Introduction
Staphylococcus aureus is a major bacterial human pathogen that causes a wide variety of clinical manifestations.[1] Infections are common both in community-acquired as well as hospital-acquired settings and treatment remains challenging to manage due to the emergence of multi-drug resistant strains such as MRSA (Methicillin-Resistant Staphylococcus aureus).[2][3] S. aureus is found in the environment and is also found in normal human flora, located on the skin and mucous membranes (most often the nasal area) of most healthy individuals.[1] S. aureus does not normally cause infection on healthy skin; however, if it is allowed to enter the bloodstream or internal tissues, these bacteria may cause a variety of potentially serious infections.[1] Transmission is typically from direct contact. However, some infections involve other transmission methods.[4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
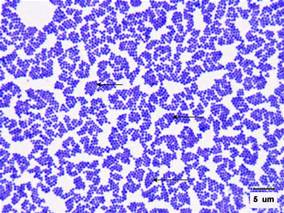
Staphylococcus aureus is Gram-positive bacteria (stain purple by Gram stain) that are cocci-shaped and tend to be arranged in clusters that are described as “grape-like.” On media, these organisms can grow in up to 10% salt, and colonies are often golden or yellow (aureus means golden or yellow). These organisms can grow aerobically or anaerobically (facultative) and at temperatures between 18 C and 40 C. Typical biochemical identification tests include catalase positive (all pathogenic Staphylococcus species), coagulase positive (to distinguish Staphylococcus aureus from other Staphylococcus species), novobiocin sensitive (to distinguish from Staphylococcus saprophyticus), and mannitol fermentation positive (to distinguish from Staphylococcus epidermidis).[4][1] MRSA strains carry a mec gene on the bacterial chromosome, which is a component of the larger Staphylococcal chromosomal cassette mec (SCCmec) region, conferring resistance to multiple antibiotics depending on the SCCmec type.[2] The mec gene encodes the protein PBP-2a (penicillin-binding protein 2a). PBP-2a is a penicillin-binding protein (PBP), or essential bacterial cell wall enzyme that catalyzes the production of the peptidoglycan in the bacterial cell wall. PBP-2A has a lower affinity to bind to beta-lactams (and other penicillin-derived antibiotics) when compared to other PBPs, so PBP-2A continues to catalyze the synthesis of the bacterial cell wall even in the presence of many antibiotics. As a result, S. aureus strains that synthesize PBP-2A can grow in the presence of many antibiotics, and these MRSA strains are resistant to many antibiotics. MRSA strains tend to be resistant to methicillin, nafcillin, oxacillin, and cephalosporins.[2][4]
Epidemiology
Staphylococcus aureus (including drug-resistant strains such as MRSA) are found on the skin and mucous membranes, and humans are the major reservoir for these organisms.[3][5] It is estimated that up to half of all adults are colonized, and approximately 15% of the population persistently carry S. aureus in the anterior nares. Some populations tend to have higher rates of S. aureus colonization (up to 80%), such as health care workers, persons who use needles on a regular basis (i.e., diabetics and intravenous (IV) drug users), hospitalized patients, and immunocompromised individuals. S. aureus can be transmitted person-to-person by direct contact or by fomites.[6][4][1]
Pathophysiology
S. aureus are one the most common bacterial infections in humans and are the causative agents of multiple human infections, including bacteremia, infective endocarditis, skin and soft tissue infections (e.g., impetigo, folliculitis, furuncles, carbuncles, cellulitis, scalded skin syndrome, and others), osteomyelitis, septic arthritis, prosthetic device infections, pulmonary infections (e.g., pneumonia and empyema), gastroenteritis, meningitis, toxic shock syndrome, and urinary tract infections.[6] Depending on the strains involved and the site of infection, these bacteria can cause invasive infections and/or toxin-mediated diseases.[6][7] The pathophysiology varies greatly depending on the type of S. aureus infection.[6] Mechanisms for evasion of the host immune response include the production of an antiphagocytic capsule, sequestering of host antibodies or antigen masking by Protein A, biofilm formation, intracellular survival, and blocking chemotaxis of leukocytes.[8][7] Binding of the bacteria to extracellular matrix proteins and fibronectin in infectious endocarditis is mediated by bacterial cell wall-associated proteins such as fibrinogen-binding proteins, clumping factors, and teichoic acids.[7] Also, Staphylococcal superantigens (TSST-1 or toxic shock syndrome toxin 1) are important virulence factors in infectious endocarditis, sepsis, as well as toxic shock syndrome.[9][10] Pneumonia infections are associated with the bacterial production of PVL (Panton-Valentine leukocidin), Protein A, and alpha-hemolysin, and infections are more common following influenza virus infection as well as a diagnosis of Cystic Fibrosis. Prosthetic device infections are often mediated by the ability of S. aureus strains to form biofilms as well as communicate using quorum sensing in a bacterial cell density-dependent manner. [11]
History and Physical
History and physical will vary greatly depending on the type of infection; however, an accurate history and physical is often required for diagnosis and treatment.[1]
Evaluation
Evaluation of an S. aureus infection involves evaluation of clinical signs and symptoms as well as the history and physical findings. In many cases, routine cultures will reveal the diagnosis (i.e., blood, sputum); however, RT-PCR (real-time PCR) for 16S rRNA genes may be necessary in some cases. Drug susceptibility testing often is required to guide treatment. If patient samples are collected for pathogen identification in the microbiology laboratory, caution must be exercised as the presence of S. aureus in the skin or mucous membrane does not necessarily indicate infection because these organisms are frequently members of the normal flora.[4]
Treatment / Management
Treatment of S. aureus infections depends largely on the type of infection as well as the presence or absence of drug resistant strains.[6] When antimicrobial therapy is needed, the duration and mode of therapy are largely dependent on the infection type as well as other factors.[6] In general, penicillin remains the drug of choice if isolates are sensitive (MSSA, or methicillin sensitive S. aureus strains) and vancomycin for MRSA strains.[3] In some cases, alternative therapy is necessary for addition to antimicrobial therapy.[6] For example, fluid-replacement management is often required for toxin-mediated illness and removal of foreign devices for prosthetic value endocarditis or catheter-associated infections. Because many MRSA strains are resistant to multiple antibiotics, MRSA infections are emerging as serious pathogens in both the hospital and the community settings.[3][5]
Differential Diagnosis
- Bacteremia
- Chemical Burns
- Impetigo
- Juvenile Idiopathic Arthritis
- Kawasaki Disease
- Leptospirosis
- Parvovirus B19 Infection
- Pediatric Bacterial Endocarditis
- IBS
- Pediatric Osteomyelitis
- Pediatric Serum Sickness
Pearls and Other Issues
Prevention of S. aureus infections remains challenging. Despite many efforts, a routine vaccination for S. aureus infections has remained elusive. As a result, efforts have relied on infection control methods such as hospital decontamination procedures, handwashing techniques, and MRSA transmission prevention guidelines. Topical antimicrobials such as mupirocin can be used to eliminate nasal colonization in some nasal carriers. However, usage is controversial.
Enhancing Healthcare Team Outcomes
Infections by S.aureus are encountered by the nurse practitioner, primary care provider, internist and the infectious disease expert on a regular basis. The key feature of treatment is to determine the presence/absence of drug-resistant strains. When prescribing antibiotics, one should limit the duration to no more than 7 to 10 days for most infections. The reason is that the empirical prescription of antibiotics has led to the development of resistant strains. Pharmacists should coordinate with the clinician to target antimicrobial therapy, and nursing can chart the progress so modification to the regimen can be made if treatment is ineffective. This kind of interprofessional coordination is necessary to treat such infections with precision.
In addition, the patient should be educated by an interprofessional team of nurses and physicians about hand hygiene to help prevent transmission of infection to others.
Media
References
Lowy FD. Staphylococcus aureus infections. The New England journal of medicine. 1998 Aug 20:339(8):520-32 [PubMed PMID: 9709046]
Centers for Disease Control and Prevention (CDC). Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections--Los Angeles County, California, 2002-2003. MMWR. Morbidity and mortality weekly report. 2003 Feb 7:52(5):88 [PubMed PMID: 12588006]
Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008 Jun 1:46 Suppl 5():S344-9. doi: 10.1086/533590. Epub [PubMed PMID: 18462089]
Rasigade JP, Vandenesch F. Staphylococcus aureus: a pathogen with still unresolved issues. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2014 Jan:21():510-4. doi: 10.1016/j.meegid.2013.08.018. Epub 2013 Aug 29 [PubMed PMID: 23994773]
Chambers HF. Community-associated MRSA--resistance and virulence converge. The New England journal of medicine. 2005 Apr 7:352(14):1485-7 [PubMed PMID: 15814886]
Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clinical microbiology reviews. 2015 Jul:28(3):603-61. doi: 10.1128/CMR.00134-14. Epub [PubMed PMID: 26016486]
DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infectious disease clinics of North America. 2009 Mar:23(1):17-34. doi: 10.1016/j.idc.2008.10.003. Epub [PubMed PMID: 19135914]
Foster TJ. Immune evasion by staphylococci. Nature reviews. Microbiology. 2005 Dec:3(12):948-58 [PubMed PMID: 16322743]
Salgado-Pabón W, Breshears L, Spaulding AR, Merriman JA, Stach CS, Horswill AR, Peterson ML, Schlievert PM. Superantigens are critical for Staphylococcus aureus Infective endocarditis, sepsis, and acute kidney injury. mBio. 2013 Aug 20:4(4):. doi: 10.1128/mBio.00494-13. Epub 2013 Aug 20 [PubMed PMID: 23963178]
Level 3 (low-level) evidenceMusser JM, Schlievert PM, Chow AW, Ewan P, Kreiswirth BN, Rosdahl VT, Naidu AS, Witte W, Selander RK. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1990 Jan:87(1):225-9 [PubMed PMID: 1967495]
Level 3 (low-level) evidenceLe KY, Otto M. Quorum-sensing regulation in staphylococci-an overview. Frontiers in microbiology. 2015:6():1174. doi: 10.3389/fmicb.2015.01174. Epub 2015 Oct 27 [PubMed PMID: 26579084]
Level 3 (low-level) evidence