Introduction
Tracheostomy is a medical procedure that involves creating an artificial passage connecting the cervical trachea to the external environment, circumventing the larynx and upper aerodigestive tract. The term "tracheostomy" encompasses the procedure and the resulting tract between the trachea and the skin. The origins of tracheostomies can be traced back to ancient Egypt and were later studied in ancient Greece and Rome; however, the first documented life-saving tracheostomy was performed by Brassavola in Italy in 1546 on a patient with a peritonsillar abscess.[1]
Severino popularized the procedure in the early seventeenth century, recognizing its efficacy during the 1610 diphtheria epidemic.[2][3] In the early twentieth century, Chevalier Jackson played a crucial role in further developing and standardizing the technique, addressing numerous challenges and controversies associated with the procedure.[3][4] However, as the twentieth century progressed, it became evident that oral or nasal intubation offered a safer and quicker alternative to tracheostomy, with lower complication rates. Consequently, contemporary tracheostomy necessitates careful consideration of indications, contraindications, risks, benefits, and standardized post-procedural care.
In the United States alone, over 83,000 tracheostomies were performed in 1999, marking a substantial increase between 1993 and 2012.[5][6] In England, the National Tracheostomy Safety Project estimates indicate that approximately 15,000 percutaneous tracheostomies are conducted annually in intensive care units, with an additional 5000 surgical tracheostomies in the operating room.
Given the escalating number of patients undergoing tracheostomy, ensuring safe care demands proficiency in cleaning, suctioning, weaning, decannulation, and managing tracheostomy-related emergencies. Routine tracheostomy tube changes are integral to ongoing care, but complications may arise more frequently without the support of experienced personnel, proper education, and a robust interprofessional tracheostomy care team. This article provides a basic understanding of the tracheostomy procedure, its relevant anatomy, and the principles behind tracheostomy care, primarily focusing on the safe exchange of the tracheostomy tube.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Tracheostomy can be performed via open surgery or by using a percutaneous technique. A fundamental grasp of cervical anatomy is imperative, as it forms the foundation for properly inserting, replacing, and removing tracheostomy tubes. A vertical or transverse skin incision can be made to perform a surgical tracheostomy. Most surgeons prefer a transverse incision for cosmetic reasons, as this incision can be hidden in the natural skin creases on the neck. The incision is made midway between the cricoid cartilage and the suprasternal notch, taking care not to injure the anterior jugular veins. To gain access to the tracheal lumen, the surgeon encounters the following anatomical structures successively: skin, subcutaneous fat, platysma muscle (may not be present in this location in some individuals), superficial or investing layer of deep cervical fascia, midline raphé of the strap muscles (sternohyoid and sternothyroid), pretracheal fascial, thyroid gland isthmus, and the trachea. A transverse incision is made into the tracheal lumen between the second and third cartilage rings. Various tracheal incision techniques exist, such as the "horizontal H" advocated by some surgeons, which spans the first through third rings. Additionally, the Bjork approach incorporates 3 incisions, forming an inferiorly-based flap that can be secured to the skin at the stoma.
Bedside percutaneous tracheostomy, guided by ultrasound or fiberoptic visualization, has emerged as an alternative to open surgical tracheostomy conducted at the bedside or in the operating room. Percutaneous tracheostomy, introduced in the 1980s, has gained popularity as an effective alternative because it eliminates the need for an operating room.[7][8] The Seldinger technique is the most frequently employed percutaneous method, involving gradually widening the tract using dilators over a guidewire. The choice between tracheostomy methods hinges on equipment availability for each procedure and institutional expertise.
Certain patient populations pose challenges during the tracheostomy procedure and tube changes. Examples include patients with a high body mass index, those who are pediatric, and those with cervical spine instability or immobility. In patients who are obese, subcutaneous adipose tissue necessitates a longer tracheostomy tract. Consequently, using a short tracheostomy tube may be inappropriate, as its lumen might not reach the center of the tracheal lumen, the area least likely to cause mucosal trauma and subsequent scarring (see Image. Cannula for Tracheostomy). However, longer tracts are more likely to create a false passage during tracheostomy tube changes.[9] In the pediatric population, where the tract is notably shorter, using shorter tubes increases the risk of inadvertently puncturing the tracheoesophageal wall if tube exchanges are not executed with care.
Results from a study conducted in 2017 by Watters found that over 90% of complications occurred more than 1 week after surgery, with 15% to 19% of tracheostomy patients experiencing complications related to the procedure.[10] The primary cause of tracheostomy-related deaths is tube obstruction, often due to thick secretions and blood clots, followed by tube misplacement and accidental decannulation. Therefore, implementing measures that prevent creating false passages, tube displacement, and tube obstruction is imperative. Rigorous monitoring of vital signs is crucial, especially in children, as they are less likely to communicate discomfort, pain, or breathing difficulties and often have less physiological reserve than adults.
Indications
The earliest recorded tracheostomies were primarily performed to address upper airway obstructions. In contemporary medical practice, the indications for tracheostomy encompass various scenarios: managing upper airway obstructions, ensuring a secure airway when the upper airway is compromised, as seen in cases of facial, head, and neck injuries; facilitating bronchial hygiene and secretion removal; safeguarding the airway, particularly in neuromuscular disorders; and for prolonged mechanical ventilation lasting more than 10 days to avert the complications associated with prolonged intubation. In pediatric cases, common indications for tracheostomy include congenital and acquired airway stenosis, neurological conditions necessitating long-term ventilation or pulmonary care, bilateral vocal fold insufficiency, and infectious compromise of the upper airway.
Tracheostomy tube changes are warranted for a variety of reasons, including confirming the maturation of the tracheocutaneous tract (especially during the first change), maintaining hygiene and managing the stoma (approximately every 30 days), adapting the tube type (eg, switching from cuffed to uncuffed when discontinuing mechanical ventilation or from nonfenestrated to fenestrated when commencing speech therapy), downsizing the tube, and facilitating the weaning or decannulation process.[11]
Contraindications
The only absolute contraindication for changing a tracheostomy tube is if the stoma is not fully matured.
Potential contraindications include:
- The patient is in an unstable condition.
- A high oxygen level and increased ventilatory support are required to maintain the patient's respiratory status.
- The tube change has a high probability of causing further tissue trauma or bleeding.
Equipment
Various studies have examined the complication rates associated with surgically performed tracheostomy compared to percutaneous procedures. Results from a study conducted by Oliver et al in 2007 showed that percutaneous tracheostomy was significantly quicker than open surgical tracheostomy, although it was associated with more early complications.[12] However, subsequent meta-analyses leaned towards favoring percutaneous tracheostomy despite similar complication rates. This preference is attributed to the shorter procedural duration and a reduced risk of infection associated with percutaneous techniques.[8][13][14]
Minor complications associated with tracheostomy include:
- Minor bleeding
- Infection
- Temporary oxygen desaturation
- Tube occlusion
- False passage
- Tracheocutaneous fistula
- Poor cosmetic scar
Major complications associated with tracheostomy include:
- Hemorrhage requiring transfusion due to tracheoinnominate fistula
- Tracheoesophageal fistula
- Subglottic stenosis
- Tracheal stenosis
- Tracheomalacia
- Death
Postoperative complications can vary depending on the timing and insertion technique. Early postoperative complications tend to arise in the first few days to weeks and include:
- Hemorrhage
- Postoperative tube dislodgement or obstruction
- Subcutaneous emphysema
- Soft tissue infection
- Pneumothorax
- Pneumomediastinum
Late complications occur more than 3 weeks after tracheostomy. They include:
- Tracheal stenosis or tracheomalacia
- Tube dislodgement or obstruction
- Equipment failure
- Tracheoinnominate fistula
- Tracheoesophageal fistula
- Infections, such as aspiration pneumonia
While rare, the most concerning complication of tracheostomy is developing a tracheoinnominate fistula between the trachea and the innominate (brachiocephalic) artery. A sentinel bleed may signal fistula onset, characterized by relatively minor hemoptysis in approximately 30% of patients; this generally occurs 24 to 48 hours before the potential onset of life-threatening bleeding into the airway.[15] Typically, the innominate artery crosses the trachea between the sixth and ninth tracheal rings, below the usual placement of a traditional tracheostomy between the second and third rings. Factors such as placing the tracheostomy too low, inflating the cuff excessively (leading to necrosis), or a history of neck radiation can elevate the risk of fistula formation.
In the event of massive hemorrhage, when a tracheostomy tube cuff is present, inflate the tube to maximum capacity and pull it toward the sternum to exert pressure on the innominate artery. Suction should be administered as necessary to sustain the airway, and preparations for transfusion should commence while urgently contacting a cardiothoracic surgeon.
Although tracheostomy tube exchanges are routinely conducted, they come with inherent risks. Therefore, a thorough understanding of the procedure and preparation for potential issues is imperative. Many institutions have implemented protocols and guidelines to ensure smooth tracheostomy tube exchanges. Continuous education and quality improvement initiatives are integral in enhancing competence and minimizing complications, particularly tube obstruction-related complications. The primary cause of tube obstruction is often traced back to the lack of cleaning and maintenance of the inner cannula. The inner cannula, designed to capture secretions, becomes critical in emergencies, enabling rapid removal to reopen the airway while the inner cannula is cleaned or replaced.
The most frequently encountered complication during a tracheostomy tube change is tube displacement or forming a false passage.[16] A false passage, indicated by subcutaneous emphysema and subsequent respiratory distress, is more likely to occur during the first tube change when the tract is not fully mature. The initial tube change should be performed under direct visualization, preferably not sooner than 5 to 7 days after the tracheostomy procedure.[17] Fortunately, the rate of accidental decannulation significantly decreased between 1985 and 2004, likely attributed to improved tracheostomy care.[18] If a false passage is suspected, promptly remove and carefully replace the tracheostomy tube. An airway exchange catheter may be used during tube changes to reduce the risk of displacement.[19]
Obstruction and accidental dislodgement of the tube seems to be more prevalent in patients who are obese, with dislodgement associated explicitly with elevated rates of morbidity and mortality. In the rare occurrence of this complication, following basic or advanced life support algorithms is crucial, and a code should be activated to bring in experienced personnel, typically anesthesiologists, surgeons, or otolaryngologists, to assist in re-establishing an airway. Attempting oral intubation may be considered to establish a definitive airway, especially in cases of respiratory arrest. However, using bag-valve masking may offer a more expedient way to provide oxygen to the patient.
Personnel
While routine tracheostomy tube changes performed on general wards are safe with adequately trained staff, equipment, and support, the first tube changes may necessitate higher nursing and ancillary support, which might not be readily available. The risk of airway loss associated with the initial tube change on a general medical-surgical ward is notably higher than changes performed in intensive care or step-down units (96.1% vs 63.6%).[20]
Numerous guidelines and local protocols outline the personnel and practice requirements for tracheostomy tube changes. Typically, the first tube change is carried out or supervised by a senior resident and is avoided during nights or weekends to ensure experienced personnel are available in an unforeseen emergency.[20] Alternatively, the procedure can be performed by various healthcare professionals, including an otolaryngologist, general surgeon, pulmonologist, anesthesiologist, tracheostomy-trained nurse practitioner, intensive care nurse, speech and language therapist, respiratory therapist, or otolaryngology nurse. An otolaryngologist or general surgeon should ideally perform the initial tube change in pediatric settings and critical airway situations.
Evidence shows collaborative and coordinated tracheostomy care is achievable, implementable, and improves care.[21][22] Garrubba's 2009 systematic review revealed that an interprofessional team approach led to notable reductions in time to decannulation, length of hospital stay, and adverse events.[23] Similarly, improved tracheostomy care outcomes are observed when various parties, including patients, surgeons, primary care physicians, nurses, and speech and language therapists, engage in a coordinated effort. The success of an interprofessional tracheostomy program implemented at Johns Hopkins Hospital in 2012 further substantiates these findings, showcasing enhanced outcomes, reduced complications, and increased cost-effectiveness.[24]
In the United Kingdom, the National Tracheostomy Safety Project represents a comprehensive nationwide initiative dedicated to advancing the care of patients with tracheostomy. Like their North American counterparts, British tracheostomy teams, comprised of otolaryngologists, anesthetists, chest physicians, specialist nurses, speech and language therapists, and respiratory therapists, are proving instrumental. The mounting evidence underscores the critical role of coordinated interprofessional teams in elevating the safety and quality of care for patients with tracheostomies and their families.[25][26]
Preparation
Ensuring all airway equipment is readily available at the patient's bedside before performing a tube exchange is essential; typically, anesthesia and sedation are not required for patient comfort during this procedure. If available, continuous pulse oximetry, cardiac monitoring, and capnography should be initiated to verify tube placement. Determining the tracheostomy tube model and size is essential before the exchange. Additionally, the obturator and inner cannula should be on hand. If a cuff is present, inflate it to check functionality, ensuring it maintains pressure without leakage, and then deflate it in preparation for tube placement. The obturator should be inserted into the tracheostomy tube, and the inner cannula should be set aside, ensuring smooth sliding in and out of the obturator. The replacement tube should be coated with a water-soluble lubricant to facilitate placement.
Proper patient positioning is paramount for a safe and efficient tracheostomy tube exchange. The bed should be adjusted to the appropriate height for the healthcare provider, and any bed rails or bedside tables should be relocated. The patient should lie supine with the neck extended over a shoulder roll or pillow, bringing the trachea as close to the skin surface as possible to shorten the tract. A right-hand dominant healthcare provider should stand to the patient's right, and vice versa for a left-hand dominant individual. An assistant should be positioned on the opposite side, ready to provide suction and assist with securing the tube. No one should be standing at the foot of the bed, as manipulating the tracheostomy tube could induce a vigorous cough, expelling sputum in that direction. For optimal coordination, healthcare team members may find it beneficial to rehearse or discuss the procedural steps before the tube exchange, especially if some team members lack experience with tracheostomy tube changes.
Technique or Treatment
Tracheostomy tube change should be performed quickly to reduce the chances of stoma collapse and patient discomfort and to minimize potential hypoxia time. The tube change is ideally performed 5 to 7 days after the initial open tracheostomy procedure or 7 to 10 days after a percutaneous approach. Some clinicians suggest changing the tube every 7 to 14 days after the initial tube exchange to prevent secretion buildup; others may recommend only changing the tube to downsize it as needed. The selection of a tracheostomy tube should be customized to the patient's specific condition, considering factors such as the anticipated weaning period, the initial reason for tracheotomy, and the nature of the secretions.
This procedure involves a 2-person technique: 1 individual supports the tube and the patient, while the other executes the change. For patients prone to aspiration, it is advisable to cease enteral feeding 3 to 4 hours before the procedure and aspirate the enteral tube immediately before the operation.
The approach to changing a tracheostomy tube depends on the circumstances surrounding the change. There are 2 commonly employed methods:
Guided Exchange Using a Tube Exchange Device
This method is typically necessary for early changes and in cases where there is a heightened risk of airway loss.
- Determine if the proper equipment is available and functional.
- Ensure the assistant understands what is expected of them during the procedure.
- Explain the procedure to the patient and obtain the patient's consent.
- Position the patient in a semi-recumbent position.
- Pre-oxygenate where required.
- Check and lubricate the tube.
- Ask the assistant to suction if required, remove the old dressing, inner cannula, tapes, and support the tube.
- Insert the exchange device into the length of the tube.
- Ask the assistant to deflate the cuff.
- Remove the old tube over the exchange device.
- Insert new tube over exchange device.
- Check for airflow through the tube and inflate the cuff.
- Remove the exchange device. Identify the presence of CO2 using a CO2 detector.
- Observe the site, swab if required, and clean while the assistant supports the tube.
- Dress the stoma and apply holder/ties.
- Replace the inner cannula.
- Ensure the patient is stable.
- Check cuff pressure.
- Document the procedure.
- Recheck the patient.
- If using a fenestrated tube, place a spare inner cannula in the emergency pack and clearly label the tube.
Blind Exchange Using an Obturator
This approach suits patients with well-formed stomas and a lower risk of airway loss.
- Determine if the proper equipment is available and functional.
- Ensure the assistant understands what is expected of them during the procedure.
- Explain the procedure to the patient and obtain the patient's consent.
- Position the patient in a semi-recumbent position.
- Pre-oxygenate where required.
- Check and lubricate the tube.
- Insert the obturator.
- Ask the assistant to suction if required, and remove the old dressing, inner cannula, tapes, and support tube.
- Deflate the cuff with the suction applied.
- Remove the tube on expiration.
- Insert the tube on expiration, remove the obturator, and inflate the cuff.
- Check for airflow through the tube. Identify the presence of CO2 using a CO2 detector.
- Ask the assistant to support the new tube.
- Dress and apply holder/ties.
- Replace the inner cannula when used.
- Check that the patient is stable.
- Check the cuff pressure.
- Document the procedure.
- Recheck the patient.
- If using a fenestrated tube, place a spare inner cannula in the emergency pack and clearly label the tube.
If the tube is unable to be reinserted successfully or the patient becomes compromised, the following steps should be taken:
- Maintain oxygenation via stoma and nose and mouth with a facemask.
- Use a tracheal dilator and attempt to reinsert the tube.
- Reposition the patient's neck and attempt to reinsert the tube.
- Consider using a smaller size tube.
- Orally intubate if needed.
- Call the on-call anesthesia provider, otorhinolaryngologist, or surgeon if appropriate to the situation to assist or orally intubate when needed.
Complications
Various studies have examined the complication rates associated with surgically performed tracheostomy compared to percutaneous procedures. In a study by Oliver et al in 2007, percutaneous tracheostomy was significantly quicker than open surgical tracheostomy, although it was associated with more early complications.[12] However, subsequent meta-analyses leaned towards favoring percutaneous tracheostomy despite similar complication rates. This preference is attributed to the shorter procedural duration and a reduced risk of infection associated with percutaneous techniques.[8][13][14]
Minor complications associated with tracheostomy include:
- Minor bleeding
- Infection
- Temporary oxygen desaturation
- Tube occlusion
- False passage
- Tracheocutaneous fistula
- Poor cosmetic scar
Major complications associated with tracheostomy include:
- Hemorrhage requiring transfusion due to tracheoinnominate fistula
- Tracheoesophageal fistula
- Subglottic stenosis
- Tracheal stenosis
- Tracheomalacia
- Death
Postoperative complications can vary depending on the timing and insertion technique. Early postoperative complications tend to arise in the first few days to weeks and include:
- Hemorrhage
- Postoperative tube dislodgement or obstruction
- Subcutaneous emphysema
- Soft tissue infection
- Pneumothorax
- Pneumomediastinum
Late complications occur more than 3 weeks after tracheostomy. They include:
- Tracheal stenosis or tracheomalacia
- Tube dislodgement or obstruction
- Equipment failure
- Tracheoinnominate fistula
- Tracheoesophageal fistula
- Infections, such as aspiration pneumonia
While rare, the most concerning complication of tracheostomy is the development of a tracheoinnominate fistula between the trachea and the innominate (brachiocephalic) artery. Its onset may be signaled by a sentinel bleed, characterized by relatively minor hemoptysis in approximately 30% of patients. It occurs 24 to 48 hours before the potential onset of life-threatening bleeding into the airway.[15] Typically, the innominate artery crosses the trachea between the sixth and ninth tracheal rings, below the usual placement of a traditional tracheostomy between the second and third rings. Factors such as placing the tracheostomy too low, inflating the cuff excessively (leading to necrosis), or a history of neck radiation can elevate the risk of fistula formation.
In the event of massive hemorrhage, if a tracheostomy tube cuff is present, it should be maximally inflated, and the tube should be pulled toward the sternum to exert pressure on the innominate artery. Suction should be administered as necessary to sustain the airway, and preparations for transfusion should commence while urgently contacting a cardiothoracic surgeon.
Although tracheostomy tube exchanges are routinely conducted, they come with inherent risks. Therefore, a thorough understanding of the procedure and preparation for potential issues is imperative. Many institutions have implemented protocols and guidelines to ensure smooth tracheostomy tube exchanges. Continuous education and quality improvement initiatives are integral in enhancing competence and minimizing complications, particularly tube obstruction-related complications. The primary cause of tube obstruction is often traced back to the lack of cleaning and maintenance of the inner cannula. The inner cannula, designed to capture secretions, becomes critical in emergencies, enabling rapid removal to reopen the airway while the inner cannula is cleaned or replaced.
The most frequently encountered complication during tracheostomy tube changes is tube displacement or forming a false passage.[16] The creation of a false passage, indicated by subcutaneous emphysema and subsequent respiratory distress, is more likely during the first tube change when the tract is not yet fully mature. It is recommended that the initial tube change be performed under direct visualization and preferably not sooner than 5 to 7 days after the tracheostomy is done.[17] Fortunately, the rate of accidental decannulation significantly decreased between 1985 and 2004, which was likely attributed to improved tracheostomy care.[18] If a false passage is suspected, prompt removal and careful tracheostomy tube replacement are essential. An airway exchange catheter may be used during tube changes to reduce the risk of displacement.[19]
Obstruction and accidental dislodgement of the tube seem to be more prevalent in obese patients, with dislodgement associated explicitly with elevated rates of morbidity and mortality. In the rare occurrence of this complication, following basic or advanced life support algorithms is crucial, and a code should be activated to bring in experienced personnel, typically anesthesiologists, surgeons, or otolaryngologists, to assist in re-establishing an airway. Attempting oral intubation may be considered to establish a definitive airway, especially in cases of respiratory arrest. However, using bag-valve masking may offer a more expedient way to provide oxygen to the patient.
Clinical Significance
Regular tracheostomy tube changes are crucial for patient care. This practice can decrease the risk of complications such as granulation tissue formation, bleeding, and infection. Competence and experience in tube exchange are essential to ensure the procedure is smooth, causing minimal discomfort to patients while maintaining a low risk of complications.
Enhancing Healthcare Team Outcomes
Tracheostomy tube changes demand a collective effort from healthcare professionals to enhance patient-centered care, outcomes, safety, and team performance. Physicians, including otolaryngologists, surgeons, anesthesiologists, and pulmonologists, set protocols, oversee training, and intervene in complex cases. Advanced practitioners give routine care, conduct assessments, educate patients, and collaborate across the healthcare team. Nurses contribute expertise in patient assessment, procedural care, and post-procedure monitoring for swift responses to complications. Pharmacists, leveraging their medication expertise, focus on drug-related aspects to enhance safety. Respiratory therapists or physiotherapists play a pivotal role in managing chest function, secretion clearance, and conducting pulmonary rehabilitation. Simultaneously, speech and language therapists are crucial during tube changes, influencing decisions on tube types and ensuring optimal airway management.
Effective interprofessional communication, facilitated by regular team meetings and structured handoffs, is crucial for information exchange. Care coordination ensures a seamless continuum, while ongoing education and simulation exercises refine skills and foster mutual understanding. This collaborative approach among diverse healthcare professionals ensures the success of tracheostomy tube changes, ultimately benefiting patient well-being, safety, and overall team performance.
Nursing, Allied Health, and Interprofessional Team Interventions
Study results have reported deficits in knowledge and comfort with tracheostomy care among healthcare providers.[27] Experience from the Texas Children’s Hospital found that a tracheostomy educational program significantly improved self-efficacy and knowledge scores.[28] To improve outcomes, members of the interprofessional tracheostomy team must be knowledgeable and comfortable with their roles.[25]
Evidence underscores the efficacy of a multidisciplinary, ward-based approach to tracheostomy care in minimizing complications and enhancing outcomes.[29] Regular weekly tracheostomy ward rounds, led by a physician (commonly an intensivist, pulmonologist, or otolaryngologist), a chest physiotherapist, a speech and language therapist, and a specialist nurse, ensure adherence to care standards, facilitate weaning or decannulation plans, and troubleshoot potential issues. Fundamental measures, including daily tracheostomy care checklists, humidification, suction clearance, stoma care, inner cannula care, oral care and swallowing, and cuff management, are routinely monitored by the interprofessional team to guarantee the safety and quality of tracheostomy care. Following tracheostomy tube changes, monitoring respiratory function and secretion management is crucial for early identification and prompt management of complications such as tube dislodgement or false passage creation. Competence and experience are paramount in tracheostomy care, especially in addressing emerging issues. The primary focus in caring for patients with tracheostomies is preventing tube obstruction.
Media
(Click Image to Enlarge)
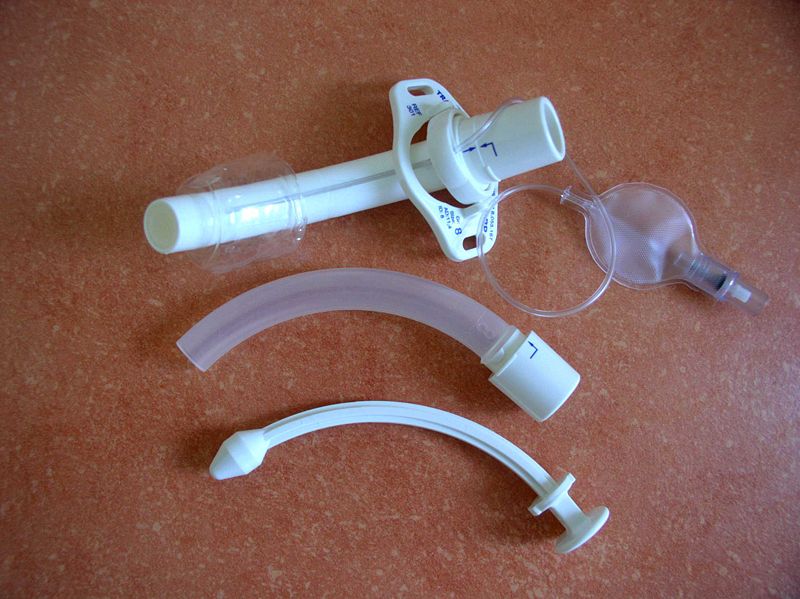
Cannula for Tracheostomy. An outer cannula (top item) with an inflatable cuff (top right), an inner cannula (center item), and an obturator (bottom item).
Klaus D Peter, Public Domain, via Wikimedia Commons
References
BORMAN J, DAVIDSON JT. A history of tracheostomy: si spiritum ducit vivit (Cicero). British journal of anaesthesia. 1963 Jun:35():388-90 [PubMed PMID: 14013998]
Sedvall G, Farde L, Nybäck H, Pauli S, Persson A, Savic I, Wiesel FA. Recent advances in psychiatric brain imaging. Acta radiologica. Supplementum. 1990:374():113-5 [PubMed PMID: 1966956]
Level 3 (low-level) evidenceFrost EA. Tracing the tracheostomy. The Annals of otology, rhinology, and laryngology. 1976 Sep-Oct:85(5 Pt.1):618-24 [PubMed PMID: 791052]
Level 3 (low-level) evidenceGarner JM, Shoemaker-Moyle M, Franzese CB. Adult outpatient tracheostomy care: practices and perspectives. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2007 Feb:136(2):301-6 [PubMed PMID: 17275559]
Level 3 (low-level) evidencePopovic JR. 1999 National Hospital Discharge Survey: annual summary with detailed diagnosis and procedure data. Vital and health statistics. Series 13, Data from the National Health Survey. 2001 Sep:(151):i-v, 1-206 [PubMed PMID: 11594088]
Level 3 (low-level) evidenceMehta AB, Syeda SN, Bajpayee L, Cooke CR, Walkey AJ, Wiener RS. Trends in Tracheostomy for Mechanically Ventilated Patients in the United States, 1993-2012. American journal of respiratory and critical care medicine. 2015 Aug 15:192(4):446-54. doi: 10.1164/rccm.201502-0239OC. Epub [PubMed PMID: 25955332]
Higgins KM, Punthakee X. Meta-analysis comparison of open versus percutaneous tracheostomy. The Laryngoscope. 2007 Mar:117(3):447-54 [PubMed PMID: 17334304]
Level 1 (high-level) evidenceJohnson-Obaseki S, Veljkovic A, Javidnia H. Complication rates of open surgical versus percutaneous tracheostomy in critically ill patients. The Laryngoscope. 2016 Nov:126(11):2459-2467. doi: 10.1002/lary.26019. Epub 2016 Apr 14 [PubMed PMID: 27075530]
Higgins D. Basic nursing principles of caring for patients with a tracheostomy. Nursing times. 2009 Jan 27-Feb 2:105(3):14-5 [PubMed PMID: 19248371]
Watters KF. Tracheostomy in Infants and Children. Respiratory care. 2017 Jun:62(6):799-825. doi: 10.4187/respcare.05366. Epub [PubMed PMID: 28546379]
Yaremchuk K. Regular tracheostomy tube changes to prevent formation of granulation tissue. The Laryngoscope. 2003 Jan:113(1):1-10 [PubMed PMID: 12514373]
Level 2 (mid-level) evidenceOliver ER, Gist A, Gillespie MB. Percutaneous versus surgical tracheotomy: an updated meta-analysis. The Laryngoscope. 2007 Sep:117(9):1570-5 [PubMed PMID: 17667139]
Level 1 (high-level) evidenceIftikhar IH, Teng S, Schimmel M, Duran C, Sardi A, Islam S. A Network Comparative Meta-analysis of Percutaneous Dilatational Tracheostomies Using Anatomic Landmarks, Bronchoscopic, and Ultrasound Guidance Versus Open Surgical Tracheostomy. Lung. 2019 Jun:197(3):267-275. doi: 10.1007/s00408-019-00230-7. Epub 2019 Apr 24 [PubMed PMID: 31020401]
Level 2 (mid-level) evidenceYaghoobi S, Kayalha H, Ghafouri R, Yazdi Z, Khezri MB. Comparison of complications in percutaneous dilatational tracheostomy versus surgical tracheostomy. Global journal of health science. 2014 Apr 20:6(4):221-5. doi: 10.5539/gjhs.v6n4p221. Epub 2014 Apr 20 [PubMed PMID: 24999127]
Level 1 (high-level) evidenceSaleem T, Anjum F, Baril DT. Tracheo Innominate Artery Fistula. StatPearls. 2024 Jan:(): [PubMed PMID: 29494111]
Mirza S, Cameron DS. The tracheostomy tube change: a review of techniques. Hospital medicine (London, England : 1998). 2001 Mar:62(3):158-63 [PubMed PMID: 11291466]
Bontempo LJ, Manning SL. Tracheostomy Emergencies. Emergency medicine clinics of North America. 2019 Feb:37(1):109-119. doi: 10.1016/j.emc.2018.09.010. Epub [PubMed PMID: 30454773]
Dal'Astra AP, Quirino AV, Caixêta JA, Avelino MA. Tracheostomy in childhood: review of the literature on complications and mortality over the last three decades. Brazilian journal of otorhinolaryngology. 2017 Mar-Apr:83(2):207-214. doi: 10.1016/j.bjorl.2016.04.005. Epub 2016 May 6 [PubMed PMID: 27256033]
Patiño MA, Truong DT, Truong A, Cata JP. Do Not Burn Your Airway Bridge: A Technique to Safely Exchange a Tracheostomy Tube for a Tracheal Tube. A & A case reports. 2016 Oct 1:7(7):155-7. doi: 10.1213/XAA.0000000000000368. Epub [PubMed PMID: 27467904]
Tabaee A, Lando T, Rickert S, Stewart MG, Kuhel WI. Practice patterns, safety, and rationale for tracheostomy tube changes: a survey of otolaryngology training programs. The Laryngoscope. 2007 Apr:117(4):573-6 [PubMed PMID: 17415123]
Level 3 (low-level) evidenceMcGrath BA, Lynch J, Bonvento B, Wallace S, Poole V, Farrell A, Diaz C, Khwaja S, Roberson DW. Evaluating the quality improvement impact of the Global Tracheostomy Collaborative in four diverse NHS hospitals. BMJ quality improvement reports. 2017:6(1):. pii: bmjqir.u220636.w7996. doi: 10.1136/bmjquality.u220636.w7996. Epub 2017 May 23 [PubMed PMID: 28607676]
Level 2 (mid-level) evidenceLavin J, Shah R, Greenlick H, Gaudreau P, Bedwell J. The Global Tracheostomy Collaborative: one institution's experience with a new quality improvement initiative. International journal of pediatric otorhinolaryngology. 2016 Jan:80():106-8. doi: 10.1016/j.ijporl.2015.11.024. Epub 2015 Nov 30 [PubMed PMID: 26746621]
Level 2 (mid-level) evidenceGarrubba M, Turner T, Grieveson C. Multidisciplinary care for tracheostomy patients: a systematic review. Critical care (London, England). 2009:13(6):R177. doi: 10.1186/cc8159. Epub 2009 Nov 6 [PubMed PMID: 19895690]
Level 1 (high-level) evidenceMirski MA, Pandian V, Bhatti N, Haut E, Feller-Kopman D, Morad A, Haider A, Schiavi A, Efron D, Ulatowski J, Yarmus L, Stevens KA, Miller CA, Papangelou A, Vaswani R, Kalmar C, Gupta S, Intihar P, Mack S, Rushing AP, Chi A, Roberts VJ Jr. Safety, efficiency, and cost-effectiveness of a multidisciplinary percutaneous tracheostomy program. Critical care medicine. 2012 Jun:40(6):1827-34. doi: 10.1097/CCM.0b013e31824e16af. Epub [PubMed PMID: 22610187]
Level 2 (mid-level) evidenceBonvento B, Wallace S, Lynch J, Coe B, McGrath BA. Role of the multidisciplinary team in the care of the tracheostomy patient. Journal of multidisciplinary healthcare. 2017:10():391-398. doi: 10.2147/JMDH.S118419. Epub 2017 Oct 11 [PubMed PMID: 29066907]
Swords C, Manji A, Ward E, Arora A. A pilot study on the provision of tracheostomy healthcare and patient engagement in quality improvement measures: a global perspective. The Journal of laryngology and otology. 2018 Dec:132(12):1093-1096. doi: 10.1017/S0022215118002177. Epub [PubMed PMID: 30674362]
Level 2 (mid-level) evidenceDorton LH, Lintzenich CR, Evans AK. Simulation model for tracheotomy education for primary health-care providers. The Annals of otology, rhinology, and laryngology. 2014 Jan:123(1):11-8. doi: 10.1177/0003489414521144. Epub [PubMed PMID: 24574418]
Level 2 (mid-level) evidenceBedwell JR, Pandian V, Roberson DW, McGrath BA, Cameron TS, Brenner MJ. Multidisciplinary Tracheostomy Care: How Collaboratives Drive Quality Improvement. Otolaryngologic clinics of North America. 2019 Feb:52(1):135-147. doi: 10.1016/j.otc.2018.08.006. Epub 2018 Oct 5 [PubMed PMID: 30297183]
Level 2 (mid-level) evidenceSpeed L, Harding KE. Tracheostomy teams reduce total tracheostomy time and increase speaking valve use: a systematic review and meta-analysis. Journal of critical care. 2013 Apr:28(2):216.e1-10. doi: 10.1016/j.jcrc.2012.05.005. Epub 2012 Aug 27 [PubMed PMID: 22951017]
Level 1 (high-level) evidence