn phenomenon is comparable to the Somogyi phenomenon, which attributes morning hyperglycemia to counterregulatory hormones from low glucose. The dawn phenomenon has been noted to occur more commonly than the Somogyi phenomenon.[1] While the two theories are not seen in all cases of insulin-dependent diabetics, it is important to note that the best way to prevent either is optimal diabetes control with the proper insulin therapy.[1]
The Somogyi phenomenon states that early morning hyperglycemia occurs due to a rebound effect from late-night hypoglycemia. However, the dawn phenomenon does not include hypoglycemic episodes to be a factor.
Insulin Release and Insulin Resistance
With recent studies attributing early morning hyperglycemia to hypoinsulinemia, there is an observable pattern in which the body secretes insulin. The theory is insulin gets secreted in a circadian pattern, with the lowest concentrations between midnight and 6 AM and the highest concentrations between noon and 6 PM.[4] This pattern of insulin secretion is the opposite of melatonin from the pineal gland. The circadian pattern of insulin secretion provides evidence for the dawn phenomenon.
The Somogyi phenomenon has been a proposed phenomenon in insulin-dependent diabetic patients. The thinking is that these patients should monitor their blood glucose levels and adjust insulin dosages as necessary to prevent hypo- or hyperglycemic episodes.
In an individual that does not have diabetes, the blood glucose and insulin concentrations stay flat and constant throughout the night, with a transient increase in insulin just before dawn to prevent hepatic glucose production through gluconeogenesis and prevent hyperglycemia.[5] This explains why non-diabetic patients do not exhibit the dawn phenomenon, as their insulin levels follow the circadian pattern necessary for optimal glucose control.
Insulin resistance, seen in diabetes or metabolic syndrome, has been associated with constant exposure to high insulin levels.[6] As patients get diagnosed with diabetes or metabolic syndrome at an earlier age, there is more exogenous insulin exposure that leads to this resistance. Because of this, the normal regulation and pattern of insulin levels make it difficult for insulin-dependent diabetics to control their blood glucose levels during their sleep. Not only is insulin necessary to regulate glucose levels, but it is also the primary hormone that inhibits gluconeogenesis.[7] Gluconeogenesis in the morning gets inhibited in a non-diabetic due to the transient increase in insulin right before dawn. As a patient becomes more and more resistant to insulin, the key inhibitor of gluconeogenesis is no longer working; this allows the body to produce more glucose, leading to a hyperglycemic state.
Clinical Significance
The Somogyi phenomenon had been considered in the past; an essential consideration for the proper diagnosis and management of blood glucose levels is vital for the body’s metabolic demands. The post-hypoglycemic hyperglycemia raises the question of whether a patient’s insulin levels should be adjusted in the evening to prevent hyperglycemia in the morning. As this is something ideally avoided, the Somogyi phenomenon occurs too infrequently to make this standardized practice.
Hypoglycemia vs. Hyperglycemia
Glucose control is vital for brain metabolism and function, as it is the primary substrate for proper brain activity. The body has different regulatory molecules that act to decrease the likelihood of low blood glucose. The first response thought to activate due to low blood glucose is the counterregulatory hormone mechanism.[8] Other mechanisms include glycogenolysis, the breakdown of glycogen into glucose, and gluconeogenesis, de novo production of glucose, which occur in the liver.[7] The ideal plasma glucose concentration ranges from 70 to 140 mg/dL throughout the day.[9] Both glycogenolysis and gluconeogenesis can activate when levels fall below this range. Another mechanism induced by hypoglycemia includes a decrease in the pancreatic beta-cell secretion of insulin and an increase in the pancreatic alpha-cell secretion of glucagon. Glucagon, epinephrine, and glucocorticoid production will increase to induce glucose production in the body.[10] A hypoglycemic episode puts patients at risk for neurological dysfunction, coma, or even death.[10] With repeated hypoglycemia, the counterregulatory system that is supposed to keep blood glucose levels in range will start to fail.[10] Signs of hypoglycemia that patients must be aware of include diaphoresis, hunger, irritability, loss of consciousness, seizures, and confusion.[10]
Hyperglycemic episodes are also to be avoided. Symptoms to look out for include polyuria, polydipsia, weakness, tachycardia, and hypotension.[11] The hyperglycemia will worsen the cytokine and inflammatory response, causing even more hyperglycemia.[11] Consequences of hyperglycemia include renal failure, polyneuropathy, retinopathy, and arrhythmias.[11] Hyperglycemia produces an increase in advanced glycation end products (AGEs), which deposit in the body, causing connective tissue crosslinking, fibrosis, and impaired relaxation of the heart.[12] People with diabetes become insulin-resistant, which keeps blood glucose levels elevated, causing an even more increase in AGEs.[12] With the failure to keep blood glucose levels at a normal range, the hyperglycemic episodes also induce the renin-angiotensin-aldosterone system (RAAS), increasing vascular resistance and arterial pressure in the body.[12]
In an interprofessional team, there are many things to consider in managing a patient’s blood glucose concentrations. Theoretically, the risks of adjusting a patient’s insulin in case they undergo the Somogyi phenomenon could risk the potential consequence of increasing a patient’s chance of becoming severely hypoglycemic.
It is essential to coordinate care by involving the patient in discussions regarding proper glucose management. Lifestyle modifications and being aware of the symptoms of hypoglycemia or hyperglycemia require discussion in great detail with the patient. It will allow them to make well-informed decisions regarding their food choices, lifestyle habits, and activity levels.
Controversy
The importance of maintaining proper blood glucose levels in any patient is apparent from both ends of blood glucose level abnormalities. Using the Somogyi phenomenon as a precaution for standard in practice does not have enough evidence. Instead, this theory can be used to help monitor patients’ blood glucose levels throughout different times of the day to see if they fit the theory.
Clinical studies suggest that underdosing of insulin from the previous night fails to prevent hyperglycemia.[2] Another study on Type 1 Diabetes patients suggested that nocturnal hypoglycemia is usually associated with early morning hypoglycemia, not hyperglycemia.[13] In clinical practice, patients with hypoglycemic episodes at night do not wake up in the morning with hyperglycemia, hence refuting Somogyi Phenomenon.
In a study done at the Washington University School of Medicine to test the hypothesis of the Somogyi phenomenon, the conclusion was that the nocturnal hypoglycemia did not cause daytime hyperglycemia.[14] It showed no correlation between increased daytime glucose levels with the concentrations of counterregulatory hormones such as glucagon, epinephrine, growth hormone, or cortisol.[14] This study disproved the theory of Dr. Somogyi.
Nursing, Allied Health, and Interprofessional Team Interventions
All interprofessional healthcare team members who interact with patients with diabetes should be aware of the Somogyi phenomenon and the dawn effect and be able to explain them to their patients if it comes up during patient counseling, as patients may have heard of it and ask questions. Interprofessional healthcare team members can explain that it is no longer considered valid and direct the patients toward more scientifically back resources to help manage their condition.
Media
References
Rybicka M, Krysiak R, Okopień B. The dawn phenomenon and the Somogyi effect - two phenomena of morning hyperglycaemia. Endokrynologia Polska. 2011:62(3):276-84 [PubMed PMID: 21717414]
Gale EA, Kurtz AB, Tattersall RB. In search of the Somogyi effect. Lancet (London, England). 1980 Aug 9:2(8189):279-82 [PubMed PMID: 6105438]
Raskin P. The Somogyi phenomenon. Sacred cow or bull? Archives of internal medicine. 1984 Apr:144(4):781-7 [PubMed PMID: 6370162]
Boden G,Ruiz J,Urbain JL,Chen X, Evidence for a circadian rhythm of insulin secretion. The American journal of physiology. 1996 Aug [PubMed PMID: 8770017]
Porcellati F, Lucidi P, Bolli GB, Fanelli CG. Thirty years of research on the dawn phenomenon: lessons to optimize blood glucose control in diabetes. Diabetes care. 2013 Dec:36(12):3860-2. doi: 10.2337/dc13-2088. Epub [PubMed PMID: 24265365]
Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes care. 2008 Feb:31 Suppl 2():S262-8. doi: 10.2337/dc08-s264. Epub [PubMed PMID: 18227495]
Level 3 (low-level) evidenceHatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. Insulin regulation of gluconeogenesis. Annals of the New York Academy of Sciences. 2018 Jan:1411(1):21-35. doi: 10.1111/nyas.13435. Epub 2017 Sep 3 [PubMed PMID: 28868790]
Bolli GB,Fanelli CG, Physiology of glucose counterregulation to hypoglycemia. Endocrinology and metabolism clinics of North America. 1999 Sep [PubMed PMID: 10500926]
Level 3 (low-level) evidenceChung ST, Chacko SK, Sunehag AL, Haymond MW. Measurements of Gluconeogenesis and Glycogenolysis: A Methodological Review. Diabetes. 2015 Dec:64(12):3996-4010. doi: 10.2337/db15-0640. Epub [PubMed PMID: 26604176]
Kalra S, Mukherjee JJ, Venkataraman S, Bantwal G, Shaikh S, Saboo B, Das AK, Ramachandran A. Hypoglycemia: The neglected complication. Indian journal of endocrinology and metabolism. 2013 Sep:17(5):819-34. doi: 10.4103/2230-8210.117219. Epub [PubMed PMID: 24083163]
Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet (London, England). 2009 May 23:373(9677):1798-807. doi: 10.1016/S0140-6736(09)60553-5. Epub [PubMed PMID: 19465235]
Jia G,Whaley-Connell A,Sowers JR, Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018 Jan [PubMed PMID: 28776083]
Guillod L, Comte-Perret S, Monbaron D, Gaillard RC, Ruiz J. Nocturnal hypoglycaemias in type 1 diabetic patients: what can we learn with continuous glucose monitoring? Diabetes & metabolism. 2007 Nov:33(5):360-5 [PubMed PMID: 17652003]
Level 2 (mid-level) evidenceHirsch IB, Smith LJ, Havlin CE, Shah SD, Clutter WE, Cryer PE. Failure of nocturnal hypoglycemia to cause daytime hyperglycemia in patients with IDDM. Diabetes care. 1990 Feb:13(2):133-42 [PubMed PMID: 2190769]