Introduction
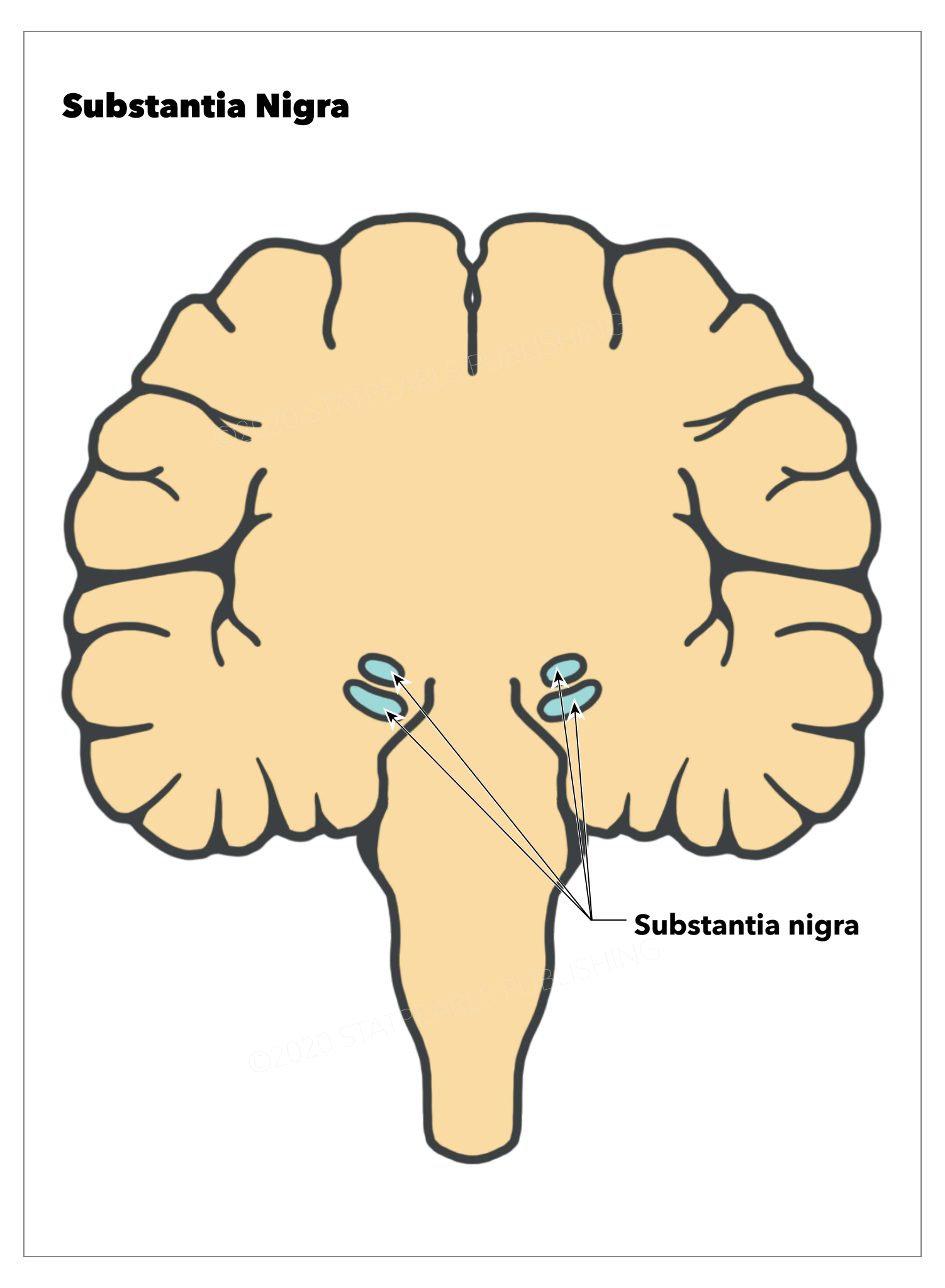
The substantia nigra (SN) is a midbrain dopaminergic nucleus, which has a critical role in modulating motor movement and reward functions as part of the basal ganglia circuitry (see Image. Substantia Nigra). Projections from the SN to the putamen, called the nigrostriatal pathway, are critically involved in the motor deficits observed in Parkinson disease.[1] These dopaminergic neural projections leave the SN via the medial forebrain bundle and forming synapses on multiple neuronal populations throughout the basal ganglia, but especially in the putamen. The basal ganglia are a grouping of interconnected subcortical nuclei that mitigate and control functions ranging from voluntary movement, cognitive planning, emotions and reward functions, and even cognition and learning. The substantia nigra is classically considered to be the primary input into the basal ganglia circuitry and a critical element to these functions. When these subcortical nuclei are damaged such as in stroke or during neurodegeneration, a multitude of neurological conditions can result, including Parkinson disease, Huntington disease, Tourette syndrome, schizophrenia, attention-deficit hyperactivity disorder, and obsessive-compulsive disorder.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Located within the midbrain posterior to the crus cerebri fibers of the cerebral peduncle, the substantia nigra can be functionally and morphologically divided into two regions, the pars compacta (SNpc) containing dopaminergic neurons and the pars reticulata (SNpr) with inhibitor gamma-aminobutyric acid-containing (or GABAergic) neurons (see . The SNpc is a densely packed nuclear region. In gross anatomical dissections, the SNpc appears dark in color because of the high neuromelanin content, which forms from the L-DOPA precursor in dopamine synthesis.[2] This characteristic is the source of the name of the region, which means "dark substance."
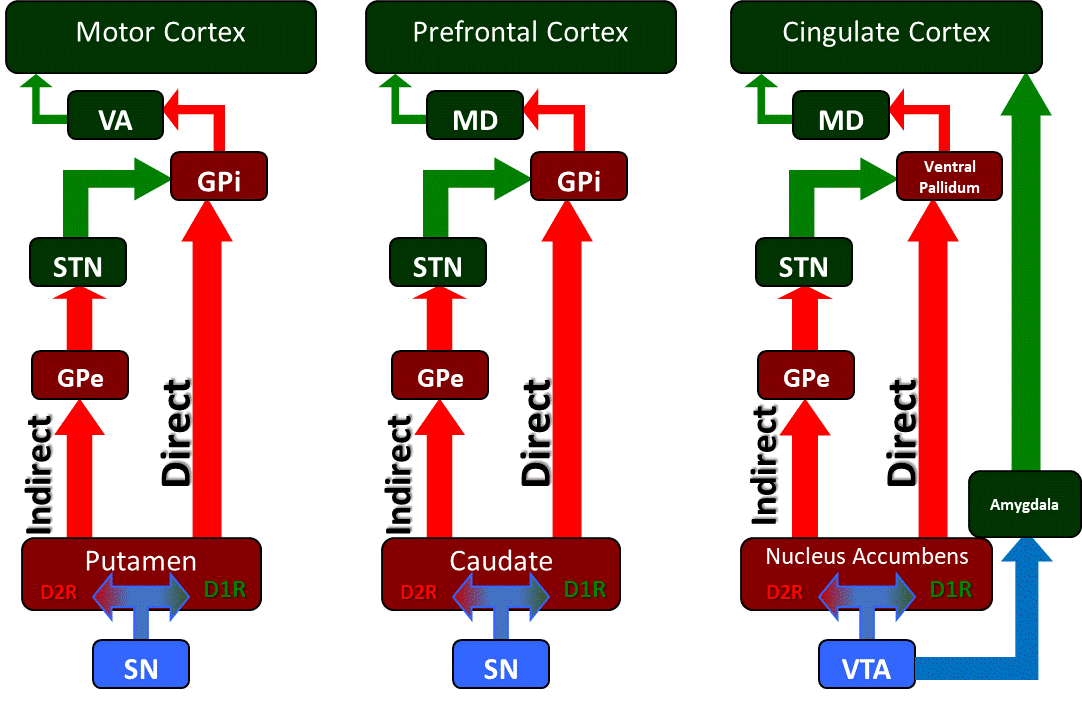
The SNpc has dopaminergic projections to the striatum, the putamen, and the caudate nuclei. Within the striatum, these dopaminergic projections synapse on two separate populations of dopamine-receptive neurons, D1-family receptor neurons, and D2-family receptor neurons (see Image. Basal Ganglia Circuitry and its Associated Motor, Cognitive, and Limbic Uutputs). The D1-family of neurons form inhibitory projections to the globus pallidus internus (GPi), which are called the "direct pathway" of the basal ganglia, while the D2-family of neurons form projections through the globus pallidus externus (GPe), which inhibit the stimulatory subthalamic nucleus (STN) that synapses on the GPi.
This second pathway is termed the "indirect pathway" because it indirectly stimulates the GPi, whereas the direct pathway directly inhibits the GPi. These pathways combined form a balance of activity on the GPI, which ultimately regulates the initiation of motor output. The GPi sends its inhibitory projections to the ventral anterior (VA) and ventral lateral (VL) nuclei of the thalamus and, depending upon the balance between the stimulatory indirect pathway and inhibitory direct pathway, will either inhibit unwanted motor output by inhibiting thalamic nuclei or will disinhibit motor output to be released by allowing the thalamus to stimulate the motor cortex. The SNpr is an inhibitory GABAergic nucleus that works in conjunction with the GPi as the final output of the basal ganglia's direct and indirect pathways. The SNpc receives input from the pedunculopontine nucleus, the primary reticular formation nucleus of the ascending reticular activating system.[3] The ascending reticular activating system is a deep neural system that receives collateral innervation from afferent sensory neurons of the body along a parallel tract which ultimately activates gain-setting nuclei (such as the SNpc) and limbic structures, the amygdala and the hypothalamus in order to prime the body's automatic functions outside of the conscious, cortical sensory pathways.
Embryology
Using the tyrosine hydroxylase (TH) enzyme, the rate-limiting enzyme in dopamine biosynthesis, as a marker for dopaminergic neurons, embryonic studies suggest that the dopaminergic substantia nigra neurons first form in the ventricular zone of the ventral mesencephalon during week 6.5 with migration occurring at week 6.[4] By week 8 the TH-positive neurons began to form neural projections of the nigrostriatal bundle. Other studies indicate that the SNpc and SNpr segregate at around week 19.[5] In rodent studies, these timelines seem to correlate with embryonic days 13 through 15 and are dependent upon the transcription factor Pitx3.[6][7][8] The PBX1 gene directly controls the Pitx3 gene expression as well as a gene involved in protection from oxidative stress, and PBX1 has been found to be less active in adult Parkinson disease patients.[9] In rodent studies, mutations in many of these genes are embryonically lethal; however, a number of genes have an association with juvenile or early-onset parkinsonism in humans, defined by the National Institute of Neurological Disorders and Stroke (NINDS) as onset before the age of 50. These include the PINK1 gene,[10] PARKIN,[11] SNCA,[12] and LRRK2.[13] Idiopathic parkinsonism typically develops late in life, with a mean age of onset of around 60 years, with approximately 1% of the population suffering from the condition (NINDS, 2016).
Blood Supply and Lymphatics
The substantia nigra has split irrigation from branches of the basilar and posterior cerebral arteries. The medial portion of the substantia nigra, along with the corticospinal tracts of the crus cerebri, receive supply from paramedian branches of the basilar artery. The oculomotor nucleus and accessory oculomotor nucleus also receive vascular supply via this branch. Short circumferential branches of the posterior choroidal arteries, a branch of the posterior cerebral arteries, supply the more lateral portions of the substantia nigra and crus cerebri, as well as the medial lemniscus, which are the crossed afferent fibers from the nucleus gracilis and fasciculus cuneatus. Parkinsonism due to stroke is a less common form of acute onset (developing over one year after a stroke) called vascular parkinsonism and, in one study, was diagnosed in 17% of stroke patients.[14]
Physiologic Variants
While most forms of Parkinson disease are due to the insidious degeneration of the substantia nigra over a lifetime or during old age, the cause of that degeneration is unknown. These forms of Parkinson disease are termed idiopathic due to their unknown cause. A great deal of research is being done to identify these causes, which can include peripheral inflammation,[15] metabolic insufficiency,[16] and various environmental toxins,[17][18][19] which have been shown to affect the substantia nigra. Several other neurological conditions exhibit parkinsonian-like signs and symptoms by affecting the dopaminergic neurons of the substantia nigra. These include drug-induced parkinsonism in which a patient is taking a pharmaceutical compound, such as antipsychotics and antihypertensive, that affects dopamine metabolism. Progressive supranuclear palsy produces balance, movement, and gait problems in addition to vertigo, slurred speech, vertical gaze paresis, and dementia and is much more rapid in its onset. Dementia with Lewy bodies is a form of parkinsonism that manifests as a progressive cognitive decline with fluctuations in alertness and attention. Multiple systems atrophy can manifest as Parkinson disease but may have degenerative elements that affect many other regions of the brain, which may include autonomic failure (Shy-Drager Syndrome), ataxia, and dementia (striatonigral degeneration) or incoordination and incontinence (olivopontocerebellar atrophy). Corticobasal degeneration also manifests as a form of parkinsonism with severe coordination impairment but may affect other systems as well. When Parkinson disease is the result of a stroke of the central nervous system, it is called vascular parkinsonism and usually develops within one year of a stroke.[14]
Surgical Considerations
In disorders that affect the substantia nigra, such as Parkinson disease, surgical intervention is an option for palliative treatment of the symptoms. Deep brain stimulation (DBS) involves the intracranial implantation of an electrode. In Parkinson disease, an electrode is placed in a target brain area of the basal ganglia, such as the subthalamic nucleus or the globus pallidus internus, to alter the activity between the direct and indirect basal ganglia pathways to disinhibit motor movement in the absence of dopamine release in the striatum. Implantation of a catheter directly into the striatum can target the putamen with a dopamine agonist such as apomorphine and reduce systemic side effects. Neurotrophic treatment of central brain nuclei such as the substantia nigra is also under investigation for their ability to halt neurodegeneration.[20]
Clinical Significance
The substantia nigra is a critical brain region for the production of dopamine, and this neurochemical affects many systems of the central nervous system ranging from movement control, cognitive executive functions, and emotional limbic activity. A loss of the dopaminergic neurons of the substantia nigra leads to Parkinson disease, and the symptoms of this disease can be treated using dopamine-supplementation approaches. One such treatment is the oral administration of L-DOPA, the precursor in the biological synthesis of dopamine which has the ability to cross the blood-brain barrier and be used to synthesize dopamine within the central nervous system. The inhibition of the metabolic breakdown of dopamine is also pharmaceutically possible, called monoamine oxidase inhibitors. Dopamine can be replaceable with dopamine receptor agonists such as apomorphine, pramipexole, and ropinirole, but the effects are short-lived, and systemic side effects such as emesis and increased sympathetic tone are common.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Hodge GK, Butcher LL. Pars compacta of the substantia nigra modulates motor activity but is not involved importantly in regulating food and water intake. Naunyn-Schmiedeberg's archives of pharmacology. 1980 Aug:313(1):51-67 [PubMed PMID: 7207636]
Level 3 (low-level) evidenceFabbri M, Reimão S, Carvalho M, Nunes RG, Abreu D, Guedes LC, Bouça R, Lobo PP, Godinho C, Coelho M, Gonçalves NC, Rosa MM, Antonini A, Ferreira JJ. Substantia Nigra Neuromelanin as an Imaging Biomarker of Disease Progression in Parkinson's Disease. Journal of Parkinson's disease. 2017:7(3):491-501. doi: 10.3233/JPD-171135. Epub [PubMed PMID: 28671143]
Galtieri DJ, Estep CM, Wokosin DL, Traynelis S, Surmeier DJ. Pedunculopontine glutamatergic neurons control spike patterning in substantia nigra dopaminergic neurons. eLife. 2017 Oct 5:6():. doi: 10.7554/eLife.30352. Epub 2017 Oct 5 [PubMed PMID: 28980939]
Freeman TB,Spence MS,Boss BD,Spector DH,Strecker RE,Olanow CW,Kordower JH, Development of dopaminergic neurons in the human substantia nigra. Experimental neurology. 1991 Sep [PubMed PMID: 1680741]
Aubert I, Brana C, Pellevoisin C, Giros B, Caille I, Carles D, Vital C, Bloch B. Molecular anatomy of the development of the human substantia nigra. The Journal of comparative neurology. 1997 Mar 3:379(1):72-87 [PubMed PMID: 9057113]
Level 2 (mid-level) evidenceAltman J, Bayer SA. Development of the brain stem in the rat. V. Thymidine-radiographic study of the time of origin of neurons in the midbrain tegmentum. The Journal of comparative neurology. 1981 Jun 1:198(4):677-716 [PubMed PMID: 7251936]
Level 3 (low-level) evidenceNunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2003 Apr 1:100(7):4245-50 [PubMed PMID: 12655058]
Level 3 (low-level) evidenceLe W, Zhang L, Xie W, Li S, Dani JA. Pitx3 deficiency produces decreased dopamine signaling and induces motor deficits in Pitx3(-/-) mice. Neurobiology of aging. 2015 Dec:36(12):3314-3320. doi: 10.1016/j.neurobiolaging.2015.08.012. Epub 2015 Aug 20 [PubMed PMID: 26363812]
Villaescusa JC, Li B, Toledo EM, Rivetti di Val Cervo P, Yang S, Stott SR, Kaiser K, Islam S, Gyllborg D, Laguna-Goya R, Landreh M, Lönnerberg P, Falk A, Bergman T, Barker RA, Linnarsson S, Selleri L, Arenas E. A PBX1 transcriptional network controls dopaminergic neuron development and is impaired in Parkinson's disease. The EMBO journal. 2016 Sep 15:35(18):1963-78. doi: 10.15252/embj.201593725. Epub 2016 Jun 28 [PubMed PMID: 27354364]
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science (New York, N.Y.). 2004 May 21:304(5674):1158-60 [PubMed PMID: 15087508]
Level 3 (low-level) evidenceKitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998 Apr 9:392(6676):605-8 [PubMed PMID: 9560156]
Level 3 (low-level) evidenceAbou-Sleiman PM, Hanna MG, Wood NW. Genetic association studies of complex neurological diseases. Journal of neurology, neurosurgery, and psychiatry. 2006 Dec:77(12):1302-4 [PubMed PMID: 17110744]
Level 2 (mid-level) evidenceMata IF, Wedemeyer WJ, Farrer MJ, Taylor JP, Gallo KA. LRRK2 in Parkinson's disease: protein domains and functional insights. Trends in neurosciences. 2006 May:29(5):286-93 [PubMed PMID: 16616379]
Manosalva HA, Pio F, Jeerakathil T, Saqqur M, Camicioli R, Suchowersky O. Vascular Parkinsonism in a Tertiary Care Stroke Prevention Clinic and the Development of a New Screening Strategy. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2018 Jan:27(1):153-161. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.020. Epub 2017 Oct 3 [PubMed PMID: 28986199]
Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological psychiatry. 2008 Jun 1:63(11):1022-9. doi: 10.1016/j.biopsych.2007.12.007. Epub 2008 Feb 1 [PubMed PMID: 18242584]
Level 1 (high-level) evidenceYang L, Wang H, Liu L, Xie A. The Role of Insulin/IGF-1/PI3K/Akt/GSK3β Signaling in Parkinson's Disease Dementia. Frontiers in neuroscience. 2018:12():73. doi: 10.3389/fnins.2018.00073. Epub 2018 Feb 20 [PubMed PMID: 29515352]
Langston JW, Irwin I, Langston EB, Forno LS. 1-Methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra. Neuroscience letters. 1984 Jul 13:48(1):87-92 [PubMed PMID: 6332288]
Level 3 (low-level) evidenceGash DM, Rutland K, Hudson NL, Sullivan PG, Bing G, Cass WA, Pandya JD, Liu M, Choi DY, Hunter RL, Gerhardt GA, Smith CD, Slevin JT, Prince TS. Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Annals of neurology. 2008 Feb:63(2):184-92 [PubMed PMID: 18157908]
Level 3 (low-level) evidenceTanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. Rotenone, paraquat, and Parkinson's disease. Environmental health perspectives. 2011 Jun:119(6):866-72. doi: 10.1289/ehp.1002839. Epub 2011 Jan 26 [PubMed PMID: 21269927]
Level 2 (mid-level) evidenceStenslik MJ, Potts LF, Sonne JW, Cass WA, Turchan-Cholewo J, Pomerleau F, Huettl P, Ai Y, Gash DM, Gerhardt GA, Bradley LH. Methodology and effects of repeated intranasal delivery of DNSP-11 in a rat model of Parkinson's disease. Journal of neuroscience methods. 2015 Aug 15:251():120-9. doi: 10.1016/j.jneumeth.2015.05.006. Epub 2015 May 18 [PubMed PMID: 25999268]