Introduction
Spondylolysis is a unilateral or bilateral defect in the region of the pars interarticularis, which may or may not be accompanied by the vertebral body slippage. It is most commonly the sequelae of repetitive trauma to the growing immature skeletal architecture among a genetically susceptible individuals. [1][2][3] The pars interarticularis is considered the isthmus or bone bridge between the inferior and superior articular surfaces of a single vertebra. [4][5][6]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Spondylolysis may be congenital or acquired. Although the exact pathogenesis remains unknown in all cases, it is most commonly secondary to a fatigue or stress fracture of the pars interarticularis that persists as a non-union. It typically develops in genetically susceptible children and adolescents with faulty biomechanics, and who also experience repetitive microtrauma on the pars interarticularis from repeated activities involving lumbar hyperextension with rotation.[3][4][5][6]
Epidemiology
The prevalence of spondylolysis is 4% by age 6 and 6% by age 14 and thereafter remains constant throughout adulthood.
There is a genetic predisposition with an increased incidence seen in:
- Males (male to female ratio of 2:1)
- Alaskan Eskimo descendants
- First-degree offspring of patients with the condition
- Concurrent pathologies such as spina bifida occulta, Marfan syndrome, osteogenesis imperfecta, and osteopetrosis.
Adolescents involved in sports have a higher prevalence compared to those not involved in sports. The mean age of diagnosis is 15 years of age.
There is an increased incidence among participants of certain higher-risk sports which involve repeated axial loading and/or lumbar hyperextension with rotation. These sports include gymnastics, dance, football (particularly linemen), rugby, wrestling, martial arts, soccer, basketball, cheerleading, pitching, golf, tennis, volleyball servers, weightlifting, and butterfly and breaststroke swimming.[3][4][7][8]
Pathophysiology
Ninety percent of the cases of spondylolysis occur at the L5 vertebra with decreasing incidence at progressively higher lumbar levels. Excessive lumbar lordosis is a risk factor for spondylolysis development. Most commonly, pars interarticularis defects occur bilaterally as opposed to unilaterally. Unhealed pars interarticularis defects may progress to lytic (isthmic) spondylolisthesis, which is an anterior displacement of the vertebral body in relation to the vertebra below. It is important to note unilateral lesions never progress to spondylolisthesis. However, in patients with bilateral spondylolysis, at the time of diagnosis, 50% to 75% will already have accompanying spondylolisthesis. Slip progression is more common in adolescents compared to adults, and although the incidence of spondylolysis is more common in males, the slip progression of spondylolisthesis occurs more frequently in females.
Additionally, multifidi muscles of the back attach to the mamillary process of the vertebra thereby stabilizing vertebral joints and providing stability at each segmental level. The mammillary process is not completely formed until 25 years of age. Full ossification of the neural arch is also not completed until the same age limit. These 2 factors play a pivotal role in the development of the condition during adolescence.[8][5][3]
History and Physical
Spondylolysis mostly remains asymptomatic, but approximately 10% of affected individuals manifest symptoms constituting of insidious onset, recurrent axial low back pain that increases with activity, is exacerbated by lumbar hyperextension, and may or may not be associated with a radicular component. The pain can range from mild to severe in intensity and is described as a dull, aching pain in the lower back, buttocks, and posterior thigh regions. If neurologic symptoms/signs are present, it is likely secondary to spondylolysis with spondylolisthesis or associated degenerative processes resulting in narrowing of the neuroforamina and the spinal nerve impingements. Since spondylolysis most commonly affects L5 on S1, the corresponding dermatomal and myotomal pattern clinical manifestations usually occur. It is important to note that if the patient’s spondylolysis has progressed to spondylolisthesis and they are presenting with pain, the degree of pain does not correlate with the degree of slippage, and this presents a diagnostic challenge and explains why the condition is often advanced at the time of diagnosis.
The neurological examination would specifically show increased lumbar lordosis, tight hamstrings, reduced trunk range of motion (particularly with extension), tenderness to palpation overlying the pars fracture site, a positive stork test (single leg hyperextension and rotation of the spine which reproduces the patient pain and is diagnostic of spondylolysis until proven otherwise), with the characteristic absence of any radiculopathy. Again, radicular symptoms can occur, but they are uncommon.[9][7][3]
Evaluation
As a general consensus rule, a patient's neurologic examination, as well as all laboratory evaluations including inflammatory markers, will be largely unremarkable.
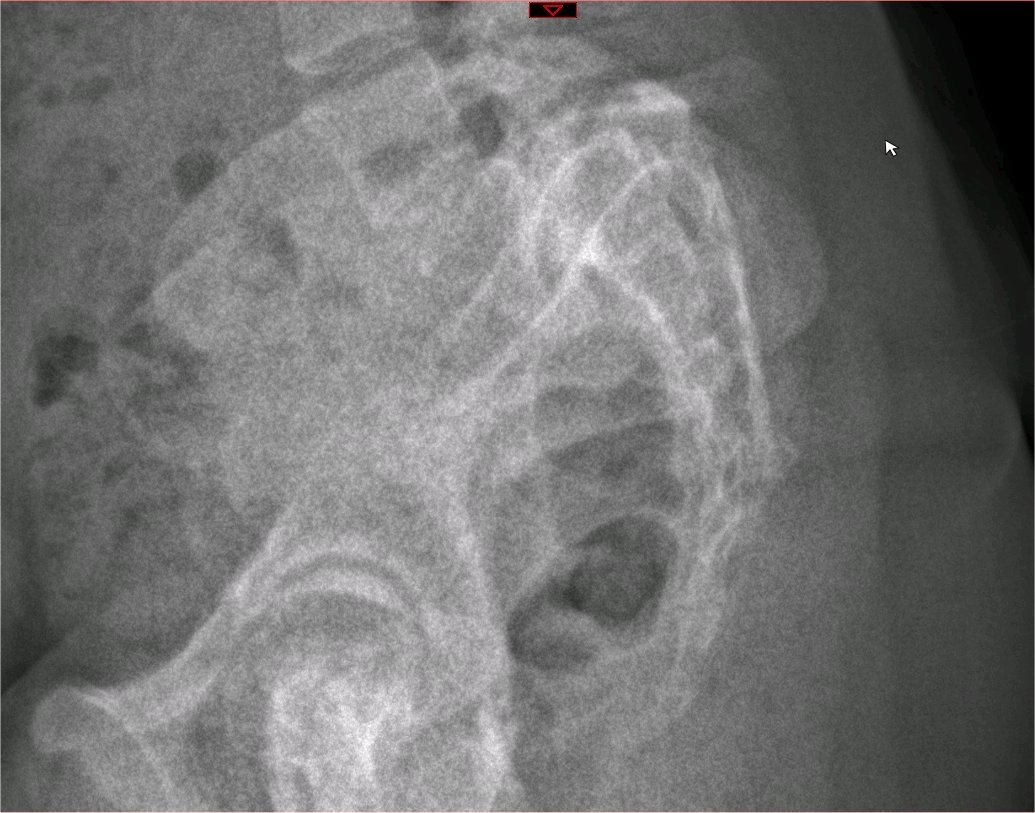
There are no universally accepted guidelines for an imaging protocol, but the initial imaging studies of choice when the condition is suspected are plain radiographs in the posteroanterior, lateral, and oblique views of the lumbosacral region in the standing position. When present, the lesion is most typically visible in the oblique view which shows the classic "collar on the Scotty dog" and represents the bony defect between the inferior articular surface and the superior articular surface of a single vertebra. As with any stress fracture, plain films may miss the lesion within the first 2 weeks of the injury. Additionally, it has been reported that plain radiographs only have a sensitivity of 33% in detecting spondylolysis. Some authorities advocate axial CT-imaging as the test of choice for spondylolysis; however, due to the high prevalence of spondylolysis in the pediatric population and concerns regarding unnecessary radiation exposure, the next advanced imaging modality is typically chosen, if plain films are negative, is the MRI. MRI is excellent at detecting bone marrow edema associated with acute pars interarticularis stress injury as well as detailing neural and soft tissue pathologies. The SPECT scan is a suitable alternative to MRI for detecting acute stress fractures and is also useful in determining the acuity of a fracture. It however carries the risk of concurrent radiation hazards, which is therefore preferably avoided in the pediatric population.[7][10][8][9]
Treatment / Management
Most patients with spondylolysis, including athletes, can be managed conservatively. In fact, patients who are asymptomatic and their condition is discovered incidentally on imaging may maintain their current level of physical activity without any activity restrictions. However, if the patient presents with acute symptomatic spondylolysis as confirmed by SPECT-scan or MRI, conservative treatments are warranted and would include:
- Relative 6 to 12 weeks of spinal bracing (Corset versus TLSO), thus, limiting spinal mobilization and stress on the pars interarticularis. However, a recent meta-analysis found that 83% of patients treated non-operatively improved clinically regardless of any spinal bracing.
- Activity modifications including cessation of activities, especially those involving a hyperextension of the spine. Athletic activities may be gradually resumed as the pain subsides.
- Physical therapy emphasizing spinal stabilization through stretching of the hip flexors, hamstrings, quadriceps, gastrocnemius-soleus complex, and strengthening of the abdominal and back muscles utilizing a pain-free range of motion with the application of the progressive resistance training protocol such as William’s flexion exercises is generally advised.
Adjunctive treatments including ice/heat therapy, NSAIDS, epidural steroid injections, massage, osteopathic or chiropractic manipulation, and cognitive-behavioral therapy (CBT) are generally well-tolerated, are of benefits, and should be considered.
With conservative treatment protocol, 75% of adolescents will improve, and invariably their lytic defects heal. Unilateral defects are more likely to heal than bilateral defects. In cases of spondylolysis with concomitant spondylolisthesis, the bony defects are unlikely to heal but the implementation of the same conservative treatment principles typically results in an abatement of the patients’ symptomatology and return to their previous level of activity. A recent randomized control trial found no difference in the clinical outcomes among patients with spondylolisthesis among cohorts treated conservatively with those managed surgically; which is clinically significant considering the cost and the potential complications associated with surgical treatments. Additionally, there is currently no clinical evidence favoring spinal bracing in preventing vertebral slippage among patients with spondylolysis and spondylolisthesis.
Surgical intervention is only warranted if the patient fails a conservative management trial of at least 6 months or has progressive neurologic symptoms with saddle anesthesia, bowel/bladder dysfunction, refractory pain, or develops spondylolisthesis of grade 3 or higher. The most commonly performed surgical treatments of spondylolysis include a direct repair of the defect in the pars interarticularis at L1 to L4 levels and an inter-transverse fusion for defects at the L5 level.[7][11](A1)
Differential Diagnosis
- Muscular strains and sprains
- Lumbar radiculopathy secondary to degenerative disc disease and resultant disc bulge and/or herniation
- Spinal canal stenosis
- Epidural abscess
- Fracture of other components of the posterior vertebral arch
- Osteoid sarcoma or other primary bone tumors
- Pathologic fracture secondary to osteoporosis, malignancy, infections, or additional intrinsic bone-weakening etiologies
- Degenerative spondylolisthesis of adulthood
- Ankylosing spondylitis[7][4]
Prognosis
The prognosis in patients with spondylolysis is usually excellent. Asymptomatic individuals require no specific treatments or any modifications to activities of daily living or athletic activities. Even patients who present with symptomatic spondylolysis usually have a very favorable prognosis as validated by a recent meta-analysis which demonstrated that the 92% of the adolescent athletes were able to return to competitions when they are treated conservatively, and 90% of the time when managed surgically.[11][1][3][4]
Complications
In the majority of patients with spondylolysis, the condition is occult and remains asymptomatic throughout their lifetimes. However, degenerative disc disease and resultant spondylosis, which typically occurs as a sequela of the aging process, have a propensity to be accelerated in patients with spondylolysis. This may lead to spinal stenosis and lumbar radiculopathies. These deleterious effects may also occur secondary to vertebral body slippage in almost 50% to 75% of patients with bilateral spondylolysis. Potential surgical complications would include a failed fusion, infections, chronic persistent pain, neurological deteriorations, and the failed back surgery syndrome.[11][1][4]
Postoperative and Rehabilitation Care
The need for surgical correction of spondylolysis is uncommon. However, if surgical correction is justified, the postoperative rehabilitative processes should include stringent protection of the surgical site until wound closure (bracing is typically surgeon dependent), proper analgesia, graded physical therapy, and education on proper biomechanics with an emphasis on maintaining corrective postures and sleeping positionings.[9][11][1]
Consultations
Orthopedic and neurosurgical consultations as indicated.[4][7]
Deterrence and Patient Education
Asymptomatic individuals, whose condition is discovered incidentally on imaging, should be reassured of the generally benign course of the disease throughout their lifetime, without any need for activity restrictions. However, symptomatic individuals with a pars interarticularis defect that is actively undergoing treatment, regardless of whether the treatment is conservative or surgical, need to have an education and understanding on proper biomechanics to optimize their comfort as well as to decrease stress and promote healing of the involved structures. Techniques that should be taught and implemented include:
- Log-rolling while getting in and out of bed and limiting truncal movement in only one plane at a time (in other words, sagittal, coronal, transverse) during transfers and ambulation
- Utilizing a lumbar roll while sitting, pillow under the knees when supine, and between the knees when side-lying helps to maintain the neutrality of the spine thereby decreasing stress on the concerned vertebra, promoting healing, as well as maximizing patient comfort.[4][7][3]
Enhancing Healthcare Team Outcomes
Spondylolysis is best managed by an interprofessional team that includes a surgeon, primary care provider, physical therapist, nurse, and sports physician. The majority of patients are managed conservatively.
Surgical correction of spondylolysis is uncommon. However, if surgical correction is necessary the postoperative rehabilitative care should include protection of the surgical site until wound closure (bracing is typically surgeon dependent), proper analgesia, physical therapy, and education on proper biomechanics with an emphasis on posture and sleeping positioning. An integral part of the postoperative rehabilitative care of athletes constitutes a graded return to sports protocol, comprising of education on maintaining proper biomechanics at first, and gradually progressing to sport-specific activities as tolerated.[9][11][1]
For those who remain compliant with physical therapy, the prognosis is good. Those who continue to lead a sedentary lifestyle usually develop chronic refractory pain and the resultant poor quality of life.[12]
Media
(Click Image to Enlarge)
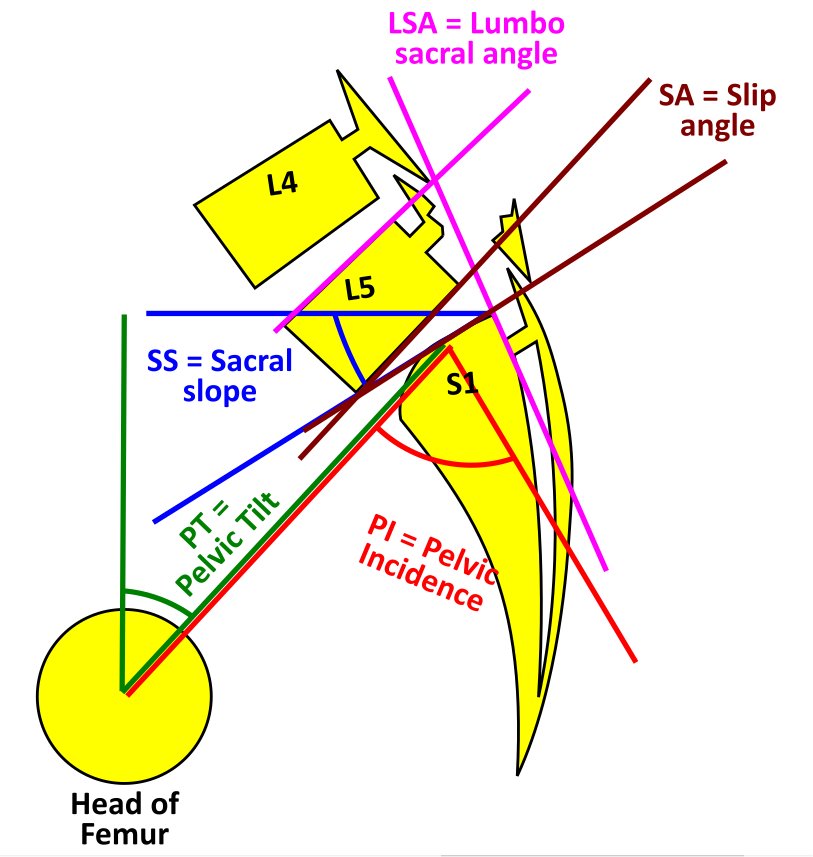
Spondylolysis with Spondylolisthesis, showing spino pelvic parameters, slip, dysplasia of upper endplate of S1, Slip angle (SA = angle between inferior endplate of L5 and line perpendicular to the S1 posterior wall) and lumbo sacral angle (LSA = angle between the superior endplate of L5 and posterior wall of the S1). Contributed by George Ampat FRCS
(Click Image to Enlarge)
References
Panteliadis P, Nagra NS, Edwards KL, Behrbalk E, Boszczyk B. Athletic Population with Spondylolysis: Review of Outcomes following Surgical Repair or Conservative Management. Global spine journal. 2016 Sep:6(6):615-25. doi: 10.1055/s-0036-1586743. Epub 2016 Aug 10 [PubMed PMID: 27556003]
Delavan JA, Stence NV, Mirsky DM, Gralla J, Fadell MF. Confidence in Assessment of Lumbar Spondylolysis Using Three-Dimensional Volumetric T2-Weighted MRI Compared With Limited Field of View, Decreased-Dose CT. Sports health. 2016 Jul:8(4):364-71. doi: 10.1177/1941738116653587. Epub 2016 Jun 9 [PubMed PMID: 27282808]
Hasler CC. Back pain during growth. Swiss medical weekly. 2013:143():w13714. doi: 10.4414/smw.2013.13714. Epub 2013 Jan 8 [PubMed PMID: 23299906]
Patel DR, Kinsella E. Evaluation and management of lower back pain in young athletes. Translational pediatrics. 2017 Jul:6(3):225-235. doi: 10.21037/tp.2017.06.01. Epub [PubMed PMID: 28795014]
Yamashita K, Sakai T, Takata Y, Hayashi F, Tezuka F, Morimoto M, Kinoshita Y, Nagamachi A, Chikawa T, Yonezu H, Higashino K, Sakamaki T, Sairyo K. Utility of STIR-MRI in Detecting the Pain Generator in Asymmetric Bilateral Pars Fracture: A Report of 5 Cases. Neurologia medico-chirurgica. 2018 Feb 15:58(2):91-95. doi: 10.2176/nmc.cr.2017-0123. Epub 2017 Dec 25 [PubMed PMID: 29276206]
Level 3 (low-level) evidenceTofte JN, CarlLee TL, Holte AJ, Sitton SE, Weinstein SL. Imaging Pediatric Spondylolysis: A Systematic Review. Spine. 2017 May 15:42(10):777-782. doi: 10.1097/BRS.0000000000001912. Epub [PubMed PMID: 27669047]
Level 1 (high-level) evidenceMortazavi J, Zebardast J, Mirzashahi B. Low Back Pain in Athletes. Asian journal of sports medicine. 2015 Jun:6(2):e24718. doi: 10.5812/asjsm.6(2)2015.24718. Epub 2015 Jun 20 [PubMed PMID: 26448841]
Mushtaq R, Porrino J, Guzmán Pérez-Carrillo GJ. Imaging of Spondylolysis: The Evolving Role of Magnetic Resonance Imaging. PM & R : the journal of injury, function, and rehabilitation. 2018 Jun:10(6):675-680. doi: 10.1016/j.pmrj.2018.02.001. Epub 2018 Feb 8 [PubMed PMID: 29428876]
Roy SL, Shaw PC, Beattie TF. Low back pain in the paediatric athlete. European journal of emergency medicine : official journal of the European Society for Emergency Medicine. 2015 Oct:22(5):348-54. doi: 10.1097/MEJ.0000000000000154. Epub [PubMed PMID: 24756086]
Level 2 (mid-level) evidenceRamadorai U, Hire J, DeVine JG, Brodt ED, Dettori JR. Incidental findings on magnetic resonance imaging of the spine in the asymptomatic pediatric population: a systematic review. Evidence-based spine-care journal. 2014 Oct:5(2):95-100. doi: 10.1055/s-0034-1386753. Epub [PubMed PMID: 25278883]
Level 1 (high-level) evidenceOverley SC, McAnany SJ, Andelman S, Kim J, Merrill RK, Cho SK, Qureshi SA, Hecht AC. Return to Play in Adolescent Athletes With Symptomatic Spondylolysis Without Listhesis: A Meta-Analysis. Global spine journal. 2018 Apr:8(2):190-197. doi: 10.1177/2192568217734520. Epub 2017 Oct 5 [PubMed PMID: 29662750]
Level 1 (high-level) evidenceCushnie D, Johnstone R, Urquhart JC, Gurr KR, Bailey SI, Bailey CS. Quality of Life and Slip Progression in Degenerative Spondylolisthesis Treated Nonoperatively. Spine. 2018 May 15:43(10):E574-E579. doi: 10.1097/BRS.0000000000002429. Epub [PubMed PMID: 28953710]
Level 2 (mid-level) evidence