Introduction
Hyperthyroidism is a common thyroid disorder. "Hyperthyroidism" defines a syndrome associated with excess thyroid hormone production.[1] It is a common misconception that the terms thyrotoxicosis and hyperthyroidism are synonyms. The term "thyrotoxicosis" refers to a state of excess thyroid hormone exposure to tissues.[1] Although hyperthyroidism can lead to thyrotoxicosis and can be used interchangeably, it is essential to note their differences. For the sake of simplicity, this review will cover a discussion of hyperthyroidism and thyrotoxicosis. Hyperthyroidism has multiple etiologies, clinical manifestations, and treatment modalities.
Hyperthyroidism can be overt or subclinical. Overt hyperthyroidism is defined as low or suppressed thyroid stimulating hormone (TSH) levels with elevated triiodothyronine (T3) levels and/or elevated thyroxine (T4) levels.[1] When T3 levels are elevated with low/suppressed TSH and normal T4 levels, this is called 'T3 toxicosis'.[2] Subclinical hyperthyroidism is low or suppressed TSH with normal T3 and T4 levels.[2] Both overt and subclinical hyperthyroidism are associated with significant long-term complications.[3][4][5][6][7]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The three most common etiologies of hyperthyroidism include:
- Graves disease (GD)
- Toxic multinodular goiter (TMNG)
- Toxic adenoma (TA)[1]
Other less common etiologies of hyperthyroidism:
- Iodine-induced hyperthyroidism[8]
- TSH (thyroid stimulating hormone)-secreting pituitary adenomas[9]
- Conditions associated with high human chorionic gonadotrophin levels: choriocarcinomas and hydatiform moles in females and germ cell tumors in males[10]
- Ectopic thyroid in struma ovarii (excess thyroid hormone production from ovarian teratomas)[11]
- Extensive metastasis from functionally differentiated thyroid carcinoma (follicular or papillary)[12]
- Drug-induced thyroiditis: amiodarone, lithium, tyrosine kinase inhibitors, interferon-alpha, immune checkpoint inhibitor therapy[13][14][15][16][17]
- Other thyroiditis: Hashitoxicosis, painless thyroiditis, painful subacute thyroiditis, suppurative thyroiditis, and Riedel thyroiditis[18][19]
- Factitious thyroiditis (due to excess exogenous thyroid hormone: intentional or unintentional use)[10]
Graves disease is the most common cause of hyperthyroidism in the United States and most Western countries.[20] As Graves disease is autoimmune in etiology, this form of hyperthyroidism tends to manifest itself in younger populations. In older adults and people living in regions of iodine deficiency, toxic multinodular goiter is the most common cause of hyperthyroidism.[21][22][23]
Factitious thyroiditis is thyrotoxicosis associated with inappropriate or excessive use of pharmaceutical thyroid hormone.[10] Due to a well-received side effect of weight loss, thyroxine has the potential for abuse. Any history of a hyperthyroid patient should include a medication list and an assessment of possible misuse (whether intentional or unintentional).
Epidemiology
The prevalence of hyperthyroidism varies worldwide, based on dietary iodine content.[12] Hyperthyroidism is more common in women compared to men.[24] Other risk factors associated with the development of hyperthyroidism include smoking, iodine deficiency, iodine excess, selenium deficiency, genetic factors, and the use of certain drugs.[12] Graves disease is typically seen in younger patients and is the most common cause of hyperthyroidism in this demographic. The incidence of GD is highest between the age group of 30 to 50 years.[25] Toxic multifocal goiter is typically seen in older individuals and is the most common cause of hyperthyroidism in this demographic.[22] Graves disease and toxic multifocal goiter have a female predilection and are typically seen in patients with pertinent family and personal medical histories. Thyroid nodular disease is also more common in women than men by 5- to 15-fold.[26] Autoimmune thyroid disorders like Graves disease are more common in iodine-replete areas, and nodular thyroid diseases are more common in iodine-deficient areas.[12]
The 1977 Whickham Survey evaluated the spectrum of thyroid disorders in County Durham in northeastern England. The Whickham Survey demonstrated a prevalence of hyperthyroidism in women, approximately ten times more than that of men (2.7% versus 0.23%).[27] An incidence of 80 cases per 100,000 women was seen at the 20-year follow-up of the Whickham cohort.[28] The prevalence of hyperthyroidism in the United States was 1.3% in the general population, with 0.5% cases of overt hyperthyroidism and 0.7% cases of subclinical hyperthyroidism.[29] A meta-analysis found the prevalence of hyperthyroidism in Europe to be 0.75%.[24] The prevalence of overt hyperthyroidism is similar in China at 0.78%.[30]
Amiodarone-induced thyrotoxicosis (AIT) is seen in about 6% of the individuals taking the medication in iodine-sufficient areas and about 10% in individuals taking the medication from iodine-deficient areas.[31][32]
Pathophysiology
The pathophysiology of hyperthyroidism depends on the particular variant of hyperthyroidism.
Graves Disease
This is an autoimmune process with antibodies against the TSH receptor. An interplay between genetic and environmental factors influences this autoimmune process. The antibodies stimulate the TSH receptor (TSHR), leading to increased production and release of thyroid hormones. The trophic effects on the thyroid also lead to the growth of the thyroid gland.[20]
Toxic Multinodular Goiter
Pathogenesis of TMNG includes the initial phase of development of the nodular disease. This phase is prolonged and present for years before the nodules develop autonomy for thyroid hormone production. The somatic mutations involving the TSHR lead to constitutive activation of the cAMP signaling pathway, resulting the thyroid autonomy.[33] There is a correlation between the size of the nodules and the development of hyperthyroidism. In a previous study, about 93.7% of the patients who developed overt hyperthyroidism had a nodule size greater than 3 cm.[34]
Toxic Adenoma
These are solitary nodules with autonomous thyroid hormone production due to somatic mutations in the TSHR
Iodine-Induced Hyperthyroidism (Jod-Basedow Phenomenon)
This is typically iatrogenic, resulting from excessive iodine intake through diet or administration of iodine-containing medications such as contrast media or amiodarone.[35][36] Individuals susceptible to this phenomenon include the ones residing in iodine-deficient regions, individuals with underlying thyroid nodular disease, or underlying occult GD or previously treated GD.[8] Hyperthyroidism develops about 2-12 weeks after exposure to excessive iodine.[37] As mentioned previously, the organification of iodide residues into precursor thyroid hormone molecules is relatively self-regulating. Excessive circulating iodide inhibits organification, a process known as the Wolff-Chaikoff effect. This autoregulation is escaped in the Jod-Basedow phenomenon leading to excess thyroid hormone in the presence of excess iodine/iodide.
Amiodarone-Induced Thyrotoxicosis
There are two subtypes of amiodarone-induced thyrotoxicosis (AIT): type 1 and type 2. Type 1 AIT leads to increased thyroid hormone production secondary to excess iodine exposure from amiodarone in the setting of pre-existing thyroid disease (as seen in the Jod-Basedow phenomenon).[38] The pre-existing thyroid disease is usually multinodular goiter or latent Graves disease. Type 2 AIT is destructive thyroiditis due to the direct toxic effects of amiodarone on the thyroid follicular cells.[39]
Thyroiditis results in the transient increase in circulating thyroid hormone resulting from inflammation or destruction of the thyroid follicular cells. Various etiologies of thyroiditis have this common pathophysiology but vary in their clinical presentations. The inflammation or destruction of the thyroid follicular cells can result from autoimmunity (Hashimoto's thyroiditis, painless sporadic thyroiditis, and painless postpartum thyroiditis) or the result of external factors (infections in painful subacute thyroiditis, suppurative thyroiditis, drug-induced thyroiditis).[19]
History and Physical
Thyroid hormone has physiological effects on multiple organ systems. As a result, the symptoms and signs of hyperthyroidism involve manifestations from multiple organ systems. Clinical manifestations are associated with a hyperadrenergic and hypermetabolic state. Common manifestations include unintentional weight loss (about 10% of patients can gain weight due to increased appetite), palpitations, tremors, heat intolerance, dyspnea on exertion, increased anxiety, irritability, fatigue, muscle weakness, increased frequency of bowel movements (some patients can have significant diarrhea), hair loss, loss of libido, and oligomenorrhea or amenorrhea in women.[20][40]
Patients with subacute thyroiditis can present with significant anterior neck pain and fever. On physical examination, patients have tachycardia (some can present with atrial fibrillation), hypertension, tremors, warm and moist skin, hyperreflexia, and an anxious appearance. Some patients might have signs of heart failure.
Eye signs of lid lad or lid retraction can be seen in all causes of hyperthyroidism due to a hyperadrenergic state.[41] Eye symptoms and signs of "true orbitopathy' are only seen in patients with Graves disease. These include diplopia, excessive tearing, conjunctival injection, and orbital or retro-orbital pressure proptosis.[42][43] Other specific physical findings associated with Graves disease are pretibial myxedema (plaques of thick, scaly skin and swelling involving the anterior aspect of lower legs) and acropachy (soft-tissue swelling of the hands and clubbing of the fingers).[44][45][46]
Examining the thyroid will reveal a diffuse non-nodular enlargement of the thyroid in Graves disease; a diffuse non-symmetric nodular enlargement can be seen in toxic multinodular goiter, and a single large nodule can be palpated in cases of a toxic adenoma. An exquisitely tender thyroid can be noted in subacute thyroiditis.[47]
Evaluation
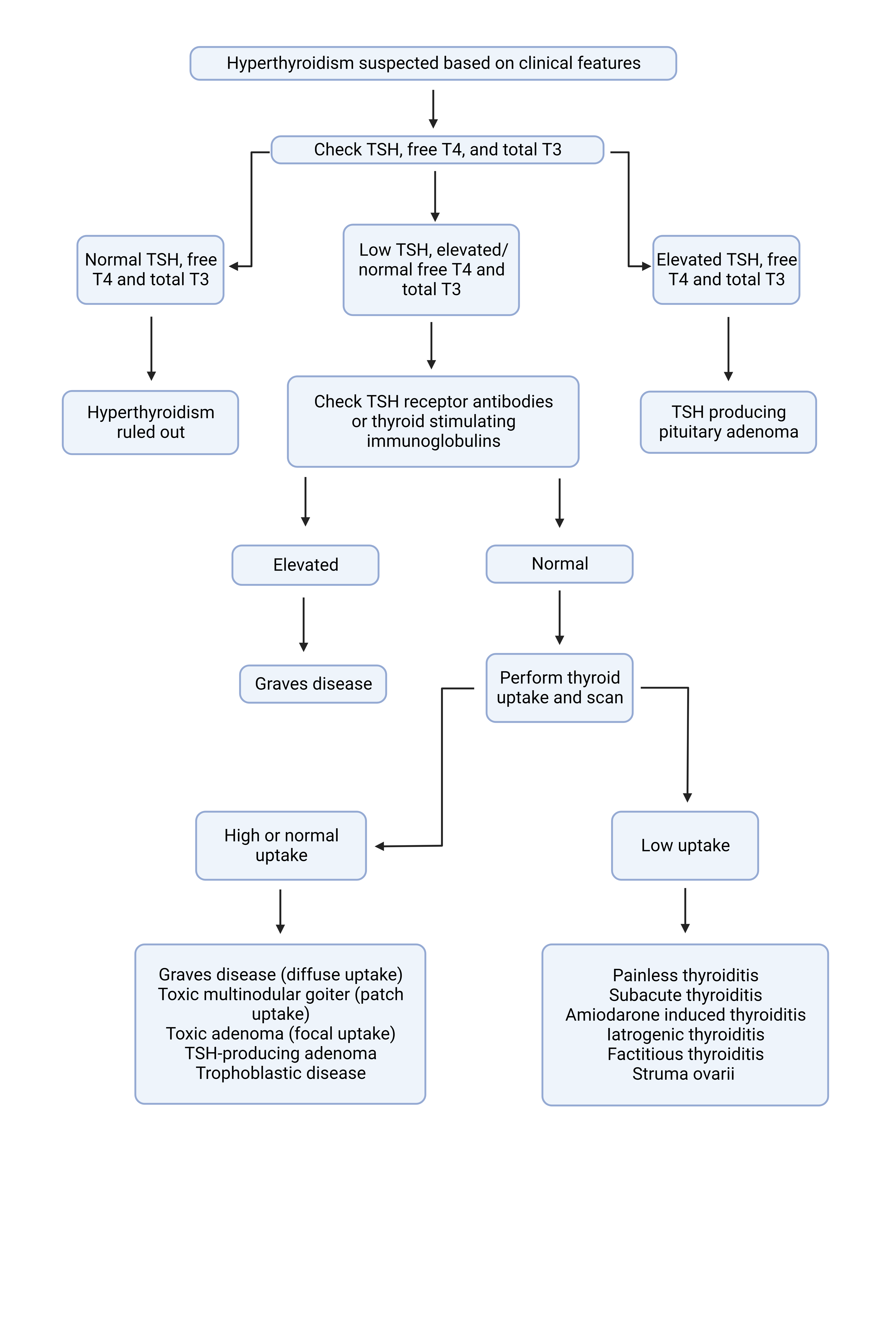
When hyperthyroidism is suspected based on clinical features, the patient should undergo an initial evaluation with measurement of TSH, free T4, and total T3 to confirm the diagnosis. Figure 1 illustrates the diagnostic algorithm for hyperthyroidism. Patients with overt hyperthyroidism will have low/suppressed TSH levels with elevated free T4 and total T3 levels. Patients with mild/subclinical hyperthyroidism will have low/suppressed TSH with normal free T4 and total T3 levels. 'T3 toxicosis' is defined as low/suppressed TSH with normal T4 and elevated T3 levels.
Conditions that can interfere with the assessment of TSH include the presence of heterophile antibodies and high biotin intake due to interference with the assays.[48] Heterophile antibodies can lead to a false elevation in TSH levels. High-dose biotin supplementation (5 to 30 mg) can result in falsely low TSH with elevated free T4 levels in vitro.[49]
After the diagnosis of hyperthyroidism has been confirmed, measurement of thyrotropin receptor antibody (TRAb) levels as an initial test for determining the etiology of hyperthyroidism has been shown to reduce the time to diagnosis and is more cost-effective.[50]. Elevated TRAb levels confirm the diagnosis of Graves disease. TRAb levels are measured using TBI or TBII (thyrotropin-binding inhibiting or thyrotropin-binding inhibitory immunoglobulin) assays and TSI (thyroid stimulating immunoglobulin) bioassays. The newer bioassay using the Immulite method for TSI measurement has a high sensitivity and specificity of 98% and 99.9%, respectively, for diagnosing GD.[51] TBII assays used for measuring TRAb levels also have a high sensitivity of 96-97% and specificity of 99% of the diagnosis of GD.[52]
If TRAb levels are normal, the patient should undergo a radioiodine thyroid uptake and scan using an I-123 isotope (enters the thyroid gland through the Na/I symporter). This test is contraindicated in pregnant and lactating women. A capsule containing an I-123 isotope is given a day before the scan is performed. The pattern of uptake of I-123 by the thyroid gland seen on the scan can help determine the diagnosis (see Figure 1). However, this test does not help differentiate between type 1 and type 2 amiodarone-induced thyrotoxicosis, as the uptake will be low in chronic amiodarone use.
- High Uptake/Normal
- Graves disease will have high or normal uptake in a diffuse pattern
- TMNG will have a high or normal uptake in a patch pattern
- TA will have a high or normal uptake with a solitary area of high uptake (corresponding to the known nodule) with low uptake in the remainder of the gland
- Low or Absent Uptake
- Any etiology of thyroiditis is associated with low or absent uptake (Na/I symporters are not functional in inflamed or destroyed thyroid follicular cells)
- Iatrogenic and factitious thyrotoxicosis
Thyroid ultrasound using the color Doppler is another important test that can help determine the underlying etiology. Intrathyroidal arterial flow velocities are measured.[53] Increased (thyroid inferno) and normal flow are seen in Graves disease. Low flow is seen in thyroiditis.[53] This test can help differentiate between type 1 and type 2 amiodarone-induced thyrotoxicosis (AIT). The flow will be high or normal in type 1 AIT (hyperthyroidism due to underlying nodular thyroid disease or occult GD) and low in type 2 AIT (destructive thyroiditis).[54]
Treatment / Management
Treatment of hyperthyroidism depends on the underlying etiology and can be divided into symptomatic and definitive therapy. The symptoms of hyperthyroidism, such as palpitations, anxiety, and tremor, can be controlled with a beta-adrenergic antagonist such as atenolol. Calcium channel blockers, such as verapamil, can be used as second-line therapy for patients who are beta-blocker intolerant or have contraindications to beta-blocker treatment.[1]
This review will only discuss the treatment for the most common causes of hyperthyroidism: Graves disease, toxic multinodular goiter, and toxic adenoma in non-pregnant patients.
Indications for treatment:
- Overt hyperthyroidism
- Subclinical hyperthyroidism with TSH <0.1 and age >65 years
- Subclinical hyperthyroidism with TSH <0.1 and age <65 years with comorbidities (cardiovascular disease, osteoporosis, or symptomatic)
- Subclinical hyperthyroidism with TSH <0.1 and age <65 years, if TSH still elevated after 3 to 6 months
- Subclinical hyperthyroidism with TSH between 0.1-0.4 and age >65 years, if TSH still elevated after 3 to 6 months
- Subclinical hyperthyroidism with TSH between 0.1-0.4 and age <65 years with comorbidities (cardiovascular disease, osteoporosis, or symptomatic), if TSH still elevated after 3-6 months
There are three definitive treatments for hyperthyroidism: radioactive iodine therapy (RAI), thionamide therapy, and subtotal thyroidectomy. The choice of which definitive treatment modality depends on the etiology, comorbidities, and patient preferences. Historically, radioactive iodine (RAI) has been the preferred treatment for managing Graves disease in the United States. Still, the trend is changing towards increased use of anti-thyroidal drugs (ATD).[55] ATDs have been the preferred treatment for Graves disease in most other countries.[1] (B3)
Antithyroid Drugs (ATDs)
Thionamide drugs include methimazole, carbimazole (precursor of methimazole), and propylthiouracil. These drugs are competitive inhibitors of the thyroid peroxidase (TPO) enzyme, resulting in their ability to block thyroid hormone synthesis. Additionally, these drugs may have additional immunosuppressive effects, as shown by their ability to induce remission in patients with Graves disease.[56][57] Methimazole and propylthiouracil both inhibit thyroid hormone synthesis by thyroid peroxidase. Thyroid peroxidase is the enzyme responsible for converting dietary iodine into iodide. Propylthiouracil (PTU) also lowers peripheral tissue exposure to active thyroid hormone by blocking the extrathyroidal conversion of T4 to T3. Thionamide therapy has no permanent effect on thyroid function, and recurrence of hyperthyroidism is common in patients who discontinue thionamide therapy.(A1)
Attaining a euthyroid status typically requires several months after initiation of thionamide therapy. Although methimazole and PTU are equally effective, methimazole is preferred due to once-daily dosing and a relatively better safety profile. An exception to this recommendation is in pregnant patients, in which PTU is preferred. Methimazole is associated with an increased risk of congenital defects, and thus PTU is preferred in managing hyperthyroidism during pregnancy.
- Doses:
- ATA (Americal Thyroid Association) guidelines provide a rough guide for the initial dose of methimazole based on free T4 levels [1]
- Free T4 1-1.5 times upper limit of normal: Start methimazole 5-10 mg daily
- Free T4 1.5-2.0 times the upper limit of normal: Start methimazole 10-20 mg daily
- Free T4 2.0-3.0 times the upper limit of normal: Start methimazole 30-40 mg daily
- PTU is administered in 2-3 doses per day due to its shorter duration of action. The initial dose of 5-150 three times daily is chosen based on the severity of hyperthyroidism. Once the disease is controlled, the dose can be decreased to a maintenance dose of 50 mg 2 to 3 times daily.
- Monitoring: TSH levels remain suppressed for almost six months in patients with Graves disease, so evaluation of free T4 and/or total T3 levels should be done every 4 to 6 weeks.
- Pregnancy: Propylthiouracil is the preferred drug in the first trimester, associated with lower incidence and severity of embryopathy than methimazole.[58][59] The treatment can be switched to methimazole after 16 weeks of gestation.
- Drug conversions:
- 10 mg of carbimazole is converted to approximately 6 mg of methimazole [1]
- An equivalent dosage ratio of propylthiouracil to methimazole is 20:1. This ratio is recommended for dose conversions when switching between these agents.[60]
These drugs should be continued for at least 12-18 months. TRAb should be assessed at that time to evaluate for remission. If TRAb levels are normal, then thionamide therapy can be discontinued. If TRAb levels are still elevated, the patient remains at high risk for relapse if medication is stopped. Other factors associated with lower remission rates: male gender, smoking, large goiters, higher TRAb titers at the time of diagnosis, presence of orbitopathy, and the need for a high dose of thionamides to maintain euthyroidism.[20]
An older study from the United States showed a 20-30% remission rate for Graves disease using thionamides.[61] European and Japanese populations noted higher remission rates of 50-60%.[62][63][64](B2)
Radioactive Iodine (RAI)
RAI (using I-131 isotope) can be the preferred therapy in most patients, especially the ones with high-risk comorbidities who are at high risk for surgery and need definitive management. Patients who have contraindications for the use of thionamides should also undergo RAI. This procedure should be avoided in patients planning a pregnancy in the six months due to the risk of inducing hypothyroidism in the fetus. RAI is also contraindicated in lactating women. Patients will a history of moderate to severe Graves orbitopathy should not undergo treatment with RAI due to the risk of worsening eye disease. Patients with underlying thyroid malignancies should not undergo RAI.
Radioactive iodine-131 leads to the destruction of thyroid follicular cells. In a female patient of reproductive age, it is highly recommended to obtain a beta-hCG to rule out pregnancy before initiation of RAI therapy. Patients on a thionamide (methimazole or propylthiouracil) should be instructed to discontinue this therapy approximately one week before RAI therapy since thionamide administration can interfere with the therapeutic benefit of RAI therapy. Several months are typically needed status post-RAI therapy to achieve euthyroid status.
- Graves disease
- A single fixed dose of 10-15 mCi (370-555 MBq) is sufficient to render a patient with GD hypothyroid. Doses of RAI can be calculated using the size of the thyroid gland and the uptake of RAI. Cure rates are higher with higher doses, up to 85%.[65][66]
- Toxic multinodular goiter
- A single dose of 15 mCi is usually sufficient.[67] A calculated dose of 150-200 microCi (5.5-7.4 MBq) per gram of thyroid tissue can be used, corrected for 24-hour radioactive iodine uptake. Cure rates are 55% at three months and 80% at six months.[68] Long-term studies have shown that the risk of hypothyroidism after RAI for TMNG is 3-5% by one year, 16% by five years, and 64% by 24 years.[69][70][71]
- Toxic adenoma
- A single fixed of 10-20 mCi (370-740 MBq) is usually sufficient. The dose can also be calculated based on nodule size: 150-200 microCi (5.5-7.5 MBq).[72] Long-term studies have shown that the risk of hypothyroidism after RAI for TA is 8% in 1 year and 60% in 20 years.[73]
Typically, patients with GD are evaluated in 4 to 6-week intervals with an assessment of TSH, free T4, and total T3 levels. The monitoring should continue for another six months or till the patient becomes hypothyroid and is on a stable dose of levothyroxine. Failure to achieve euthyroidism after RAI therapy may indicate the need for either repeat RAI therapy (for symptomatic hyperthyroidism) or the initiation of thyroxine therapy (for hypothyroidism).
RAI therapy involves the release of stored thyroid hormone due to the destruction of thyroid follicular cells, leading to transient hyperthyroidism. This is generally well tolerated, although this transient hyperthyroidism is of concern in patients with significant cardiac disease. For patients with cardiac disease, pretreatment with a thionamide to deplete the stored hormone is recommended to avoid the potential exacerbation of the cardiac disease. In addition, the use of beta-blocker therapy is also essential in these patients to minimize beta-adrenergic symptoms.
Surgery
Preferred in women planning a pregnancy in less than six months, presence of active Graves orbitopathy, patients who experience significant adverse effects with the use of thionamides, when thyroid malignancy is suspected, presence of large compressive goiters, and the presence of co-existing hyperparathyroidism needing surgery. The surgical option should be avoided in patients with significant comorbidities deemed high-risk for undergoing surgery.
Euthyroidism should be achieved before surgery with the use of thionamides. Preoperative SSKI (saturated solutions of potassium iodide), KI (potassium iodide), or Lugol's iodine should be used in patients with Graves disease and TMNG to decrease gland vascularity and decrease intraoperative blood loss.[74][75] (A1)
- Graves disease: Near-total or total thyroidectomy is the surgical procedure of choice in patients with Graves disease, with excellent cure rates. The risk of recurrence or disease persistence with total thyroidectomy is almost 0% versus 8% with sub-total thyroidectomy after five years.[76][77][78]
- Toxic multinodular goiter: Surgical option of choice is near-total or total thyroidectomy to avoid recurrences.[79][80]
- Toxic adenoma: Preferred surgical option is ipsilateral thyroid lobectomy or isthmusectomy, with excellent cure rates and a risk of the treatment failure rate of less than 1%.[81] (A1)
After patients undergo near-total or total thyroidectomy, they should be started on weight-based levothyroxine replacement therapy (0.8 mcg/lb or 1.6 mcg/kg). Lower doses should be used in the elderly, especially in patients with a history of cardiovascular disease or arrhythmia.
Differential Diagnosis
Hyperthyroidism presents with relatively nonspecific signs and symptoms such as palpitations, increased frequency of bowel movements, and weight loss, among others. Therefore, other pathologies should be ruled out as possible explanations for the patient’s symptomatology.
For etiologies of hyperthyroidism, differential diagnoses can be made based on the physical findings of the thyroid gland. Palpation of a normal thyroid gland in the context of hyperthyroidism can be due to Graves disease, painless thyroiditis, or factitious hyperthyroidism (thyrotoxicosis factitia). Graves disease can also present as a non-tender, enlarged thyroid.
Palpation of a tender enlarged thyroid may indicate De Quervain thyroiditis (subacute thyroiditis). Palpation of a single thyroid nodule is likely indicative of thyroid adenoma, and palpation of multiple thyroid nodules strongly indicates toxic multinodular goiter.
Other differential diagnoses include euthyroid hyperthyroxinemia (in which serum total T4 and T3 are elevated, but the TSH level is within normal limits) and struma ovarii.
Toxicity and Adverse Effect Management
Antithyroid drugs or thionamides are associated with rare but serious adverse effects of agranulocytosis, hepatotoxicity, and vasculitis. Hepatotoxicity is more common with the use of propylthiouracil (2.7%) than methimazole (0.4%).[82] Hepatotoxicity due to methimazole is more likely to be cholestatic, while hepatotoxicity due to PTU is more likely to be hepatocellular.[83] Hematological complications have an incidence of 0.1-0.15% with the use of PTU or methimazole. Of these patients, 89% had agranulocytosis, and 11% had pancytopenia or aplastic anemia.[84] Patients taking PTU and rarely methimazole can develop p-ANCA (anti-neutrophil cytoplasmic antibody) positive small vessel vasculitis.[85] Up to 40% of those taking PTU can develop c-ANCA positivity, but very few develop vasculitis.[86][87] These medications are also associated with the development of drug-induced lupus.[88][89] Few cases of hypoglycemia secondary to autoimmune insulin syndrome have been reported using methimazole.[90][91]
If patients develop an acute febrile illness with symptoms of pharyngitis, they should get blood work done to check complete blood cell counts along with differentials to rule out the development of agranulocytosis. Liver function tests should be assessed in patients who develop a pruritic rash, abdominal pain or bloating, anorexia, nausea, vomiting, fatigue, jaundice, light-colored stool, or dark urine.
The most common complications following total or near-total thyroidectomy include hypocalcemia due to hypoparathyroidism in less than 2% of cases (can be transient or permanent), recurrent or superior laryngeal nerve paralysis in less than 2% of cases (can be temporary or permanent), hemorrhage, and complications related to anesthesia.[92][93][94]
Prognosis
Hyperthyroidism secondary to Graves disease or toxic multinodular goiter has overall good outcomes due to high success rates of definitive treatment and efficacy of symptom management. However, as with any disease, the prognosis of particular disease pathology is patient-oriented and reflects management, response to therapy, and compliance with prescribed treatments.
Complications
Untreated or unmanaged hyperthyroidism can lead to an extreme case of hyperthyroidism, referred to as a thyroid storm. Reflecting the hypermetabolic state of hyperthyroidism, the patient experiencing thyroid storm will present with tachycardia, increased GI motility, diaphoresis, anxiety, fever, and manifestations of multiple organ dysfunction. Thyroid storm is a potentially life-threatening complication of hyperthyroidism, thus requiring immediate attention. The mortality rate is high in individuals more than 60 years of age, of about 16%.[95]
Prolonged untreated or undertreated hyperthyroidism is associated with an increased risk of acute cardiovascular events, atrial fibrillation, ischemic stroke, osteoporosis, infertility, abnormalities of menstrual cycles, and mortality.[96][97][98][99] Subclinical hyperthyroidism has been associated with an increased risk of arrhythmias such as atrial fibrillation, osteoporosis, hip fractures, and mortality.[100][2][101]
Deterrence and Patient Education
Patient education regarding hyperthyroidism is similar to other diseases. Patients should be educated on the importance of compliance with therapy and on the signs and symptoms of extreme hyperthyroidism (thyroid storm).
Pearls and Other Issues
Acute coronary syndrome (ACS) may be complicated by thyroid dysfunction. A recent study has shown that thyroid dysfunction is seen in up to 23.3% of patients with coronary artery disease and both overt and subclinical hyperthyroidism in 2.5%.[102]
Pregnancy and concurrent thyroid pathology can pose medical management challenges. PTU is recommended in pregnant women presenting with hyperthyroidism due to methimazole’s association with congenital defects. Close monitoring is recommended with PTU administration, as overcorrection can potentially cause fetal hypothyroidism. The thyroid hormone is particularly important due to its role in fetal neurodevelopment. Recent literature indicates that previously recommended TSH cutoffs in pregnant women lead to overcorrection of thyroid disease in pregnant patients.[103] As fetal exposure to thyroid hormone plays a significant role, careful monitoring and close supervision are warranted.
Neonatal thyrotoxicosis results from fetal tissue exposure to excessive thyroid hormone. There are typically two variants of neonatal thyrotoxicosis: autoimmune-mediated and non-autoimmune-mediated. Autoimmune fetal hyperthyroidism involves the transplacental passage of TSH receptor-stimulating antibodies. Hyperthyroidism is usually transient as symptoms cease 5 to 6 months after birth following clearance of maternal antibodies. Non-autoimmune fetal hyperthyroidism is associated with an activating mutation of either the TSH receptor or the GNAS gene (leading to McCune-Albright syndrome). Unlike the autoimmune etiology, the non-autoimmune variant is permanent, long persisting after birth.[104]
Enhancing Healthcare Team Outcomes
Except for thyroid storm, hyperthyroidism in itself is rarely life-threatening but can pose a significant burden on a patient’s day-to-day routine. Hyperthyroidism can present with many symptoms and, if not managed, can lead to poor quality of life. Because there are many causes of hyperthyroidism, the condition is best managed by an interprofessional team.
Primary care clinicians should educate patients on the importance of medication compliance. In addition, the patient should be informed by the pharmacist that certain products like contrast dyes, expectorants, food supplements, and seaweed tablets may contain high levels of iodine and interfere with therapy.
Inpatient management of a patient with hyperthyroidism does not always necessarily require consultation with an endocrinologist. Still, thyroid storm strongly warrants consultation with an endocrinologist and possible admission to the intensive care unit due to potentially life-threatening complications such as tachycardia and hypertensive crisis. Therefore, nurses and physician assistants involved with patient care should be vigilant about the signs and symptoms of thyroid storm.
As mentioned, any consideration of RAI therapy in a female of reproductive potential should follow a negative beta-hCG, as pregnancy is an absolute contraindication to RAI therapy. Therefore, incorporating a mandatory pregnancy test into an overall care plan would help avoid potentially damaging radiation exposure.
Patients with Graves disease will need an ophthalmology consult. For those who undergo thyroidectomy, lifelong treatment with levothyroxine is required. Pharmacists must review prescriptions, check for drug interactions, and educate patients.
The interprofessional team must communicate with other members to ensure the patient receives the current standard of care treatment.
Media
(Click Image to Enlarge)
References
Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid : official journal of the American Thyroid Association. 2016 Oct:26(10):1343-1421 [PubMed PMID: 27521067]
Biondi B, Cooper DS. Subclinical Hyperthyroidism. The New England journal of medicine. 2018 Jun 21:378(25):2411-2419. doi: 10.1056/NEJMcp1709318. Epub [PubMed PMID: 29924956]
Biondi B, Palmieri EA, Fazio S, Cosco C, Nocera M, Saccà L, Filetti S, Lombardi G, Perticone F. Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. The Journal of clinical endocrinology and metabolism. 2000 Dec:85(12):4701-5 [PubMed PMID: 11134131]
Level 2 (mid-level) evidenceVadiveloo T, Donnan PT, Cochrane L, Leese GP. The Thyroid Epidemiology, Audit, and Research Study (TEARS): morbidity in patients with endogenous subclinical hyperthyroidism. The Journal of clinical endocrinology and metabolism. 2011 May:96(5):1344-51. doi: 10.1210/jc.2010-2693. Epub 2011 Feb 23 [PubMed PMID: 21346066]
Selmer C, Olesen JB, Hansen ML, Lindhardsen J, Olsen AM, Madsen JC, Faber J, Hansen PR, Pedersen OD, Torp-Pedersen C, Gislason GH. The spectrum of thyroid disease and risk of new onset atrial fibrillation: a large population cohort study. BMJ (Clinical research ed.). 2012 Nov 27:345():e7895. doi: 10.1136/bmj.e7895. Epub 2012 Nov 27 [PubMed PMID: 23186910]
Level 2 (mid-level) evidenceCappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006 Mar 1:295(9):1033-41 [PubMed PMID: 16507804]
Selmer C, Olesen JB, Hansen ML, von Kappelgaard LM, Madsen JC, Hansen PR, Pedersen OD, Faber J, Torp-Pedersen C, Gislason GH. Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. The Journal of clinical endocrinology and metabolism. 2014 Jul:99(7):2372-82. doi: 10.1210/jc.2013-4184. Epub 2014 Mar 21 [PubMed PMID: 24654753]
Level 2 (mid-level) evidenceRoti E, Uberti ED. Iodine excess and hyperthyroidism. Thyroid : official journal of the American Thyroid Association. 2001 May:11(5):493-500 [PubMed PMID: 11396708]
Brucker-Davis F, Oldfield EH, Skarulis MC, Doppman JL, Weintraub BD. Thyrotropin-secreting pituitary tumors: diagnostic criteria, thyroid hormone sensitivity, and treatment outcome in 25 patients followed at the National Institutes of Health. The Journal of clinical endocrinology and metabolism. 1999 Feb:84(2):476-86 [PubMed PMID: 10022404]
Mittra ES, Niederkohr RD, Rodriguez C, El-Maghraby T, McDougall IR. Uncommon causes of thyrotoxicosis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008 Feb:49(2):265-78. doi: 10.2967/jnumed.107.041202. Epub 2008 Jan 16 [PubMed PMID: 18199610]
Level 3 (low-level) evidenceDunzendorfer T, deLas Morenas A, Kalir T, Levin RM. Struma ovarii and hyperthyroidism. Thyroid : official journal of the American Thyroid Association. 1999 May:9(5):499-502 [PubMed PMID: 10365682]
Level 3 (low-level) evidenceTaylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, Okosieme OE. Global epidemiology of hyperthyroidism and hypothyroidism. Nature reviews. Endocrinology. 2018 May:14(5):301-316. doi: 10.1038/nrendo.2018.18. Epub 2018 Mar 23 [PubMed PMID: 29569622]
Tsang W, Houlden RL. Amiodarone-induced thyrotoxicosis: a review. The Canadian journal of cardiology. 2009 Jul:25(7):421-4 [PubMed PMID: 19584973]
Lazarus JH. Lithium and thyroid. Best practice & research. Clinical endocrinology & metabolism. 2009 Dec:23(6):723-33. doi: 10.1016/j.beem.2009.06.002. Epub [PubMed PMID: 19942149]
Level 3 (low-level) evidenceIllouz F, Braun D, Briet C, Schweizer U, Rodien P. Endocrine side-effects of anti-cancer drugs: thyroid effects of tyrosine kinase inhibitors. European journal of endocrinology. 2014 Sep:171(3):R91-9. doi: 10.1530/EJE-14-0198. Epub 2014 May 15 [PubMed PMID: 24833135]
Level 3 (low-level) evidenceTomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinology and metabolism clinics of North America. 2007 Dec:36(4):1051-66; x-xi [PubMed PMID: 17983936]
Guaraldi F, La Selva R, Samà MT, D'Angelo V, Gori D, Fava P, Fierro MT, Savoia P, Arvat E. Characterization and implications of thyroid dysfunction induced by immune checkpoint inhibitors in real-life clinical practice: a long-term prospective study from a referral institution. Journal of endocrinological investigation. 2018 May:41(5):549-556. doi: 10.1007/s40618-017-0772-1. Epub 2017 Oct 17 [PubMed PMID: 29043574]
Iddah MA, Macharia BN. Autoimmune thyroid disorders. ISRN endocrinology. 2013:2013():509764. doi: 10.1155/2013/509764. Epub 2013 Jun 26 [PubMed PMID: 23878745]
Pearce EN, Farwell AP, Braverman LE. Thyroiditis. The New England journal of medicine. 2003 Jun 26:348(26):2646-55 [PubMed PMID: 12826640]
Smith TJ, Hegedüs L. Graves' Disease. The New England journal of medicine. 2016 Oct 20:375(16):1552-1565 [PubMed PMID: 27797318]
Vitti P, Rago T, Tonacchera M, Pinchera A. Toxic multinodular goiter in the elderly. Journal of endocrinological investigation. 2002:25(10 Suppl):16-8 [PubMed PMID: 12508907]
Laurberg P, Pedersen KM, Vestergaard H, Sigurdsson G. High incidence of multinodular toxic goitre in the elderly population in a low iodine intake area vs. high incidence of Graves' disease in the young in a high iodine intake area: comparative surveys of thyrotoxicosis epidemiology in East-Jutland Denmark and Iceland. Journal of internal medicine. 1991 May:229(5):415-20 [PubMed PMID: 2040867]
Abraham-Nordling M, Byström K, Törring O, Lantz M, Berg G, Calissendorff J, Nyström HF, Jansson S, Jörneskog G, Karlsson FA, Nyström E, Ohrling H, Orn T, Hallengren B, Wallin G. Incidence of hyperthyroidism in Sweden. European journal of endocrinology. 2011 Dec:165(6):899-905. doi: 10.1530/EJE-11-0548. Epub 2011 Sep 9 [PubMed PMID: 21908653]
Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F, Galofré JC. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. The Journal of clinical endocrinology and metabolism. 2014 Mar:99(3):923-31. doi: 10.1210/jc.2013-2409. Epub 2014 Jan 1 [PubMed PMID: 24423323]
Level 1 (high-level) evidenceFranklyn JA. The management of hyperthyroidism. The New England journal of medicine. 1994 Jun 16:330(24):1731-8 [PubMed PMID: 7910662]
Hegedüs L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocrine reviews. 2003 Feb:24(1):102-32 [PubMed PMID: 12588812]
Level 3 (low-level) evidenceTunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T, Smith PA. The spectrum of thyroid disease in a community: the Whickham survey. Clinical endocrinology. 1977 Dec:7(6):481-93 [PubMed PMID: 598014]
Level 3 (low-level) evidenceVanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clinical endocrinology. 1995 Jul:43(1):55-68 [PubMed PMID: 7641412]
Level 3 (low-level) evidenceHollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). The Journal of clinical endocrinology and metabolism. 2002 Feb:87(2):489-99 [PubMed PMID: 11836274]
Level 3 (low-level) evidenceWang C, Li Y, Teng D, Shi X, Ba J, Chen B, Du J, He L, Lai X, Li Y, Chi H, Liao E, Liu C, Liu L, Qin G, Qin Y, Quan H, Shi B, Sun H, Tang X, Tong N, Wang G, Zhang JA, Wang Y, Xue Y, Yan L, Yang J, Yang L, Yao Y, Ye Z, Zhang Q, Zhang L, Zhu J, Zhu M, Shan Z, Teng W. Hyperthyroidism Prevalence in China After Universal Salt Iodization. Frontiers in endocrinology. 2021:12():651534. doi: 10.3389/fendo.2021.651534. Epub 2021 May 28 [PubMed PMID: 34122333]
Level 2 (mid-level) evidenceBogazzi F, Tomisti L, Bartalena L, Aghini-Lombardi F, Martino E. Amiodarone and the thyroid: a 2012 update. Journal of endocrinological investigation. 2012 Mar:35(3):340-8. doi: 10.3275/8298. Epub 2012 Mar 19 [PubMed PMID: 22433945]
Martino E, Safran M, Aghini-Lombardi F, Rajatanavin R, Lenziardi M, Fay M, Pacchiarotti A, Aronin N, Macchia E, Haffajee C. Environmental iodine intake and thyroid dysfunction during chronic amiodarone therapy. Annals of internal medicine. 1984 Jul:101(1):28-34 [PubMed PMID: 6428291]
Level 2 (mid-level) evidenceKrohn K, Führer D, Bayer Y, Eszlinger M, Brauer V, Neumann S, Paschke R. Molecular pathogenesis of euthyroid and toxic multinodular goiter. Endocrine reviews. 2005 Jun:26(4):504-24 [PubMed PMID: 15615818]
Level 3 (low-level) evidenceHamburger JI. Evolution of toxicity in solitary nontoxic autonomously functioning thyroid nodules. The Journal of clinical endocrinology and metabolism. 1980 Jun:50(6):1089-93 [PubMed PMID: 7372787]
Stanbury JB, Ermans AE, Bourdoux P, Todd C, Oken E, Tonglet R, Vidor G, Braverman LE, Medeiros-Neto G. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid : official journal of the American Thyroid Association. 1998 Jan:8(1):83-100 [PubMed PMID: 9492158]
Bervini S, Trelle S, Kopp P, Stettler C, Trepp R. Prevalence of Iodine-Induced Hyperthyroidism After Administration of Iodinated Contrast During Radiographic Procedures: A Systematic Review and Meta-Analysis of the Literature. Thyroid : official journal of the American Thyroid Association. 2021 Jul:31(7):1020-1029. doi: 10.1089/thy.2020.0459. Epub 2021 Mar 15 [PubMed PMID: 33327840]
Level 1 (high-level) evidenceDunne P, Kaimal N, MacDonald J, Syed AA. Iodinated contrast-induced thyrotoxicosis. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2013 Feb 5:185(2):144-7. doi: 10.1503/cmaj.120734. Epub 2012 Nov 12 [PubMed PMID: 23148056]
Level 3 (low-level) evidenceFradkin JE, Wolff J. Iodide-induced thyrotoxicosis. Medicine. 1983 Jan:62(1):1-20 [PubMed PMID: 6218369]
Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. The Journal of clinical endocrinology and metabolism. 2010 Jun:95(6):2529-35. doi: 10.1210/jc.2010-0180. Epub [PubMed PMID: 20525904]
Level 3 (low-level) evidenceBahn Chair RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, Montori VM, Rivkees SA, Ross DS, Sosa JA, Stan MN, American Thyroid Association, American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid : official journal of the American Thyroid Association. 2011 Jun:21(6):593-646. doi: 10.1089/thy.2010.0417. Epub 2011 Apr 21 [PubMed PMID: 21510801]
Şahlı E, Gündüz K. Thyroid-associated Ophthalmopathy. Turkish journal of ophthalmology. 2017 Apr:47(2):94-105. doi: 10.4274/tjo.80688. Epub 2017 Apr 1 [PubMed PMID: 28405484]
Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, Marinò M, Vaidya B, Wiersinga WM, EUGOGO †. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. European journal of endocrinology. 2021 Aug 27:185(4):G43-G67. doi: 10.1530/EJE-21-0479. Epub 2021 Aug 27 [PubMed PMID: 34297684]
Level 1 (high-level) evidenceBahn RS. Graves' ophthalmopathy. The New England journal of medicine. 2010 Feb 25:362(8):726-38. doi: 10.1056/NEJMra0905750. Epub [PubMed PMID: 20181974]
Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. American journal of clinical dermatology. 2005:6(5):295-309 [PubMed PMID: 16252929]
Jadidi J, Sigari M, Efendizade A, Grigorian A, Lehto SA, Kolla S. Thyroid acropachy: A rare skeletal manifestation of autoimmune thyroid disease. Radiology case reports. 2019 Aug:14(8):917-919. doi: 10.1016/j.radcr.2019.04.021. Epub 2019 May 23 [PubMed PMID: 31193617]
Level 3 (low-level) evidenceFatourechi V, Ahmed DD, Schwartz KM. Thyroid acropachy: report of 40 patients treated at a single institution in a 26-year period. The Journal of clinical endocrinology and metabolism. 2002 Dec:87(12):5435-41 [PubMed PMID: 12466333]
Level 2 (mid-level) evidenceDe Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet (London, England). 2016 Aug 27:388(10047):906-918. doi: 10.1016/S0140-6736(16)00278-6. Epub 2016 Mar 30 [PubMed PMID: 27038492]
Favresse J, Burlacu MC, Maiter D, Gruson D. Interferences With Thyroid Function Immunoassays: Clinical Implications and Detection Algorithm. Endocrine reviews. 2018 Oct 1:39(5):830-850. doi: 10.1210/er.2018-00119. Epub [PubMed PMID: 29982406]
Li D, Radulescu A, Shrestha RT, Root M, Karger AB, Killeen AA, Hodges JS, Fan SL, Ferguson A, Garg U, Sokoll LJ, Burmeister LA. Association of Biotin Ingestion With Performance of Hormone and Nonhormone Assays in Healthy Adults. JAMA. 2017 Sep 26:318(12):1150-1160. doi: 10.1001/jama.2017.13705. Epub [PubMed PMID: 28973622]
McKee A, Peyerl F. TSI assay utilization: impact on costs of Graves' hyperthyroidism diagnosis. The American journal of managed care. 2012 Jan 1:18(1):e1-14 [PubMed PMID: 22435785]
Autilio C, Morelli R, Locantore P, Pontecorvi A, Zuppi C, Carrozza C. Stimulating TSH receptor autoantibodies immunoassay: analytical evaluation and clinical performance in Graves' disease. Annals of clinical biochemistry. 2018 Jan:55(1):172-177. doi: 10.1177/0004563217700655. Epub 2017 Oct 9 [PubMed PMID: 28388869]
Kahaly GJ. Bioassays for TSH Receptor Antibodies: Quo Vadis? European thyroid journal. 2015 Mar:4(1):3-5. doi: 10.1159/000375445. Epub [PubMed PMID: 25960955]
Chung J, Lee YJ, Choi YJ, Ha EJ, Suh CH, Choi M, Baek JH, Na DG, Korean Society of Thyroid Radiology (KSThR), Korean Society of Radiology. Clinical applications of Doppler ultrasonography for thyroid disease: consensus statement by the Korean Society of Thyroid Radiology. Ultrasonography (Seoul, Korea). 2020 Oct:39(4):315-330. doi: 10.14366/usg.20072. Epub 2020 Aug 25 [PubMed PMID: 32892523]
Level 3 (low-level) evidenceDaniels GH. Amiodarone-induced thyrotoxicosis. The Journal of clinical endocrinology and metabolism. 2001 Jan:86(1):3-8 [PubMed PMID: 11231968]
Level 3 (low-level) evidenceBurch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves' disease. The Journal of clinical endocrinology and metabolism. 2012 Dec:97(12):4549-58. doi: 10.1210/jc.2012-2802. Epub 2012 Oct 5 [PubMed PMID: 23043191]
Level 3 (low-level) evidenceBalazs C, Kiss E, Leövey A, Farid NR. The immunosuppressive effect of methimazole on cell-mediated immunity is mediated by its capacity to inhibit peroxidase and to scavenge free oxygen radicals. Clinical endocrinology. 1986 Jul:25(1):7-16 [PubMed PMID: 3024872]
Level 3 (low-level) evidenceLechpammer M, Lukac J, Lechpammer S, Kusić Z. Antithyroid drug-induced immunomodulation in Graves' disease patients. Acta medica Croatica : casopis Hravatske akademije medicinskih znanosti. 2002:56(1):21-6 [PubMed PMID: 12455450]
Level 1 (high-level) evidenceLaurberg P, Andersen SL. Therapy of endocrine disease: antithyroid drug use in early pregnancy and birth defects: time windows of relative safety and high risk? European journal of endocrinology. 2014 Jul:171(1):R13-20. doi: 10.1530/EJE-14-0135. Epub 2014 Mar 24 [PubMed PMID: 24662319]
Hackmon R, Blichowski M, Koren G. The safety of methimazole and propylthiouracil in pregnancy: a systematic review. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2012 Nov:34(11):1077-1086. doi: 10.1016/S1701-2163(16)35438-X. Epub [PubMed PMID: 23231846]
Level 2 (mid-level) evidenceBurch HB, Cooper DS. ANNIVERSARY REVIEW: Antithyroid drug therapy: 70 years later. European journal of endocrinology. 2018 Oct 12:179(5):R261-R274. doi: 10.1530/EJE-18-0678. Epub 2018 Oct 12 [PubMed PMID: 30320502]
Klein I, Becker DV, Levey GS. Treatment of hyperthyroid disease. Annals of internal medicine. 1994 Aug 15:121(4):281-8 [PubMed PMID: 7518659]
Mohlin E, Filipsson Nyström H, Eliasson M. Long-term prognosis after medical treatment of Graves' disease in a northern Swedish population 2000-2010. European journal of endocrinology. 2014 Mar:170(3):419-27. doi: 10.1530/EJE-13-0811. Epub 2014 Feb 4 [PubMed PMID: 24366943]
Level 2 (mid-level) evidenceKomiya I, Yamada T, Sato A, Kouki T, Nishimori T, Takasu N. Remission and recurrence of hyperthyroid Graves' disease during and after methimazole treatment when assessed by IgE and interleukin 13. The Journal of clinical endocrinology and metabolism. 2001 Aug:86(8):3540-4 [PubMed PMID: 11502776]
Suzuki N, Noh JY, Yoshimura R, Mikura K, Kinoshita A, Suzuki A, Mitsumatsu T, Hoshiyama A, Fukushita M, Matsumoto M, Yoshihara A, Watanabe N, Sugino K, Ito K. Does Age or Sex Relate to Severity or Treatment Prognosis in Graves' Disease? Thyroid : official journal of the American Thyroid Association. 2021 Sep:31(9):1409-1415. doi: 10.1089/thy.2020.0881. Epub 2021 May 26 [PubMed PMID: 33882721]
Allahabadia A, Daykin J, Sheppard MC, Gough SC, Franklyn JA. Radioiodine treatment of hyperthyroidism-prognostic factors for outcome. The Journal of clinical endocrinology and metabolism. 2001 Aug:86(8):3611-7 [PubMed PMID: 11502786]
Level 2 (mid-level) evidenceSantos RB, Romaldini JH, Ward LS. A randomized controlled trial to evaluate the effectiveness of 2 regimens of fixed iodine (¹³¹I) doses for Graves disease treatment. Clinical nuclear medicine. 2012 Mar:37(3):241-4. doi: 10.1097/RLU.0b013e31823ea6e0. Epub [PubMed PMID: 22310249]
Level 1 (high-level) evidenceRoque C, Santos FS, Pilli T, Dalmazio G, Castagna MG, Pacini F. Long-term Effects of Radioiodine in Toxic Multinodular Goiter: Thyroid Volume, Function, and Autoimmunity. The Journal of clinical endocrinology and metabolism. 2020 Jul 1:105(7):. pii: dgaa214. doi: 10.1210/clinem/dgaa214. Epub [PubMed PMID: 32320467]
Kang AS, Grant CS, Thompson GB, van Heerden JA. Current treatment of nodular goiter with hyperthyroidism (Plummer's disease): surgery versus radioiodine. Surgery. 2002 Dec:132(6):916-23; discussion 923 [PubMed PMID: 12490836]
Level 2 (mid-level) evidenceHolm LE, Lundell G, Israelsson A, Dahlqvist I. Incidence of hypothyroidism occurring long after iodine-131 therapy for hyperthyroidism. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1982 Feb:23(2):103-7 [PubMed PMID: 7057248]
Level 2 (mid-level) evidenceYano Y, Sugino K, Akaishi J, Uruno T, Okuwa K, Shibuya H, Kitagawa W, Nagahama M, Ito K, Ito K. Treatment of autonomously functioning thyroid nodules at a single institution: radioiodine therapy, surgery, and ethanol injection therapy. Annals of nuclear medicine. 2011 Dec:25(10):749-54. doi: 10.1007/s12149-011-0526-7. Epub 2011 Oct 5 [PubMed PMID: 21971604]
Level 2 (mid-level) evidenceNygaard B, Hegedüs L, Ulriksen P, Nielsen KG, Hansen JM. Radioiodine therapy for multinodular toxic goiter. Archives of internal medicine. 1999 Jun 28:159(12):1364-8 [PubMed PMID: 10386513]
Ferrari C, Reschini E, Paracchi A. Treatment of the autonomous thyroid nodule: a review. European journal of endocrinology. 1996 Oct:135(4):383-90 [PubMed PMID: 8921817]
Ceccarelli C, Bencivelli W, Vitti P, Grasso L, Pinchera A. Outcome of radioiodine-131 therapy in hyperfunctioning thyroid nodules: a 20 years' retrospective study. Clinical endocrinology. 2005 Mar:62(3):331-5 [PubMed PMID: 15730415]
Level 2 (mid-level) evidenceErbil Y, Ozluk Y, Giriş M, Salmaslioglu A, Issever H, Barbaros U, Kapran Y, Ozarmağan S, Tezelman S. Effect of lugol solution on thyroid gland blood flow and microvessel density in the patients with Graves' disease. The Journal of clinical endocrinology and metabolism. 2007 Jun:92(6):2182-9 [PubMed PMID: 17389702]
Level 1 (high-level) evidenceAnsaldo GL, Pretolesi F, Varaldo E, Meola C, Minuto M, Borgonovo G, Derchi LE, Torre GC. Doppler evaluation of intrathyroid arterial resistances during preoperative treatment with Lugol's iodide solution in patients with diffuse toxic goiter. Journal of the American College of Surgeons. 2000 Dec:191(6):607-12 [PubMed PMID: 11129808]
Level 2 (mid-level) evidenceGuo Z, Yu P, Liu Z, Si Y, Jin M. Total thyroidectomy vs bilateral subtotal thyroidectomy in patients with Graves' diseases: a meta-analysis of randomized clinical trials. Clinical endocrinology. 2013 Nov:79(5):739-46. doi: 10.1111/cen.12209. Epub 2013 Apr 19 [PubMed PMID: 23521078]
Level 1 (high-level) evidenceLimonard EJ, Bisschop PH, Fliers E, Nieveen van Dijkum EJ. Thyroid function after subtotal thyroidectomy in patients with Graves' hyperthyroidism. TheScientificWorldJournal. 2012:2012():548796. doi: 10.1100/2012/548796. Epub 2012 Feb 1 [PubMed PMID: 22448136]
Level 2 (mid-level) evidenceAl-Adhami A, Snaith AC, Craig WL, Krukowski ZH. Changing trends in surgery for Graves' disease: a cohort comparison of those having surgery intended to preserve thyroid function with those having ablative surgery. Journal of otolaryngology - head & neck surgery = Le Journal d'oto-rhino-laryngologie et de chirurgie cervico-faciale. 2013 May 29:42(1):37. doi: 10.1186/1916-0216-42-37. Epub 2013 May 29 [PubMed PMID: 23718902]
Dogan L, Karaman N, Yilmaz KB, Ozaslan C, Atalay C. Total thyroidectomy for the surgical treatment of multinodular goiter. Surgery today. 2011 Mar:41(3):323-7. doi: 10.1007/s00595-009-4272-6. Epub 2011 Feb 23 [PubMed PMID: 21365410]
Level 2 (mid-level) evidenceHussain M, Hisham AN. Total thyroidectomy: the procedure of choice for toxic goitre. Asian journal of surgery. 2008 Apr:31(2):59-62. doi: 10.1016/S1015-9584(08)60059-7. Epub [PubMed PMID: 18490216]
Vidal-Trecan GM, Stahl JE, Eckman MH. Radioiodine or surgery for toxic thyroid adenoma: dissecting an important decision. A cost-effectiveness analysis. Thyroid : official journal of the American Thyroid Association. 2004 Nov:14(11):933-45 [PubMed PMID: 15671772]
Otsuka F, Noh JY, Chino T, Shimizu T, Mukasa K, Ito K, Ito K, Taniyama M. Hepatotoxicity and cutaneous reactions after antithyroid drug administration. Clinical endocrinology. 2012 Aug:77(2):310-5. doi: 10.1111/j.1365-2265.2012.04365.x. Epub [PubMed PMID: 22332800]
Level 1 (high-level) evidenceWang MT, Lee WJ, Huang TY, Chu CL, Hsieh CH. Antithyroid drug-related hepatotoxicity in hyperthyroidism patients: a population-based cohort study. British journal of clinical pharmacology. 2014 Sep:78(3):619-29 [PubMed PMID: 25279406]
Level 2 (mid-level) evidenceNakamura H, Miyauchi A, Miyawaki N, Imagawa J. Analysis of 754 cases of antithyroid drug-induced agranulocytosis over 30 years in Japan. The Journal of clinical endocrinology and metabolism. 2013 Dec:98(12):4776-83. doi: 10.1210/jc.2013-2569. Epub 2013 Sep 20 [PubMed PMID: 24057289]
Level 3 (low-level) evidenceNoh JY, Yasuda S, Sato S, Matsumoto M, Kunii Y, Noguchi Y, Mukasa K, Ito K, Ito K, Sugiyama O, Kobayashi H, Nihojima S, Okazaki M, Yokoyama S. Clinical characteristics of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis caused by antithyroid drugs. The Journal of clinical endocrinology and metabolism. 2009 Aug:94(8):2806-11. doi: 10.1210/jc.2008-2700. Epub 2009 Jun 2 [PubMed PMID: 19491223]
Gao Y, Zhao MH, Guo XH, Xin G, Gao Y, Wang HY. The prevalence and target antigens of antithyroid drugs induced antineutrophil cytoplasmic antibodies (ANCA) in Chinese patients with hyperthyroidism. Endocrine research. 2004 May:30(2):205-13 [PubMed PMID: 15473130]
Level 2 (mid-level) evidenceYazisiz V, Ongüt G, Terzioğlu E, Karayalçin U. Clinical importance of antineutrophil cytoplasmic antibody positivity during propylthiouracil treatment. International journal of clinical practice. 2010 Jan:64(1):19-24. doi: 10.1111/j.1742-1241.2007.01485.x. Epub 2008 Feb 14 [PubMed PMID: 18284438]
Aloush V, Litinsky I, Caspi D, Elkayam O. Propylthiouracil-induced autoimmune syndromes: two distinct clinical presentations with different course and management. Seminars in arthritis and rheumatism. 2006 Aug:36(1):4-9 [PubMed PMID: 16887463]
Level 3 (low-level) evidenceHess E. Drug-related lupus. The New England journal of medicine. 1988 Jun 2:318(22):1460-2 [PubMed PMID: 3259288]
Gomez Cruz MJ, Jabbar M, Saini N, Eng D, Crawford B, Vazquez DM, Menon R, Chen M. Severe hypoglycemia secondary to methimazole-induced insulin autoimmune syndrome in a 16 year old African-American male. Pediatric diabetes. 2012 Dec:13(8):652-5. doi: 10.1111/j.1399-5448.2012.00884.x. Epub 2012 Jul 3 [PubMed PMID: 22759245]
Level 3 (low-level) evidenceJain N, Savani M, Agarwal M, Kadaria D. Methimazole-induced insulin autoimmune syndrome. Therapeutic advances in endocrinology and metabolism. 2016 Aug:7(4):178-81. doi: 10.1177/2042018816658396. Epub 2016 Jul 19 [PubMed PMID: 27540463]
Level 3 (low-level) evidenceRosato L, Avenia N, Bernante P, De Palma M, Gulino G, Nasi PG, Pelizzo MR, Pezzullo L. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World journal of surgery. 2004 Mar:28(3):271-6 [PubMed PMID: 14961204]
Level 2 (mid-level) evidenceBhattacharyya N, Fried MP. Assessment of the morbidity and complications of total thyroidectomy. Archives of otolaryngology--head & neck surgery. 2002 Apr:128(4):389-92 [PubMed PMID: 11926912]
Level 2 (mid-level) evidenceSafioleas M, Stamatakos M, Rompoti N, Mouzopoulos G, Iannescu R, Salichou V, Skandalakis P. Complications of thyroid surgery. Chirurgia (Bucharest, Romania : 1990). 2006 Nov-Dec:101(6):571-81 [PubMed PMID: 17283832]
Thiyagarajan A, Platzbecker K, Ittermann T, Völzke H, Haug U. Estimating Incidence and Case Fatality of Thyroid Storm in Germany Between 2007 and 2017: A Claims Data Analysis. Thyroid : official journal of the American Thyroid Association. 2022 Nov:32(11):1307-1315. doi: 10.1089/thy.2022.0096. Epub 2022 Sep 28 [PubMed PMID: 36006371]
Level 3 (low-level) evidenceDekkers OM, Horváth-Puhó E, Cannegieter SC, Vandenbroucke JP, Sørensen HT, Jørgensen JO. Acute cardiovascular events and all-cause mortality in patients with hyperthyroidism: a population-based cohort study. European journal of endocrinology. 2017 Jan:176(1):1-9 [PubMed PMID: 27697972]
Abrahamsen B, Jørgensen HL, Laulund AS, Nybo M, Bauer DC, Brix TH, Hegedüs L. The excess risk of major osteoporotic fractures in hypothyroidism is driven by cumulative hyperthyroid as opposed to hypothyroid time: an observational register-based time-resolved cohort analysis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015 May:30(5):898-905. doi: 10.1002/jbmr.2416. Epub [PubMed PMID: 25431028]
Level 2 (mid-level) evidenceMintziori G, Kita M, Duntas L, Goulis DG. Consequences of hyperthyroidism in male and female fertility: pathophysiology and current management. Journal of endocrinological investigation. 2016 Aug:39(8):849-53. doi: 10.1007/s40618-016-0452-6. Epub 2016 Mar 8 [PubMed PMID: 26956000]
Siu CW, Pong V, Zhang X, Chan YH, Jim MH, Liu S, Yiu KH, Kung AW, Lau CP, Tse HF. Risk of ischemic stroke after new-onset atrial fibrillation in patients with hyperthyroidism. Heart rhythm. 2009 Feb:6(2):169-73. doi: 10.1016/j.hrthm.2008.10.023. Epub 2008 Nov 1 [PubMed PMID: 19187905]
Level 2 (mid-level) evidenceXu N, Wang Y, Xu Y, Li L, Chen J, Mai X, Xu J, Zhang Z, Yang R, Sun J, Chen H, Chen R. Effect of subclinical hyperthyroidism on osteoporosis: A meta-analysis of cohort studies. Endocrine. 2020 Jul:69(1):39-48. doi: 10.1007/s12020-020-02259-8. Epub 2020 Mar 23 [PubMed PMID: 32207036]
Level 1 (high-level) evidenceCollet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, Iervasi G, Åsvold BO, Sgarbi JA, Völzke H, Gencer B, Maciel RM, Molinaro S, Bremner A, Luben RN, Maisonneuve P, Cornuz J, Newman AB, Khaw KT, Westendorp RG, Franklyn JA, Vittinghoff E, Walsh JP, Rodondi N, Thyroid Studies Collaboration. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Archives of internal medicine. 2012 May 28:172(10):799-809. doi: 10.1001/archinternmed.2012.402. Epub [PubMed PMID: 22529182]
Level 2 (mid-level) evidenceAbdulaziz Qari F. Thyroid Hormone Profile in Patients With Acute Coronary Syndrome. Iranian Red Crescent medical journal. 2015 Jul:17(7):e26919. doi: 10.5812/ircmj.26919v2. Epub 2015 Jul 22 [PubMed PMID: 26421178]
Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nature reviews. Endocrinology. 2017 Oct:13(10):610-622. doi: 10.1038/nrendo.2017.93. Epub 2017 Aug 4 [PubMed PMID: 28776582]
Samuels SL, Namoc SM, Bauer AJ. Neonatal Thyrotoxicosis. Clinics in perinatology. 2018 Mar:45(1):31-40. doi: 10.1016/j.clp.2017.10.001. Epub 2017 Dec 16 [PubMed PMID: 29406005]