Introduction
Ataxia is the absence of voluntary muscle coordination and loss of control of movement that affects gait stability, eye movement, and speech. Spinocerebellar ataxia (SCA) is an inherited (autosomal dominant), progressive, neurodegenerative, and heterogeneous disease that mainly affects the cerebellum. SCA is a subset of hereditary cerebellar ataxia and is a rare disease. To date, more than 40 distinct genetic SCAs have been identified which are classified according to the genetic loci in order of identification. SCA1 was the first SCA described and then further subtypes are identified sequentially. SCA doesn't compulsorily mean that it is restricted to the cerebellum and spinal cord. It may involve the other parts of the central nervous system as well, such as pontine nuclei, spinal cord, peripheral nerves, cortex, basal ganglia, etc. SCA6 is restricted to the cerebellum whereas SCA2 spares cerebellum.[1] Well defined and common types are SCA1, SCA2, SCA3, and SCA6 which accounts for more than half of cases and other rare variants constitute the remaining cases.[1][2] SCA is very complex to understand both genotypically and phenotypically and very difficult to describe all variants at one time.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Several types of spinocerebellar ataxia are associated with anticipation, in which there is a tendency of gradual expansion of CAG repeats in a consecutive generation. CAG repeat expansion occurs in SCA1, 2, 3, 6, 7, 8, 12, and 17. Similarly, SCA 10 is caused by the expansion of ATTCT (pentanucleotide), SCA 31, 36, 37 involve amplification of TGGAA (pentanucleotide), GGCCTG (hexanucleotide), and ATTTT (pentanucleotide) respectively. Other SCA subtypes are rarer and involve other repeat expansions or single nucleotide variants.[1] SCA5, SCA13, SCA14, and SCA19 engage the missense mutation, and SCA15, SCA20, and SCA39 involve the deletion or duplication of genes.[3]
Epidemiology
The global prevalence of spinocerebellar ataxia is 1 to 5 per100000 and overall European prevalence is 0.9 to 3 per 100000 with some geographical variation i.e 2/100000 in Italy to 4/100000 in Norway and 5/100000 in Portugal.[4][1]
SCA3 (25 to 50%) is most prevalent followed by descending prevalence SCA2 (13 to 18%), SCA6 (13-15%), and SCA7.[5] The frequency of different types varies from region to region. Asian countries have limited published data; however, studies have been performed in India, China, Singapore, Japan, and Korea. Despite SCA3 being most common worldwide, it was found that SCA2 is most common in South Korea and India.[6]
Pathophysiology
The exact pathogenesis of spinocerebellar ataxia is still not known. But many study series promulgated that common mechanisms of SCA are genetic mutations causing abnormal protein products, transcriptional dysregulation, dysfunction of autophagy, channelopathies, mitochondrial dysfunction, toxic RNA gain of function.[4]
Six forms of SCA involve CAG repeat amplification encode glutamine, which gets assembled into ataxins that alters the protein configuration into the beta-pleated structure and toxic gain of function with autosomal inheritance. Ataxins are misfolded proteins from the expansion of a polyglutamine (more than 40 glutamines), which is abnormally translocated and accumulated in nuclei that interact with other proteins and oligomerize forming intranuclear inclusions in Purkinje cells.[6] Normally, ataxins are present in CNS, which regulates normal protein homeostasis and cytoskeleton regulation. Biochemical studies have shown cytoplasmic aggregations in SCA2, the nucleus in SCA1, SCA3, and SCA7 and nucleolar in SCA7. Ataxins are targeted by ubiquitin-proteasome proteolytic complex in an attempt to degrade a remove and form the aggregations. Also, cellular interactions with abnormal ataxins have some role in pathogenesis. Ataxins bind to other proteins, including the TATA-binding transcription protein and the CREB-binding protein, impairing their functions disrupt the normal transcription regulation, which leads to abnormal and uncontrolled transcription. Some hypothesis declares that intranuclear inclusions are not supposed to be the sole cause of cellular dysfunction. In SCA subtype 1, Ataxin-1 is an uninterrupted expansion of polyglutamine. The polyglutamine tract interrupted by histidines are not affected and does not show any pathological effects. In addition to ataxin-1, the immune response against the 1C2 monoclonal antibody has some role in the pathogenesis of SCA type 1.[7] 14-3-3 protein binds and stabilizes the ataxin-1 regulated by Akt phosphorylation decreases normal proteolysis of ataxin-1 that increases neurotoxicity.[8]
The principal cell involved in degeneration is Purkinje cells, and other cells, such as granule cells, astrocytes, Golgi cells, and oligodendrocytes are not involved.[9] Purkinje cells regulate fine movement and muscle coordination. So, the degeneration of Purkinje cells is highly associated with ataxia. Some studies support that reason behind the involvement and vulnerability of only Purkinje cells is due to its large cell body with abundant cytoplasm and granules, long and prominent dendrites with many extensions (arborization). Protein aggregation also impairs axonal transport.[10] Proper functioning and energy supply by mitochondria are vital for survival, neuronal conduction, and development of the dendritic tree of active Purkinje cells. Mitochondrial dysfunction leads to the degeneration of Purkinje cells. Other parts of the CNS such as dentate nuclei, and substantia nigra cause parkinsonism, motor cortex provoke voluntary muscle movements, and brainstem motor nuclei induce abnormality of ocular motility and tongue movement.[11] The involvement of the spinal cord, thalamus, and peripheral sensory neurons is variable.
Channelopathies involving a mutation of voltage-gated calcium channel cause the release of calcium from calcium stores such as endoplasmic reticulum in SCA15, 16, and 29 and mitochondrial calcium influx in SCA28 which lead to enzyme activation and apoptosis of Purkinje cells. Pre- and postsynaptic calcium signaling consists of the alpha1A subunit of calcium channel encoded by the CACNA1A gene, and mutation of the CACNA1A gene occurs in SCA6, which disrupts the normal transmission of impulse in the synaptic junction of Purkinje cells. Normal functioning of voltage-gated potassium channels required for electrophysiological firing during nerve conduction. In SCA13, mutation of voltage-gated potassium channel KCNC3 impairs the normal conduction of Purkinje cells.[3]
Loss of autophagy, another built-in proteolysis mechanism, and accumulation of misfolded long polyglutamine peptide may be the cause of neurodegeneration.[12] Length and number of the polyglutamine chain determine the extent of neurotoxicity, subsequent apoptosis and degeneration, and severity of the disease, earlier onset.
Some studies say that codon repeats containing RNA also accumulated forming RNA-foci in SCA8, 31, and 36. This RNA-foci sequester RNA binding proteins which alter transcriptional regulation, and RNA splicing ability leading to neurotoxicity. Transcriptional dysregulation directly causes neurodegeneration in SCA1, 3, 7, and 17.
Recent studies affirmed that DNA repair modifies CAG with expansions.[4] So, defects in other nonproteins coding abnormality such as DNA damage, altered chromatin acetylation impairs DNA repair, which leads to CAG expansion.[5]
Histopathology
Gross examination shows atrophy of the cerebellum and lateral ventricle enlargement, especially in SCA2, SCA3, and SCA7, atrophy of brainstem and cerebral cortex, loss of pigments from substantia nigra and grayish discoloration of cerebral white matter.[13]
Histopathology shows obvious loss of neurons mainly Purkinje cells in the cerebellum and other parts of CNS likely pons, spinal cord, vermis, dentate nucleus, and medulla, loss of myelin in the anterior horn of spinal cord, motor neurons of cranial nerves in the brain stem, and axonal loss. In some cases, the immunohistochemical study shows ubiquitin and 1C2 positive intranuclear and cytoplasmic inclusions.[6][14] Patients having parkinsonism and dementia show Lewy body, neuritic plaques, and tangles.[13]
History and Physical
Family history is vital and should not be missed except in cases of nonpaternity and adoption, which baffles the actual history. Onset and duration of symptoms are variable though the history of gradual onset and slow progression over the years have a positive impact. The duration of such progressive disease is important since it takes years to manifest in full extent. Clinical features may vary significantly among individual members of a single-family. There is a huge overlap of phenotypic features among the various spinocerebellar ataxia subtypes, even within family members or interfamilial cases. Clinical manifestation is usually more severe and early onset in pediatric and adolescents phenotypes.
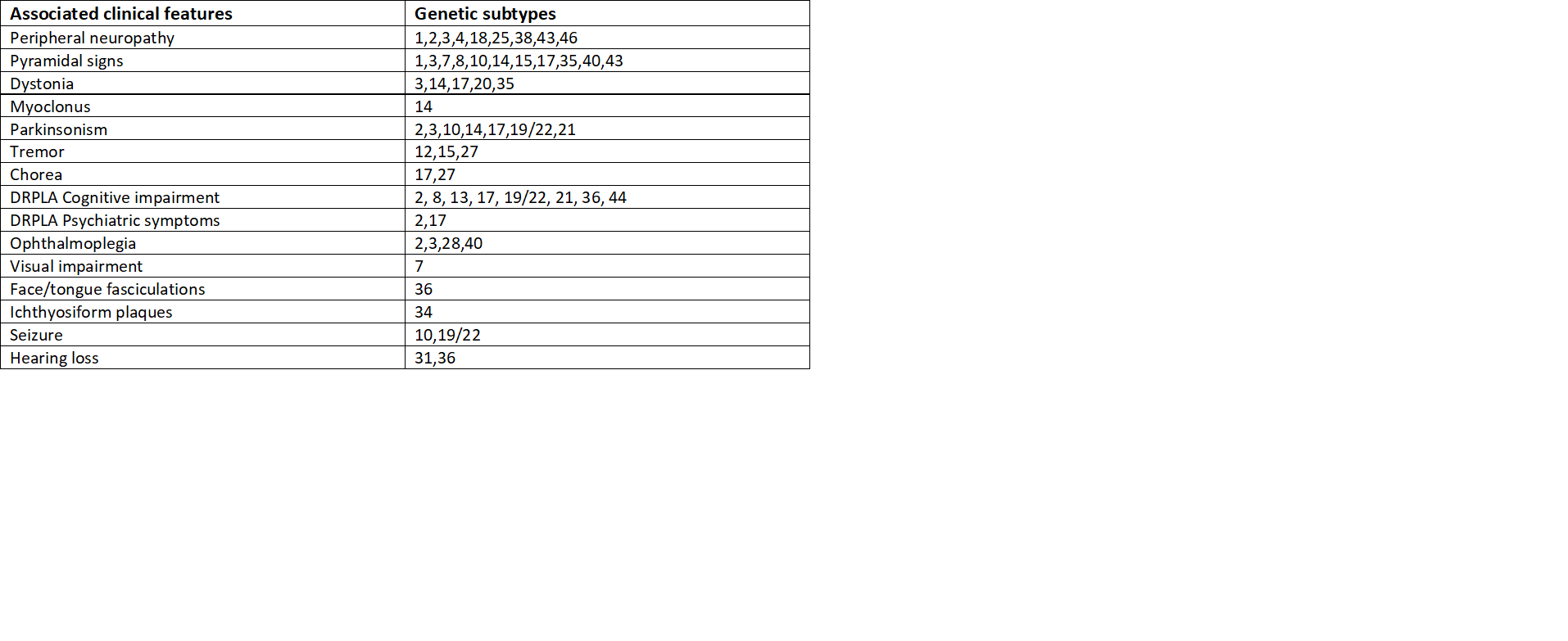
Some studies concluded that the size of triplet repeat expansion affects the severity and onset of disease and has a direct relationship, i.e., larger the size of the triplet repeat, the more severe and early onset is the presentation.[1] Certain signs and symptoms differ according to the genetic differences and subtypes and some characteristic features of each subtype.[6]
On neurological examination, hyperreflexia and saccadic eye movements suggest certain SCA types and make the diagnosis of SCA type 2 unlikely. Weakness, numbness, and pain (peripheral neuropathy) are more consistent among SCA1, 2, 3, 4, and 18, but sensory neuropathy is most characteristic with SCA4. Macular degeneration and visual impairment are more consistent with SCA7.
Likewise, ocular muscle weakness, involuntary eye movement(nystagmus), protruded eyes, muscle fasciculations, and parkinsonism are typical for SCA1, 2, and 3. Pyramidal signs such as spasticity, muscle weakness, hyperreflexia are overlapped with most of the subtypes. SCA10 is more prevalent with epileptic seizures, SCA14 with jerky contractions of muscles (myoclonus), brief contractions of face and tongue (fasciculations) with SCA36, abnormal involuntary movement (chorea) and tremor in SCA12, 27, and ichthyosiform plaques with SCA 34. Abnormal movements or postures, cognitive and psychiatric problems, tremor, quick involuntary movements (chorea), hearing loss are other common findings.[4]
Evaluation
Clinical manifestation and characterization are imperative before genetic analysis. But phenotypes of various SCA subtypes overlap, so, genotype has become the gold standard for diagnosis. In recent advances, more descriptions of phenotypic differentiation aids in sorting out variants.
Genetic Testing
Advances in molecular genetic analysis and testing expedite the definite early classification and diagnosis. Also, recognition of a specific mutated gene helps to test the same gene in other family members. In the setting of positive family history, genetic testing is the definitive way of identifying spinocerebellar ataxia subtypes. Polymerase chain reaction (PCR) of nucleotide repeats in different SCA gene loci helps to identify the specific gene and nucleotide repeats involved.[14][15] In clinically suspected patients, genetic testing should be at first carried out in most common SCAs such as SCA1, 2, and 3 and then should proceed to other subtypes if the first series test is negative. This is it is more convenient and technical as there are high chances of positive testing outcomes in dominant SCAs and reduces financial burden and time. However, in cases with complex or unique phenotypic features, a further genetic evaluation may be necessary that guide specific gene testing of definitive subtype.[6] Prenatal screening can be done through genetic testing, but there is a risk of termination of pregnancy and a lack of follow-up. In most common and well-known subtypes such as SCA1, SCA2, SCA3, SCA6, SCA7, SCA8, and SCA10, blood testing for mutation is also performed.
Neuroimaging
Neuroimaging demonstrates the gross cerebellar atrophy most prominent in SCA2 and least in other subtypes, enlargement of ventricles, and atrophy of other parts of the brain as well. Some specific focal or regional atrophies appreciated in certain SCAs are pontocerebellar atrophy with enlargement of the fourth ventricle in SCA3, atrophy of vermis sparing brainstem in SCA5, isolated cerebellar atrophy in SCA6, atrophy of the cerebellar vermis and hemispheres in SCA8, and SCA10, cerebral atrophy in SCA12, etc. Some subtle metabolic abnormalities, not obvious in structural imaging, may be detected by positron emission tomography (PET) scanning and magnetic resonance spectroscopy; however, rarely routinely performed due to availability and expense.
Electrophysiologic Testing
With the loss of function of Purkinje cells and axonal neuropathy of sensory neurons, the state of nerve conduction action potential can be checked by electrophysiologic testing. But this testing cannot differentiate the subtypes of SCAs.
Treatment / Management
Spinocerebellar ataxia is a genetic disease that has no definitive cure. Treatment is mainly symptomatic to alleviate symptoms like seizures, tremors, depression, ataxia, and eye symptoms. Antiepileptic drugs for seizures, botulinum toxin injections for dystonia, beta-blockers, and primidone for tremors, antidepressants for depression, and levodopa in parkinsonism, etc. can be utilized for symptomatic treatment. The use of N-acetyl cysteine in neurological disorders and SCA showed some benefits. Citalopram benefits by decreasing the level of ataxin-3 and improves the behavioral status of patients. Dantrolene inhibits the release of calcium from its stores and protects the Purkinje cells. Chlorzoxazone is the FDA approved activator of calcium-activated potassium channels, which normalizes the electrophysiology and action potential firing of Purkinje cells.[16] Zolpidem has been reported to improve cerebellar dysfunction transiently in some cases of SCA2, and a controlled trial of varenicline in SCA3 patients has shown improvement of cerebellar dysfunction in some patients.[4]
But any therapeutic procedure that clears the accumulated misfolded mutant protein can be a potential treatment option.[12] Ubiquitin-proteosome and autophagy are the two main pathways for the removal of aggregated misfolded proteins. Several agents that can counteract the misfolded protein-mediated process have been investigated. Chemical chaperones such as dimethyl sulfoxide, trimethylamine N-oxide and glycerol can accelerate the degradation of the mutant protein.[12][17] It showed that the proteasome catalytic subunit could not cleave polyglutamine efficiently. So, proteasome activity reinforced by the non-catalytic method has been used. Mammalian target of rapamycin (mTOR) is the signaling pathway for regulating autophagy. Rapamycin, an mTOR inhibitor, upregulates autophagy and prevents the accumulation of mutated proteins by degradation. Additionally, some other agents, such as perhexiline, niclosamide, amiodarone, can inhibit mTORC1. Other mechanisms are increased intracellular glucose, which increases glucose-5-phosphate that inhibits mTOR, and class I PI3K and AKT regulation by dexamethasone induces autophagy.(B3)
Use of antisense oligonucleotides in patients demonstrated a decrease in cerebellar ataxin expression below 75%, delayed the onset of SCA, increased firing frequency of Purkinje cells, and improvement in motor function.[4][18] Other RNA based therapies such as interfering RNAs and microRNAs suppressing the polyglutamine induced neurodegeneration.[16] To some extent, antioxidants and NMDA may also prevent neurodegeneration.
Neurorehabilitation and physical therapy for improving motor functions have a crucial role in the management of SCA.[19][20] Physical therapy focuses on regaining and maintaining the postural balance, gait, and physical strength of patients which assists them to retain independence. Research showed that mild stages of ataxia have significant improvement in balance and gait after six months of physical therapy. Occupational therapies consist of adaptive devices such as wheelchair support, crutches, walker, writing, and feeding devices to help them to make daily living easier, which reduces the burden of asking for help. Speech therapy can be augmented with communication devices and behavioral intervention.[5](A1)
Differential Diagnosis
Differential diagnosis of spinocerebellar ataxia is complex due to its wide range of clinical presentations.
Nongenetic Ataxia
One of the most distinctive features of SCA is ataxia. Ataxia can be caused by diverse etiologies, such as Wilson disease, Vitamin B12 deficiency, hypothyroidism, radiation exposure, Arnold-Chiari malformation, alcohol use disorder, metastatic tumors, paraneoplastic diseases, several enzyme deficiencies, etc. Further evaluation for cancer, endocrine dysfunction, metabolic derangements, nutritional deficits, toxins, and multisystem involvement is essential to rule out the diseases.[1]
Drug-induced Movement Disorder
Neuroleptic drug-induced dystonia (blepharospasm, torticollis) may be confused with dystonia associated with SCA. Benzodiazepines, diphenhydramines, dopamine antagonists, etc. are a few examples of these drugs that induce dystonia as their side effects.
Acute Viral Cerebellitis and Postinfectious Ataxia
Viral infection (Epstein-Barr virus, coxsackievirus) resulting in cerebellitis and several bacterial infections affecting cerebellum such as Lyme disease, syphilis, legionella though rare, may lead to cerebellar ataxia.
Focal Ataxia
Vascular insults such as infarction, hemorrhage, subdural hematoma culminate ipsilateral focal cerebellar ataxia along with other relevant symptoms.
Other Autosomal Recessive Ataxias
Clinically ataxia is the dominant sign, whether it is autosomal recessive or autosomal dominant. Friedreich's ataxia, mitochondrial ataxia, and ataxia-telangiectasia are the main recessive ataxias. It is difficult to distinguish recessive ataxia from SCA clinically since most of the clinical signs and symptoms are similar and overlapping, which necessitates the genetic testing for differentiation.[21]
Dentatorubral Pallidoluysian Atrophy
It has variable manifestation such as progressive ataxia, dystonia, myoclonus, choreoathetosis, seizures, dystonia, etc. which are similar to SCA. It also involves CAG triplet repeat anticipation.[22]
Fragile X Associated Tremor Ataxia Syndrome
It mainly occurs in older age groups and caused by the expansion of GCC repeat in the FMR1 gene. Besides tremor-ataxia, other specific signs present in this syndrome are a premature ovarian failure, which is not present in SCAs.[23]
Gerstmann-Straussler-Schenker Syndrome
It is a group of prion diseases, presents with the ataxia mimic with SCA. It is differentiated by the presence of pathologic prion-protein containing amyloid plaques.[24]
Episodic Ataxia
Type 1 and type 2 episodic ataxias are dominantly inherited disorders, which usually precipitated by certain stimulants such as exercise or the sudden change in posture.
Chorea
Huntington chorea, Sydenham chorea, chorea acanthosis are associated with motor, oculomotor, cognitive disorders, and dystonia like in SCA.
Immune-mediated Ataxia
It is a group of rare diseases that manifest cerebellar ataxia. In these diseases, autoantibodies are found in serum. Some of the diseases include gluten ataxia, Hashimoto encephalopathy, multiple sclerosis, etc.[25]
Prognosis
Differentiation of the genotype-phenotype relationship of spinocerebellar ataxia subtypes helps to improve the prognosis. Although it takes a long time to appreciate the full range of the signs and symptoms, it is almost irreversible once it is evident. But, the symptomatic treatment may improve the prognosis. Survival depends upon the length of CAG repeat expansion. One longitudinal cohort study concluded that the average 10-year survival rate of SCA1 is only 57%, while that of SCA6 is 87%. SCA complicated by dysphagia has the shortest survival rate.[26] Most patients require wheelchair support bye 10 to 15 years of disease onset, but physical therapy can delay the wheelchair requirement.
Complications
Sequelae and complications of spinocerebellar ataxia present late and depend upon the spread of disease as well as parts of CNS involvement. Most of the subtypes of spinocerebellar ataxia spread beyond the cerebellum and spinal cord. In many cases, discrepancies between symptoms and complications are arbitrary yet symptoms may be considered when it is mild but complications are the late consequences of symptoms when it severe that interfere the normal life activities.
Parkinsonism
Due to associated neurodegeneration of dopaminergic neurons of substantia nigra and most commonly seen in SCA2, and rarely in SCA3 and SCA17.[27]
Progression of Dystonia, Tremors, and Movement Disorders
Ataxia accompanied by dystonia increases the severity of the disease.[28] Dystonia is one of the most frequent co-existing disorders of SCA.[29]
Depression, Dementia, and Cognitive Disorders
Depression is the primary long-term factor affecting the subjective health notion of patients in neurodegenerative diseases including SCAs with impairment of emotional function.[2] Depressive symptoms are affected by sexual and urinary dysfunction, personality disturbance, and cognitive impairment. Cognitive impairment mainly associated with SCA1. Dementia was found in one case study in Japan which is present with a severe terminal stage of SCA31.[13]
Ophthalmic Complications
Impaired ocular motility and retinopathy are the common ocular features in SCA7. Central retinal degeneration involving the macula with subsequent spread towards the periphery has been reported. So, central vision impairment followed by progressively complete visual loss can be present.[30][31] Blepharospasm is a rare manifestation of SCA31.[32] Abnormal saccadic movements and nystagmus are noted in a few cases.
Dysphagia and Dysarthria
Degeneration of motor nuclei in the brainstem leading to difficulty in swallowing and slurring of voice can be seen in SCA2, SCA3, SCA6, and SCA7 subtypes. Death due to aspiration pneumonia is common in these patients.[33]
Seizure and Narcolepsy
Pentanucleotide repeats expansions of SCA10 increases the chances of epilepsy by 6 to 10 fold.[34]
Deterrence and Patient Education
Patients and family members should be informed about genetic inheritance, course of the disease, treatment, risks to other members, and the importance of family history. Information about the disease should be shared in a way that the patient and family can understand. But some fundamental messages should be common to all patients. Patient education is essential to clear the confusion about their diseases and enhances patient compliance towards treatment and follow up. This automatically comforts the treatment overload and maintains a good patient-doctor relationship. Patients always have the desire to clear any doubt and to know about their diseases, and their queries should be answered honestly as it is their right. Taking the consent before any intervention, discussing the treatment plans and options, and letting them choose the best one after full description and counseling are vital parts of the management plan. Having knowledge about signs and symptoms makes them aware at the time of onset of disease or any progression of complications that allow them to consult the clinicians earlier that may improve the outcomes.
Enhancing Healthcare Team Outcomes
The interprofessional health team is efficient in covering all aspects of disease diagnosis and management. Physicians have a key role in disease management. Diagnosis of spinocerebellar ataxia is helped by pathologists, radiologists, neurologists, and geneticists. Nursing care is essential in hospitalized patients, and pharmacists evaluate the drug administration and side effects. Since psychological problems are common among patients with SCA, which necessitates deliberate management by experts, including genetic counselors and psychologists.[6]
Although in developed countries, these patients are evaluated longitudinally in multispecialty clinics, significant education is necessary among physicians, patients, and families to obtain all the necessary care in resource-scarce regions. These patients need social workers to increase active participation in the treatment. Occupational therapists, physical therapists, speech-language pathologists, ophthalmologists, are invaluable in the symptomatic treatment of the disease.
Media
(Click Image to Enlarge)
References
Soong BW, Morrison PJ. Spinocerebellar ataxias. Handbook of clinical neurology. 2018:155():143-174. doi: 10.1016/B978-0-444-64189-2.00010-X. Epub [PubMed PMID: 29891056]
Jacobi H, du Montcel ST, Bauer P, Giunti P, Cook A, Labrum R, Parkinson MH, Durr A, Brice A, Charles P, Marelli C, Mariotti C, Nanetti L, Sarro L, Rakowicz M, Sulek A, Sobanska A, Schmitz-Hübsch T, Schöls L, Hengel H, Baliko L, Melegh B, Filla A, Antenora A, Infante J, Berciano J, van de Warrenburg BP, Timmann D, Szymanski S, Boesch S, Nachbauer W, Kang JS, Pandolfo M, Schulz JB, Melac AT, Diallo A, Klockgether T. Long-term evolution of patient-reported outcome measures in spinocerebellar ataxias. Journal of neurology. 2018 Sep:265(9):2040-2051. doi: 10.1007/s00415-018-8954-0. Epub 2018 Jun 29 [PubMed PMID: 29959555]
Huang M, Verbeek DS. Why do so many genetic insults lead to Purkinje Cell degeneration and spinocerebellar ataxia? Neuroscience letters. 2019 Jan 1:688():49-57. doi: 10.1016/j.neulet.2018.02.004. Epub 2018 Feb 5 [PubMed PMID: 29421540]
Level 3 (low-level) evidenceSullivan R, Yau WY, O'Connor E, Houlden H. Spinocerebellar ataxia: an update. Journal of neurology. 2019 Feb:266(2):533-544. doi: 10.1007/s00415-018-9076-4. Epub 2018 Oct 3 [PubMed PMID: 30284037]
Klivényi P, Horváth Z, Vécsei L. [Clinical features and diagnosis of spinocerebellar ataxia]. Ideggyogyaszati szemle. 2004 Jan 20:57(1-2):11-22 [PubMed PMID: 15042864]
Tan EK. Autosomal dominant spinocerebellar ataxias: an Asian perspective. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2003 Nov:30(4):361-7 [PubMed PMID: 14672269]
Level 3 (low-level) evidenceCalabresi V, Guida S, Servadio A, Jodice C. Phenotypic effects of expanded ataxin-1 polyglutamines with interruptions in vitro. Brain research bulletin. 2001 Oct-Nov 1:56(3-4):337-42 [PubMed PMID: 11719269]
Chen HK, Fernandez-Funez P, Acevedo SF, Lam YC, Kaytor MD, Fernandez MH, Aitken A, Skoulakis EM, Orr HT, Botas J, Zoghbi HY. Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 2003 May 16:113(4):457-68 [PubMed PMID: 12757707]
Level 3 (low-level) evidenceKoeppen AH. The pathogenesis of spinocerebellar ataxia. Cerebellum (London, England). 2005:4(1):62-73 [PubMed PMID: 15895563]
Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rüb U. Brain pathology of spinocerebellar ataxias. Acta neuropathologica. 2012 Jul:124(1):1-21. doi: 10.1007/s00401-012-1000-x. Epub 2012 Jun 9 [PubMed PMID: 22684686]
Koeppen AH. The Neuropathology of Spinocerebellar Ataxia Type 3/Machado-Joseph Disease. Advances in experimental medicine and biology. 2018:1049():233-241. doi: 10.1007/978-3-319-71779-1_11. Epub [PubMed PMID: 29427106]
Level 3 (low-level) evidenceJimenez-Sanchez M, Thomson F, Zavodszky E, Rubinsztein DC. Autophagy and polyglutamine diseases. Progress in neurobiology. 2012 May:97(2):67-82. doi: 10.1016/j.pneurobio.2011.08.013. Epub 2011 Sep 10 [PubMed PMID: 21930185]
Level 3 (low-level) evidenceAdachi T, Kitayama M, Nakano T, Adachi Y, Kato S, Nakashima K. Autopsy case of spinocerebellar ataxia type 31 with severe dementia at the terminal stage. Neuropathology : official journal of the Japanese Society of Neuropathology. 2015 Jun:35(3):273-9. doi: 10.1111/neup.12184. Epub 2014 Dec 11 [PubMed PMID: 25495291]
Level 3 (low-level) evidenceMuñoz E, Rey MJ, Milà M, Cardozo A, Ribalta T, Tolosa E, Ferrer I. Intranuclear inclusions, neuronal loss and CAG mosaicism in two patients with Machado-Joseph disease. Journal of the neurological sciences. 2002 Aug 15:200(1-2):19-25 [PubMed PMID: 12127671]
Level 3 (low-level) evidenceTsai HF, Liu CS, Leu TM, Wen FC, Lin SJ, Liu CC, Yang DK, Li C, Hsieh M. Analysis of trinucleotide repeats in different SCA loci in spinocerebellar ataxia patients and in normal population of Taiwan. Acta neurologica Scandinavica. 2004 May:109(5):355-60 [PubMed PMID: 15080863]
Ashizawa T, Öz G, Paulson HL. Spinocerebellar ataxias: prospects and challenges for therapy development. Nature reviews. Neurology. 2018 Oct:14(10):590-605. doi: 10.1038/s41582-018-0051-6. Epub [PubMed PMID: 30131520]
Yoshida H, Yoshizawa T, Shibasaki F, Shoji S, Kanazawa I. Chemical chaperones reduce aggregate formation and cell death caused by the truncated Machado-Joseph disease gene product with an expanded polyglutamine stretch. Neurobiology of disease. 2002 Jul:10(2):88-99 [PubMed PMID: 12127147]
Level 3 (low-level) evidenceScoles DR, Meera P, Schneider MD, Paul S, Dansithong W, Figueroa KP, Hung G, Rigo F, Bennett CF, Otis TS, Pulst SM. Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature. 2017 Apr 20:544(7650):362-366. doi: 10.1038/nature22044. Epub 2017 Apr 12 [PubMed PMID: 28405024]
Rodríguez-Díaz JC, Velázquez-Pérez L, Rodríguez Labrada R, Aguilera Rodríguez R, Laffita Pérez D, Canales Ochoa N, Medrano Montero J, Estupiñán Rodríguez A, Osorio Borjas M, Góngora Marrero M, Reynaldo Cejas L, González Zaldivar Y, Almaguer Gotay D. Neurorehabilitation therapy in spinocerebellar ataxia type 2: A 24-week, rater-blinded, randomized, controlled trial. Movement disorders : official journal of the Movement Disorder Society. 2018 Sep:33(9):1481-1487. doi: 10.1002/mds.27437. Epub 2018 Aug 22 [PubMed PMID: 30132999]
Level 1 (high-level) evidenceSynofzik M, Ilg W. Motor training in degenerative spinocerebellar disease: ataxia-specific improvements by intensive physiotherapy and exergames. BioMed research international. 2014:2014():583507. doi: 10.1155/2014/583507. Epub 2014 Apr 27 [PubMed PMID: 24877117]
Synofzik M, Németh AH. Recessive ataxias. Handbook of clinical neurology. 2018:155():73-89. doi: 10.1016/B978-0-444-64189-2.00005-6. Epub [PubMed PMID: 29891078]
Tsuji S. Dentatorubral-pallidoluysian atrophy. Handbook of clinical neurology. 2012:103():587-94. doi: 10.1016/B978-0-444-51892-7.00041-3. Epub [PubMed PMID: 21827919]
Hoem G, Koht J. Fragile X-associated tremor/ataxia syndrome. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2017 Oct 31:137(20):. doi: 10.4045/tidsskr.17.0317. Epub 2017 Oct 30 [PubMed PMID: 29094559]
Keuss SE, Ironside JW, O'Riordan J. Gerstmann-Sträussler-Scheinker disease with atypical presentation. BMJ case reports. 2017 Nov 1:2017():. pii: bcr-2017-220907. doi: 10.1136/bcr-2017-220907. Epub 2017 Nov 1 [PubMed PMID: 29092967]
Level 3 (low-level) evidenceJoubert B, Rostásy K, Honnorat J. Immune-mediated ataxias. Handbook of clinical neurology. 2018:155():313-332. doi: 10.1016/B978-0-444-64189-2.00021-4. Epub [PubMed PMID: 29891069]
Diallo A, Jacobi H, Cook A, Labrum R, Durr A, Brice A, Charles P, Marelli C, Mariotti C, Nanetti L, Panzeri M, Rakowicz M, Sobanska A, Sulek A, Schmitz-Hübsch T, Schöls L, Hengel H, Melegh B, Filla A, Antenora A, Infante J, Berciano J, van de Warrenburg BP, Timmann D, Boesch S, Pandolfo M, Schulz JB, Bauer P, Giunti P, Kang JS, Klockgether T, Tezenas du Montcel S. Survival in patients with spinocerebellar ataxia types 1, 2, 3, and 6 (EUROSCA): a longitudinal cohort study. The Lancet. Neurology. 2018 Apr:17(4):327-334. doi: 10.1016/S1474-4422(18)30042-5. Epub 2018 Mar 13 [PubMed PMID: 29553382]
Park H, Kim HJ, Jeon BS. Parkinsonism in spinocerebellar ataxia. BioMed research international. 2015:2015():125273. doi: 10.1155/2015/125273. Epub 2015 Mar 19 [PubMed PMID: 25866756]
Kuo PH, Gan SR, Wang J, Lo RY, Figueroa KP, Tomishon D, Pulst SM, Perlman S, Wilmot G, Gomez CM, Schmahmann JD, Paulson H, Shakkottai VG, Ying SH, Zesiewicz T, Bushara K, Geschwind MD, Xia G, Subramony SH, Ashizawa T, Kuo SH. Dystonia and ataxia progression in spinocerebellar ataxias. Parkinsonism & related disorders. 2017 Dec:45():75-80. doi: 10.1016/j.parkreldis.2017.10.007. Epub 2017 Oct 23 [PubMed PMID: 29089256]
van Gaalen J, Giunti P, van de Warrenburg BP. Movement disorders in spinocerebellar ataxias. Movement disorders : official journal of the Movement Disorder Society. 2011 Apr:26(5):792-800. doi: 10.1002/mds.23584. Epub 2011 Mar 2 [PubMed PMID: 21370272]
Campos-Romo A, Graue-Hernandez EO, Pedro-Aguilar L, Hernandez-Camarena JC, Rivera-De la Parra D, Galvez V, Diaz R, Jimenez-Corona A, Fernandez-Ruiz J. Ophthalmic features of spinocerebellar ataxia type 7. Eye (London, England). 2018 Jan:32(1):120-127. doi: 10.1038/eye.2017.135. Epub 2017 Aug 11 [PubMed PMID: 28799562]
Miller RC, Tewari A, Miller JA, Garbern J, Van Stavern GP. Neuro-ophthalmologic features of spinocerebellar ataxia type 7. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2009 Sep:29(3):180-6. doi: 10.1097/WNO.0b013e3181b1b3f8. Epub [PubMed PMID: 19726938]
Level 2 (mid-level) evidenceItaya S, Kobayashi Z, Ozaki K, Sato N, Numasawa Y, Ishikawa K, Yokota T, Matsuda H, Shintani S. Spinocerebellar Ataxia Type 31 with Blepharospasm. Internal medicine (Tokyo, Japan). 2018 Jun 1:57(11):1651-1654. doi: 10.2169/internalmedicine.0068-17. Epub 2018 Feb 9 [PubMed PMID: 29434122]
Rüb U, Brunt ER, Petrasch-Parwez E, Schöls L, Theegarten D, Auburger G, Seidel K, Schultz C, Gierga K, Paulson H, van Broeckhoven C, Deller T, de Vos RA. Degeneration of ingestion-related brainstem nuclei in spinocerebellar ataxia type 2, 3, 6 and 7. Neuropathology and applied neurobiology. 2006 Dec:32(6):635-49 [PubMed PMID: 17083478]
McFarland KN, Liu J, Landrian I, Zeng D, Raskin S, Moscovich M, Gatto EM, Ochoa A, Teive HA, Rasmussen A, Ashizawa T. Repeat interruptions in spinocerebellar ataxia type 10 expansions are strongly associated with epileptic seizures. Neurogenetics. 2014 Mar:15(1):59-64. doi: 10.1007/s10048-013-0385-6. Epub 2013 Dec 7 [PubMed PMID: 24318420]
Level 2 (mid-level) evidence