Introduction
Typhoid fever and paratyphoid fever are clinically indistinguishable febrile multisystemic illnesses caused by Salmonella enterica serotypes Typhi (S Typhi) and Paratyphi (S Paratyphi) A, B, and C. Collectively known as enteric fever, more than 9 million people are sickened, and 110,000 die from the disease every year around the globe.[WHO. Typhoid Fact Sheet. 2023] Enteric fever is the leading cause of community-acquired bloodstream infections in South and Southeast Asia.[1] A reportable disease in the United States and many other developed nations, enteric fever is second only to malaria as a cause of severe and sometimes life-threatening infection in travelers.[2]
Following an incubation period of 6 to 30 days, enteric fever presents insidiously with the gradual onset of fever with fatigue, anorexia, headache, malaise, and abdominal symptoms. If treatment is delayed or inadequate, meningitis, sepsis, or intestinal perforation can occur. With a history of S Typhi and S Paratyphi strains rapidly developing antimicrobial resistance with the widespread use of successive antibiotics, the recent emergence of extensively drug-resistant strains has greatly complicated treatment and raised alarms.
S Typhi and S Paratyphi are said to spread by the "4 Fs" (flies, fingers, feces, and fomites). They afflict people living or traveling in low- and middle-income countries around the globe that lack clean water, adequate sanitation, and hygiene, known collectively as WASH. Improved WASH infrastructure is the foundation for decreasing the incidence of enteric fever and other diseases spread via the fecal-oral route.
Historically, enteric fever has received less investment and attention than the "big 3" (human immunodeficiency virus/acquired immunodeficiency syndrome, tuberculosis, and malaria). However, with the specter of untreatable variants on the horizon, enteric fever control efforts have been renewed. Recently developed typhoid conjugate vaccines, improved surveillance and understanding of antimicrobial resistance patterns, and WASH initiatives have decreased the disease burden.
This activity covers the epidemiology, pathophysiology, treatment, management, complications, patient education, prevention measures, and the role of the interprofessional team in improving patient care and decreasing the burden of this disease. While several barriers to controlling this disease exist, recent advancements provide hope that the impact of enteric fevers can be limited or eliminated in the future.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Like other members of the Enterobacteriaceae family, Salmonellae are gram-negative, acid-fast, facultative anaerobic bacillae. Salmonella comprises 2 main species, Salmonella enterica and Salmonella bongori (prev S enterica subspecies V). S enterica, in turn, comprises 6 subspecies, of which S enterica subsp enterica has the most serovars (or serotypes) and is the most important to human infection.[1] This includes S Typhi and S Paratyphi, with the complete scientific names Salmonella enterica subspecies enterica Typhi and Salmonella enterica subspecies enterica Paratyphi, respectively. Unlike many other Salmonella species, S Typhi and S Paratyphi are exclusively human pathogens.[CDC. Yellow Book. 2024] Other species within the genus Salmonella are collectively known as nontyphoidal salmonella (NTS), many of which are pathogenic to humans or other animals. NTS infections are mainly limited to gastroenteritis in humans, although invasive nontyphoidal serovars (iNTS) exist.
S Typhi and S Paratyphi serovars have been classified by phage typing since the early 1900s within an established, continuously evolving taxonomic structure as new variants arise.[2] With the advent of genetic techniques, enteric fever classification is increasingly based on genotypes for research, surveillance, and clinical purposes. Genotyping improves the ability to identify and track outbreaks and antimicrobial resistance and monitor for emerging antimicrobial resistance mechanisms. When combined with emerging clinical antimicrobial susceptibility patterns, this provides additional guidance for empiric therapy. Single nucleotide variants are used to assign S Typhi genomes into the GenoTyphi scheme, comparable to existing phenotypic taxonomic structures.[3]
See StatPearls' companion article "Salmonella" for more information on the spectrum of Salmonella bacteria and illness.
Epidemiology
Transmission
Transmission is primarily through the fecal-oral route, via consumption of food or water contaminated with the feces of a convalescent or chronic asymptomatic carrier. The importance of chronic carriage to ongoing transmission is well documented in non-endemic countries; however, the role in endemic countries is not well understood. Sexual transmission has rarely been documented among men who have sex with men.[CDC. Yellow Book. 2024] S Typhi and S Paratyphi can exist in the environment for a prolonged period in a nonculturable, nonreplicative state. This creates a persistent environmental reservoir that can lead to infection and outbreaks, such as via contaminated crops.[2]
Risk Factors
Different factors determine the risk of enteric fever in endemic versus non-endemic countries. In non-endemic countries, the acquisition of enteric fever is related to travel, contact with a traveler from an endemic country, or exposure to food prepared by a chronic carrier. In contrast, risk factors in endemic countries include individual host factors, environmental exposures, and climate and geographic factors.
Individual host factors
On average, the incidence of enteric fever peaks between the ages of 5 and 9. However, this masks great variability in the age of onset in different locations, with a younger peak age incidence correlating to a higher prevalence of enteric fever. In very high prevalence areas, peak incidence may occur in infants due to increased exposures and the greater accumulated immunity acquired with repeat clinical, subclinical, or asymptomatic infections as people age. Reinfections demonstrate that only a moderate level of protection is conferred by clinical infection.[2]
Typhoid fever is more severe in debilitated and immunocompromised patients such as those with human immunodeficiency virus, on glucocorticoid therapy, or with altered phagocyte function (eg, patients with malaria and sickle cell anemia). Salmonellae are most commonly acid-sensitive bacteria and are destroyed in the stomach by gastric acid unless a large dose is ingested.[4] Achlorhydria and intake of antacids and antihistamines increase susceptibility to infection with smaller doses.
Normal flora of the gut is protective against the infection. The use of broad-spectrum antibiotics that destroy the normal flora allows increased invasion. Malnutrition decreases normal gut flora and also increases the susceptibility to infection.[5] Human genetics likely plays a role in susceptibility to typhoid, with a marker mapping to the HLA class II region strongly associated with enteric fever resistance.[2]
Environmental exposures
A meta-analysis demonstrated that having an improved water source with a protected well can reduce the risk of culture-confirmed typhoid infection by half.[6] Having surface water as a water source doubles the risk of typhoid fever compared to other unimproved water sources, such as an unprotected well or spring. Using any household water treatment reduces the risk compared to no treatment. Using metal lids and keeping water containers covered is associated with an 80% lower odds of typhoid infection than keeping water in open containers, while using dirty containers doubles the risk.
People using unimproved pit latrines had 50 times the risk of enteric fever than those having limited, basic, or safely managed sanitation facilities. Those defecating in the open had 1.2 times the risk. A lack of a handwashing facility with soap and water at home increases the odds by 2.3 compared to those without such facilities.[6] Independent of vaccination, living in a house with better WASH facilities significantly decreases the risk of enteric fever.
Climatic and geographic factors
A distinct seasonal pattern of enteric fever incidence exists globally, with variability accentuated further from the equator. Similarly, a higher incidence of enteric fever occurs with increased temperatures. Between 11° and 35° N, previous rainfall events are positively associated with the incidence of enteric fever. Climate change will likely increase the incidence of enteric fever and other gastrointestinal illnesses via increased flooding, drought, and temperatures, compromising food and water safety.[2]
Burden of Disease
Enteric fever is a prime example of the "infectious divide" between high-income countries (gross national income per capita in 2021 of >$12,695) and those with low and middle incomes (≤$1046 and >$1047-$12,695, respectively).[7] In high-income countries, where improvements to sanitation started in the early 1900s, enteric fever is now seen primarily in travelers returning from endemic areas. Incidence remains high in many low- and middle-income countries with poor WASH.
Due to diagnostic challenges, underdeveloped surveillance systems, and the lack of access to universal health care in many areas, the true incidence of S Typhi and S Paratyphi in much of the world can only be estimated. The 2019 Global Burden of Disease Study models rates using existing epidemiological data and case counts from population-based studies.[8] The vast majority of enteric fever cases worldwide are due to S Typhi. In 2019, an estimated 9.2 million (95% CI 5.9-14.1) typhoid fever cases and 110,000 (95% confidence interval; 53,000-191,000) deaths occurred worldwide. This compares to 11 to 21 million cases and 148,000 to 161,000 deaths in 2015.[8] While S Typhi comprises the majority of enteric fever cases worldwide, the percentage of cases due to S Paratyphi varies widely across regions. S Paratyphi accounts for about 25% of all enteric fever cases in South Asia.[2] Approximately 3.8 million S Paratyphi infections occurred globally in 2019.[8]
High-Income Countries
As an example of a high-income country, about 400 cases of typhoid fever and 50 to 100 cases of paratyphoid fever were reported each year in the United States from 2016 to 2018. Of these, 85% and 92% are attributed to international travel, with the remainder due to local transmission.[CDC. Yellow Book. 2024] Fewer cases were reported in 2020, likely reflecting decreased travel due to the coronavirus pandemic.[CDC. Typhoid and Paratyphoid Surveillance. 2020] The median age was 26 and 24 years for typhoid and paratyphoid cases, respectively. No deaths were reported, although about 82% of cases for which data is available were hospitalized.
In 76% of cases, international travel was reported where a travel history was available. More than 75% of those with a single destination identified travel to India or Pakistan. The most common reason for international travel was to visit friends and relatives (VFR), with 48% of S Typhi infections and 64% of S Paratyphi infections reporting VFR. Just under 6% of people with S Typhi reported receiving the typhoid fever vaccine in the previous 5 years.[CDC. Typhoid and Paratyphoid Surveillance. 2020] Longer travel duration is associated with a higher incidence of infection, although infection can occur even during short visits of less than 1 week to highly endemic areas.[CDC. Yellow Book. 2024]
Low- and Middle-Income Countries
The 2019 Global Burden of Disease Report identifies 44 countries with a high estimated typhoid burden (≥100 cases/100,000 persons/year).[8] The highest estimated incidences of enteric fever occur in Southeast Asia, Eastern Mediterranean (187 cases/100,000 persons/year), and African World Health Organization superregions (respectively 306, 187, and 111 cases/100,000 persons/year).[8] Together, typhoid and paratyphoid were the 18th leading causes of disability-adjusted life-years (DALYs) globally for children aged 0 to 9 years and 14th for those aged 10 to 24 years in 2019. While typhoid and paratyphoid fever represent a greater proportion of all DALYs in 2019 than in 1990, the age-standardized rates for DALYs declined 51% and 16%, respectively, since 1990.[8] In South and Southeast Asia, S Typhi is the leading cause of nonhospital acquired bloodstream infections.[2]
Outbreaks are an additional signal of disease burden. The US Centers for Disease Control (CDC) Global Disease Detection Operation Center reported 7 confirmed outbreaks globally between 2017 and 2022, with 14,056 becoming ill in the Philipines in 2022; 1312, 3187, 7134 people affected in 3 separate outbreaks in Zimbabwe in 2017 and 2018; 14,894 sickened with XDR Typhi in Pakistan in 2018 and 2019; and 23 infections occurring in 1 building in China in 2022.[7]
Antimicrobial Resistance
In low- to middle-income countries, antimicrobial resistance patterns often follow the pattern of antibiotic use and the acquisition and spread of mutations. In high-income countries where travel is the primary risk factor for the acquisition of enteric fever, the prevalence and the pattern of drug resistance among isolates reflect the destination countries for travel. In the United States in 2020, 99% of isolates had a decreased susceptibility to fluoroquinolones. None were multiple drug resistant (MDR), and none were resistant to ceftriaxone, although extremely drug resistant (XDR) cases have previously been reported.[CDC. Typhoid and Paratyphoid Surveillance. 2020] Up-to-date information on typhoid and paratyphoid resistance patterns in the United States can be found on the CDC's National Antimicrobial Resistance Monitoring System website.
Multidrug Resistance
Worldwide, an estimated 2.5 million MDR S Typhi infections and 7.4 million fluoroquinolone non-susceptibility (FQNS) S Typhi infections occurred in 2019.[9] MDR strains are common in sub-Saharan Africa, increasing from an estimated 6% of all typhoid strains in 1990 to 72.7% in 2019.[9] The rate of MDR typhoid is declining in South and Southeast Asia, comprising 55.4% of all typhoid infections in South Asia in 1990 and 26.4% in 2019.[9] Rates of MDR are also more variable in Asia than in Africa, ranging from an estimated 3% in India and Nepal to 68% in Pakistan and 76% in Cambodia.[3] The Southeast Asian, East Asian, and Oceania superregion has a lower overall prevalence of MDR typhoid compared to South Asia, declining from an estimated 19.0% to 4.7% between 1990 and 2019.[9] Northern Africa and the Middle East have shown a stable rate in MDR, with little change from the 19.4% estimated in 1990.[9] MDR Typhi is virtually absent in Micronesia and Latin America.[3] Globally, the prevalence of MDR in S Paratyphi strains was an estimated 9.2% in 1990, while in 2019, it was 0.2%. FQNS was present in 0.6% of S Paratyphi in 1990 and 95.0% in 2019.[9]
Fluoroquinolone Non-Susceptibility
Nonsusceptibility to fluoroquinolones is widespread among S Typhi strains. Globally, 1.1% of strains were estimated to be FQNS in 1995.[9] Rates have increased since then, although they vary highly across countries and regions. The estimated prevalence of FQNS strains in South Asia overall was 95.2% in 2019, while in the superregion comprising Southeast Asia, East Asia, and Oceania, it was 36.4%. In 2019, Pakistan had an estimated rate of 99.1% FQNS, while in Indonesia, it was 10.4%.[9]
The increase in FQNS started later in Africa than in Asia, and the prevalence continues to be lower. In 2019, rates of FQNS in Africa were variable across regions and countries, often reflecting travel interconnections with Southeast Asia.[10] In sub-Saharan Africa, 24 of 38 countries had a prevalence of FQNS greater than 15%. In North Africa and the Middle East, the overall rate of FQNS was estimated to be 35.0%.[9] FQNS is emerging in some areas of Latin America, such as Chile.[3]
Extensive Drug Resistance
In Pakistan, about 70% of all isolates were XDR in 2020, with resistance to chloramphenicol, ampicillin, co-trimoxazole, ciprofloxacin, and 3rd generation cephalosporins.[3] While large epidemiological studies have not determined XDR strains to be widespread in other countries, this must be interpreted cautiously, as modeling studies derive estimates based on a fraction of total cases.[3]
In 2019, 16 countries were known to have imported cases of XDR typhoid.[10] Diagnosis of XDR S Typhi cases in people without a history of travel to Pakistan indicates a wider spread than can be detected by surveillance systems. For example, in the UK, XDR S Typhi infection in a traveler to India indicates XDR has been introduced at least once. Nine XDR typhoid cases without travel outside the United States were identified between November 2019 and October 2020; the S Typhi genotype matched those circulating in Pakistan.[11] In 2022, 23 cases were linked to an apartment building in Beijing.[10]
Countries that have a high incidence of typhoid and extensive travel with Pakistan have an extremely high risk of XDR transmission. For example, Afghanistan has one of the highest estimated typhoid fever burdens globally and was the destination for 3.37 million trips in 2021.[10] While Saudi Arabia and the United Arab Emirates have reported no or 1 case of XDR typhoid, this likely reflects low detection and reporting of cases. A high rate of travel exists between these highly populated Gulf States and Pakistan, yet reports far fewer cases than in countries with fewer travel connections (eg, the United States, Ireland, and the United Kingdom).[10] Note that cephalosporin resistance also occurs in strains without other antimicrobial resistance. Levels of cephalosporin resistance were low in all endemic countries except for Pakistan.[3]
Pathophysiology
The pathophysiologies of S Typhi and S Paratyphi infections have not been fully elucidated, mainly due to the complexity of pathogenic mechanisms and the restriction of infection to humans. S Typhimurium infection in a mouse model and controlled human challenge studies (CHIM) have significantly contributed to the current understanding of enteric fever pathogenesis. In the CHIM studies, S Paratyphi requires a 10 times lower dose of bacteria than S Typhi (1000 cf 10,000 cfu) to induce clinical infection in 60% and 67% of volunteers, respectively.[2] The median onset of symptoms is 8 days. Bacteremia is longer in S Paratyphi than in S Typhi, and asymptomatic infection was more common.
S Typhi and S Paratyphi have diverse mechanisms to address the critical tasks in their pathogenesis and survival: evasion of stomach acidity, invasion of the intestinal epithelium, dissemination and intracellular survival, excretion and transmission, and the development of antimicrobial resistance. Virulence genes are carried in Salmonella pathogenicity islands, chromosomal elements acquired through horizontal transmission from other pathogenic bacteria. To date, 15 Salmonella pathogenicity islands have been identified, carrying genes affecting pathogen survival, virulence, adhesion, evasion of host defenses, cellular death, proinflammatory mediators, bacterial multiplication, and others.[4]
The Vi antigen is well known as an exopolysaccharide capsule possessed by S Typhi, along with several other Salmonella and non-Salmonella spp. The target of modern conjugate vaccines, Vi is considered important to S Typhi pathogenesis; however, the precise role remains unclear, notably as S Paratyphi lacks a Vi capsule and yet produces a clinically indistinguishable illness. S Typhi and S Paratyphi do not share any other unique virulence factors. Moreover, Vi antigen-negative S Typhi bacteria have also been identified.[4]
Ingestion and Invasion
Following ingestion, food and beverages act as buffers against gastric acid, facilitating bacteria reaching the small bowel.[5] S Typhi and S Paratyphi then rapidly cross the gut epithelium. Invasion likely occurs via several routes as with other Salmonellae: transcellular or by direct invasion of enterocytes and the M cells that overlie Peyer patches. In contrast to noninvasive salmonella, invasion causes a minimal inflammatory response. In S Typhi, this is mediated by a protein that downregulates flagellin (also known as flagellar H antigen), which is associated with inflammation and upregulates Vi production.[4]
Initial Dissemination
Intracellular dissemination occurs during the asymptomatic incubation period of enteric fever and is of primary importance in its pathogenesis. Two-thirds of the S Typhi or S Paratyphi load during an infection is estimated to be intracellular.[6] Intracellular dissemination occurs via CD18 cells of the reticuloendothelial system, including macrophages, dendritic cells, polymorphonuclear monocytes, and phagocytes.[4] Invasive Salmonellae can live intracellularly by forming a modified phagosome that does not allow normal fusion with the cell's phagocyte oxidase complex.[2] In S Typhi infection, the Vi antigen capsule is thought to play a role. The intracellular nature of the bacteria safeguards against extracellular antibiotics, limiting the available options for treatment.[7]
A transient primary bacteremia, detectable by the presence of bacterial deoxyribonucleic acid, occurs within the first 24 hours of ingestion, possibly coinciding with this bacterial dissemination.[4] A systemic cytokine response occurs, whether or not systemic illness ensues. The eosinophil count begins to drop 5 days before symptoms develop.[2]
Clinical Illness
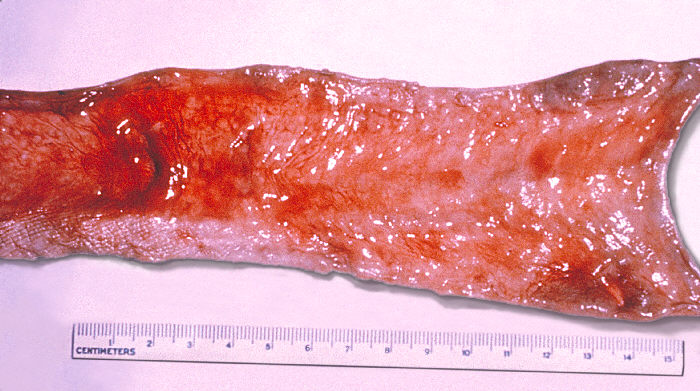
Increasing fever begins with the persistent secondary bacteremia of established infection.[4] The gallbladder is colonized through hematogenous or local spread, more commonly if gallstones or structural abnormalities are present (see Image. Gallbladder Affected by Typhoid Fever). Lymphoid tissue within Peyer patches is a site of primary infection, reinfection, and chronic infection, becoming a secondary source for fecal excretion and transmission. The proliferation of lymphoid tissue may cause constipation. Endotoxin-mediated necrosis may occur, resulting in intestinal bleeding, perforation, or tertiary bacteremia with enteric microorganisms.[4] The total white count, lymphocytes, platelets, and neutrophils begin to drop with the onset of symptoms. Immunoglobulin (Ig) IgG, IgM, and IgA antibodies develop against flagellin and lipopolysaccharide in those who develop clinical disease but not against Vi.[2]
Host Immunity
CHIM studies demonstrate that immunity to S Typhi and S Paratyphi is incomplete following clinical infection. If challenged with the same organism an average of 19 months following the initial S Typhi or S Paratyphi infection, prior infection reduced the risk of clinical illness by 36% and 57%, respectively.[2] No symptoms or clinical severity change was seen in those previously challenged. Likewise, there was no reduction in risk if the second challenge was with the alternate organism.
Antimicrobial Resistance
Antimicrobial resistance has been a significant threat to the control of enteric fever since the advent of antibiotic treatment. Multidrug resistance to all 3 first-line drugs, ampicillin, chloramphenicol, and co-trimoxazole, was first identified in 1972 and became common by the 1980s. The accumulated resistance genes of MDR strains are encoded on a large conjugative (self-transmissible) plasmid.[3]
Estimated to have originated in South Asia in the mid-1980s, H58 isolates are thought to have spread widely due to plasmid-encoded MDR. More susceptible than other S Typhi genotypes to acquiring mutations or mobile genetic elements encoding resistance determinants, H58 (renamed clade 4.3.1, along with its derived genotypes) strains are now found throughout Asia and Southern Africa.[3] With the decreased use of the original first-line antibiotics, the reproductive fitness cost of maintaining the MDR-encoding plasmid has led to its lower prevalence in most of Asia. However, the plasmid has been integrated into the Typhi chromosome several times, resulting in the fixation of the MDR phenotype in multiple lineages found mainly in Pakistan and East Africa.
Fluoroquinolone nonsusceptibility, or FQNS, arose as the use of these antibiotics subsequently became more common.[8] Genomic studies have shown a variety of mechanisms that confer FQNS:
- The stepwise accumulation of 1 to 3 mutations in core chromosomal genes that directly impact fluoroquinolone binding (QRDR mutations).
- The presence of plasma-mediated quinolone resistance (PMQR) genes in strains already carrying a QRDR mutation.
- Or this could include a combination of the above.
A wide variety of QRDR mutations and PMQR plasmids confer fluoroquinolone resistance. Core chromosomal mutations have arisen independently in at least 80 different strains around the globe, more commonly in H58 strains.[3] The number of mutations and the presence of a PMQR plasmid correlate with the degree of resistance. For example, 1 mutation may only result in a small increase in minimal inhibitory concentration (MIC) and minimal clinical impact. In contrast, those strains with 3 QRDR mutations or a PMQR plasmid and 1 or more PMQR mutations may have a MIC of 1 mg/L or more of ciprofloxacin, denoting a high degree of resistance. Genomic studies have used the term fluoroquinolone-resistant to describe these highly nonsusceptible strains.[3]
Most common strains carry genes that confer nonsusceptibility across a wide range of cephalosporins. However, different mutations may confer differing levels of non-susceptibility to other cephalosporins. As such, the utility of nalidixic acid in determining fluoroquinolone resistance levels has been challenged.[6] Third-generation cephalosporins (eg, ceftriaxone and cefixime) and azithromycin increased in use following the emergence of ciprofloxacin resistance. Azithromycin resistance has emerged primarily in Bangladesh, with at least 13 independent events resulting in 13 different genotypes, each having 1 to 3 mutations. The gene for extended beta-lactamase resistance has migrated to the chromosome in some strains.[3] An XDR strain of Typhi arose in Hyderabad, Pakistan, in 2016. This is a typical combination of chromosomal MDR genes, IncY plasmid-mediated ciprofloxacin, and extended beta-lactamase resistance. The XDR strain has since spread throughout Pakistan and neighboring countries.[3]
History and Physical
Typhoid and paratyphoid present with febrile, nonspecific illnesses indistinguishable from each other, as well as other febrile illnesses in travelers and endemic areas. The history and physical exam seek to confirm or exclude risk factors, signs, and symptoms of typhoid, paratyphoid, malaria, meningitis, dengue, or other febrile illnesses that the patient may be at risk of depending on geographic and other exposure risks.
History
After an incubation period of 10 to 14 days (range 6-30), enteric fever has an insidious onset, with a stepwise increase in fever and fatigue, reaching 38 to 40 °C by the third or fourth day of illness.[CDC. Yellow Book. 2024] The fever is often lowest in the morning and peaks in the afternoon or evening. Travelers may have a more abrupt onset of fever than people who live in endemic areas.[6] Anorexia, headache, and malaise are nearly universal, and abdominal symptoms such as pain, bloating, constipation, and diarrhea are common. Diarrhea ranges from mild to severe, with or without blood. Dry cough, myalgias, and sore throat may also be present.[2][9]
Children present with diarrhea, vomiting, febrile seizures, or other neurological symptoms more often than adults. Immunocompromised patients with human immunodeficiency virus, particularly those with low CD4 counts, more commonly present with severe diarrhea and tend to have more serious metastatic infections.[6][10]
The history must include the onset of symptoms, progression of illness, and a full review of symptoms. Symptoms that could indicate severe illness include an altered level of consciousness, severe abdominal pain exacerbated by movement, or severe bloody diarrhea. Pale stool and dark urine may signal hepatitis or biliary complications, while chest pain may be associated with myocarditis or pericarditis. Pancreatitis may present with severe epigastric pain radiating to the back. Osteomyelitis is associated with bone pain. Symptoms of abscesses will depend on their location in the body.[11]
A travel history is essential to confirm risks for enteric fever, exclude other infectious diseases in the differential diagnosis, and guide empiric treatment. This should include the following:
- History of residence in or travel to endemic and outbreak areas in the previous 30 days: departure date, time since departure from a risk area
- Type of travel: work-related, extreme sport, immigration, volunteer, missionary
- Potential exposures for infectious diseases: drinking potentially contaminated water, eating undercooked or poorly prepared food, animal contact, insect bites, sexual contact, medical care, drug use, or poor accommodations [12][CDC. Yellow Book. 2024]
Past medical history may indicate risks for complications or more severe illness. Other factors to consider are immunization history, socioeconomic status, and any previous or ongoing treatment. This includes prior antibiotics or malaria chemoprophylaxis, including the dose and interval of the medication. Patients should be asked whether there are other ill individuals in their household or with whom they traveled. For treatment purposes, the WHO classifies mild disease as not critically ill with no signs of intestinal perforation, peritonitis, or septic shock. Severe illness is defined as those who have confirmed or suspected intestinal perforation, peritonitis, sepsis, or septic shock.[WHO. Aware. 2022]
Physical Exam
Physical examination findings in enteric fever can be nonspecific. Initially, patients may look pale, lethargic, and dehydrated. If the disease progresses untreated, patients may appear toxic and with notable weight loss. Rose spots, such as blanching and 2 to 4 mm erythematous maculopapular lesions, are present in less than a quarter of enteric fever patients (see Image. Rose Spots on Chest, Patient with Typhoid Fever), primarily over the chest and abdomen.[13] They are very difficult to see in people with darker skin tones. Some patients have jaundice with yellowish skin and sclera with more severe disease. Pulmonary and cardiac exams should seek signs of pneumonitis, pulmonary abscess, or myocarditis, such as tachypnea, crackles over the lung base, or cardiac rubs. Relative bradycardia may accompany the fever in the first week but is neither sensitive nor specific for typhoid. On abdominal exam, hepatosplenomegaly may be found in 29% to 50% of cases.[2] While diffuse abdominal distension and tenderness are common, rebound tenderness, rigidity, and guarding of the abdomen later in the illness indicate intestinal bleeding or perforation.
Evaluation
A timely and accurate diagnosis of enteric fever is important to minimize complications, hospitalizations, and death. However, laboratory confirmation in the first week of illness is difficult due to the poor performance of available laboratory testing methodologies. The development of rapid multiplex diagnostic tests with improved sensitivity and specificity is urgently needed.[9]
The initial workup must include ruling out other potentially critical causes of undifferentiated fever as appropriate, such as malaria and meningitis. Without another obvious diagnosis, a fever on at least 3 of 7 days is sufficient to suspect enteric fever and initiate treatment for anyone living in an endemic area, traveling from an endemic area within 28 days, or being a household contact within 28 days of someone who is a confirmed case of typhoid (acute, convalescent, or chronic).[WHO. Aware. 2022] Based on this broad definition of a suspect case, a diversity of undifferentiated febrile illnesses are unavoidably treated as enteric fever in endemic countries.
Confirmation of typhoid or paratyphoid fever requires S Typhi or S Paratyphi bacteria or deoxyribonucleic acid from the organism to be isolated from a normally sterile site by culture or molecular test, respectively. Laboratory confirmation should be obtained wherever possible. In resource-constrained settings, the WHO's AWaRe (Access, Watch, Reserve) antibiotic book does not deem laboratory diagnosis necessary in uncomplicated cases of enteric fever.[WHO. Aware. 2022]
Culture
The culture of S Typhi or S Paratyphi from a normally sterile site, usually the blood or bone marrow, is the gold standard for diagnosing enteric fever. Blood or bone marrow culture is 100% specific and essential to determining antimicrobial susceptibility and the appropriateness of ongoing treatment. However, expense and limited technical capacity worldwide limit the widespread use of blood or bone marrow culture in endemic countries.
Low sensitivity and long lag time further limit the use of culture to diagnose enteric fever. The preferred and most common culture method, a single blood culture, is positive in only about 50% to 66% of cases in endemic areas.[6][CDC. Yellow Book. 2024] Studies have found a median of 1 to 2.5 cfu/mL in mixed blood or bone marrow samples.[6] Multiple cultures and larger samples (7 mL) are recommended to increase sensitivity.[2][14][CDC. Yellow Book. 2024] Cultures are most sensitive in the first week of infection when the viral load in the blood is high; observation for as long as 7 days may be needed before confirming a negative result. In travelers, blood culture is much more sensitive (>90%).[6]
Due to the larger number of microorganisms in the bone marrow, bone marrow culture is the most sensitive culture method at 80% to 96% [CDC. Yellow Book. 2024][6][15] Bone marrow cultures may continue to show growth for several weeks after the onset of illness and are relatively unaffected by prior antibiotic use. However, the test is highly invasive and not routinely used.
Stool culture is inappropriate for diagnosing acute S Typhi and S Paratyphi as results can also be positive in convalescent disease or chronic carriage. Stool culture has a low sensitivity and generally does not yield positive results until after the first week of the disease. Other culture diagnostic methods, including duodenal content culture via string capsule and urine culture, are not regularly used to identify S Typhi or S Paratyphi. Where characteristic rose spots are present, a punch biopsy may yield culturable S Typhi or S Paratyphi.
Immunological Tests
The Widal agglutination test is a classic serological test that has been controversial since its development in the late nineteenth century due to its low sensitivity and specificity.[13] However, it is the most commonly available test in endemic countries. An antibody titer of greater than 1:160 and greater than 1:80 for anti-H antigen and anti-O antigen is considered the cutoff level to predict recent infection of S Typhi or S Paratyphi A in an endemic area—although cutoffs vary somewhat across regions.[15] An increase in titer over 4 weeks improves test performance but is often omitted due to cost and the need for a prompt diagnosis.
Various serological tests for diagnosing typhoid are commercially available and increasingly used due to their low cost and rapid results. However, sensitivity and specificity are only moderate due to cross-reactivity with other Salmonella serovars and preexisting antibodies from prior infection.[2] The best-performing test in a 2017 Cochrane review had a sensitivity of 73.8% and a specificity of 94.5%.[2] Recent identification of alternate antigens for diagnosis has led to promising results. For example, a test using lipopolysaccharide and HlyE-specific IgA, validated in Bangladesh and Nepal, provides greater than 90% sensitivity and specificity.[2]
Some authors have advised against using currently available rapid diagnostic tests in endemic countries to prevent their misuse. However, the World Health Organization's Strategic Advisory Group of Experts on in vitro diagnostics rejected this proposal due to the lack of better alternatives. Diagnostic algorithms may improve the utility of rapid diagnostic tests.[14]
Molecular Tests
Nucleic acid detection methods such as multiplex polymerase chain reaction (PCR) or whole genome sequencing allow the genetic signature of the specific genotype to be determined, including the resistance pattern. Direct testing of clinical samples results in low sensitivities due to the low concentrations of bacteria in the blood; combined culture-PCR may improve sensitivity.[6] Having replaced serotyping in many developed nations, nucleic acid testing is cost-prohibitive in many settings. Stool PCR may indicate chronic infection or recent oral typhoid vaccine.
Additional metabolite biomarkers for diagnosing enteric fever and differentiating acute from chronic infection are under investigation.[16] For example, the metabolites ethanolamine, phenylalanine, gluconic acid, monosaccharide, and saccharide show differences in those with S Typhi infection compared to healthy controls and other febrile illnesses.[17] Upregulation of hepcidin and altered breakdown of tryptophan are also highly correlated with S Typhi infection. Molecular studies may also be combined with other tests. For example, various diagnostic biomarkers and IgG, IgM, and IgA antibodies could distinguish typhoid from other febrile illnesses in CHIM studies. Work is ongoing to optimize composite panels and cutoff standards to maximize sensitivity and specificity.
Other Laboratory Tests
Thick and thin blood smears should be ordered if malaria is a risk. For those with a prominent headache or neck stiffness, a lumbar puncture with fluid should be sent for gram stain, culture, and sensitivity. Cerebrospinal fluid is often normal in enteric fever, although mild pleocytosis (<35 cells/mL) may be present.[18] About 15% to 25% of people with enteric fever will have leukopenia and neutropenia. Among children younger than 5, 41% have leukocytosis, and 71% have anemia.[2] Liver function tests may be elevated, similar to viral hepatitis. An electrocardiogram, ultrasound, or x-ray may be required to rule out complications such as myocarditis, abscess, or intestinal perforation.
Treatment / Management
Antibiotic therapy is the mainstay of treatment for enteric fever. Treatment should be started as soon as the diagnosis is suspected, as delays prolong the course of illness and result in a higher risk of complications and severe disease. While most patients with enteric fever are hospitalized in the United States and other nonendemic areas, most people with uncomplicated illness in endemic countries receive outpatient treatment.[2]
Empiric Antibiotic Treatment
The AWaRe (access, watch, reserve) antibiotic book provides global recommendations for empiric treatment choice based on the severity of illness at presentation and the prevalence of ciprofloxacin resistance in the likely place of acquisition.[3][WHO. Aware. 2022] A cutoff of 10% is common, although this was not defined in the AWaRe document.[6] Antibiotic dosages should be adjusted for renal function in adults and children. Treatment should be extended if clinical improvement has not occurred and the patient has not been afebrile for at least 48 hours.[WHO. Aware. 2022](A1)
Where the prevalence of ciprofloxacin resistance is high, including in most of Asia and sub-Saharan Africa, azithromycin is the drug of choice for adults with mild cases of enteric fever. A loading dose of 1 g azithromycin is given on day 1, followed by 500 mg once daily for 7 days. For severe cases, ceftriaxone 2 g intraveneously daily for 10 days is the empiric drug of choice. In areas with a low prevalence of fluoroquinolone resistance, ciprofloxacin is the drug of choice. In adults, ciprofloxacin 500 mg orally every 12 hours is given for 7 days in mild cases and 10 days in severe.[WHO. Aware. 2022]
Based on several Cochrane reviews, these antibiotics have roughly comparable effectiveness against enteric fever caused by susceptible organisms in terms of tolerance and clinical and biological failure.[6] [10] Azithromycin has fewer failures than fluoroquinolones (odds ratio, .48; 95% confidence interval, .26-.89). Ceftriaxone may have a higher relapse rate when compared to azithromycin.[6]
The WHO recommends the same empiric antibiotics for children. The antibiotic dosage must be adjusted to the child's weight. Ciprofloxacin is given orally at a dosage of 15 mg/kg/dose every 12 hours; 20 mg/kg/dose of azithromycin is given orally every 24 hours; and 80 mg/kg/dose of ceftriaxone is given intravenously every 24 hours.[WHO. Aware. 2022]
Azithromycin is recommended as empiric treatment for uncomplicated illness, and carbapenem for more severe disease for travelers and residents in areas with high rates of XDR, such as Pakistan or Iraq.[CDC. Yellow Book. 2024] Azithromycin is given as a loading dose of 1g on day 1 and then 500 mg once daily for 7 days. Meropenem is the most commonly used carbapenem, with 20 mg/kg given 3 times daily.[11]
Where available, locally determined AMR patterns or national guidelines provide additional insight into the choice of empiric treatment. In the United States, most S Typhi and S Paratyphi infections are not susceptible to ciprofloxacin, with greater than 90% of resistant infections occurring among travelers returning from South Asia.[CDC. Yellow Book. 2024] Azithromycin and ceftriaxone are recommended for empiric treatment. If the patient has traveled to Pakistan or Iraq or has not traveled internationally, empiric treatment for XDR should be initiated.
(Refer to the Epidemiology section for more information on the global distribution of AMR).
Ongoing Treatment
If the initial antibiotic is effective, fever decreases over the following 3 to 5 days, and treatment is continued for the recommended interval. If fever persists for more than 5 days, a search for a persistent locus of infection or treatment with alternate antibiotics should be considered, based on bacterial susceptibility whenever possible.[CDC. Yellow Book. 2024] Defervescence can be slow with cephalosporins.[12] Cefixime can be an alternative to ceftriaxone, although its relative effectiveness may be lower than fluoroquinolones.[10] This is likely due to cefixime's low intracellular penetrance. Ofloxacin demonstrates similar performance to ciprofloxacin.[6]
Other Antibiotic Regimens
While susceptibility to chloramphenicol, ampicillin, and co-trimoxazole has re-emerged in some areas, they are no longer recommended as first-line agents due to the persistent threat of MDR.[2] They can be used as alternatives only in areas or infections with known sensitivity.[13] Studies comparing current WHO-recommended therapies to chloramphenicol have shown these to be equally effective.[2][6]
Due to S Typhi and S Paratyphi's dual intracellular and extracellular nature, dual antibiotic use targeting these 2 spaces is proposed to improve clinical outcomes and decrease the probability of resistance emerging.[6] A small randomized control trial in Nepal found that adding azithromycin (principally intracellular activity) to oral cefixime or iv ceftriaxone (principally extracellular activity) halved the time it took to clear the fever.[12] A large-scale outpatient double-blinded one-to-one randomized control trial is ongoing to compare azithromycin and cefixime versus azithromycin alone in uncomplicated typhoid.[6] Results are expected in late 2024.
For XDR strains, a combination of azithromycin and meropenem versus meropenem or azithromycin alone was not found to significantly change the time to defervescence or the treatment failure rate.[11] The cost of additional therapy must be a consideration in treatment choice in resource-constrained settings, with meropenem costing an order of magnitude more than azithromycin and requiring inpatient treatment.
The oral carbamazepine tebipenem is effective in vitro against S Typhi and S Paratyphi, alone or synergistically with azithromycin.[6] Licensed in Japan for pediatric respiratory infections, its use in enteric fever is being explored. The risks and benefits of deploying an oral carbapenem must be carefully considered in areas with unregulated prescribing and significant resistance in other Enterobacteriaceae.[6]
Supportive and Other Treatment
Outpatient treatment consists of oral antibiotics, antipyretics, and commercially available oral replacement fluids when required for vomiting and diarrhea. For severe illness where supportive measures are needed, treatment must be on an inpatient basis. Intravenous fluids and blood products should aggressively replace losses due to diarrhea or bleeding. Ventilation and oxygenation may be required for pulmonary complications. Steroids are recommended for severe illness, particularly where central nervous system involvement and shock are found.[12] This is given as dexamethasone 3 mg/kg intravenously over 30 minutes, followed by 1 mg/kg every 6 hours for a total of 8 doses. (Refer to the Complications section for further discussion on managing complications).
Differential Diagnosis
Enteric fever presents as an undifferentiated acute febrile illness resembling multiple other infectious diseases (most commonly confused with malaria). Factors such as time since returning home from travel, ill travel partners, or unusual exposures may suggest or exclude particular diagnoses. While symptoms vary in frequency across different conditions, most are not sufficiently specific upon presentation to be useful to rule in or out particular conditions. The CDC Yellow Book describes the approach to a febrile returning traveler, with a complete list of potential illnesses and their associated incubation periods.[CDC. Yellow Book. 2024]
The parasite Plasmodium falciparum causes the most deadly form of malaria. This parasite has an incubation period of 8 to 11 days, while most other Plasmodium species have an incubation period of 9 to 17 days. For P malaria, the incubation period is usually 18 to 40 days but can rarely last several years. Rigors are more commonly seen with malaria than with enteric fever. The use of preventive medication does not rule out the diagnosis. Amoebiasis is a less common parasitic infection in the differential. Like enteric fever, amoebiasis is transmitted via the fecal-oral route and can present with fever, bloody diarrhea, and abdominal pain.
The many bacterial illnesses on the differential include leptospirosis, scrub and murine typhus, bacterial meningitis, brucellosis, and bacterial gastroenteritis. Those with leptospirosis may have a history of contact with animals or adventure sports and contact with mud. People with typhus may be found to have a bite mark initially resembling a cigarette burn but developing into black eschar. Those with bacterial gastroenteritis may be more likely to have profuse diarrhea.
A wide variety of viral illnesses can also present with an undifferentiated fever. These include influenza, COVID-19, dengue, viral hepatitis, Chikungunya, viral meningitis, yellow fever, Ebola viruses, and many others, depending on the location of residence or travel. Cough is usually more prominent in influenza and COVID, with associated upper viral symptoms. People with dengue or "breakbone" fever may have severe arthralgias. Returning home more than 6 days before the onset of symptoms would exclude yellow fever, dengue, and Chikungunya.
Prognosis
Enteric fever can result in serious complications and death, particularly if antibiotics are delayed or inadequate. The overall case-fatality rate for S Typhi infection has fallen drastically from 10% to 30% in the pre-antibiotic era to less than 1% in patients who receive early treatment today. The overall pooled case fatality rate is an estimated 4.2% in non-surgical hospital sites and varies significantly across regions: 0.9% in Asia, 5.4% in Africa, 7.2% in Oceania, 6.7% in the Americas, and 1% in Europe.[10] Among cases in global non-hospital sites, the estimate is 0.2%.
Complications
Complications usually occur 2 to 3 weeks after the onset of illness. In a 2020 global systematic review and meta-analysis of typhoid fever complications, 26% of lab-confirmed, predominantly hospitalized cases of typhoid experienced a complication.[10] Reviews completed in 2018 and 2019 found similar results.[2][6][10] The frequency of specific complications depends on age, sex, pre-existing medical conditions, and geographic region.
Gastrointestinal Complications
Children younger than 5 present more frequently with diarrhea and dehydration than adults and older children. Hepatitis is also reported to occur in 36% of children in this age group.[2] Terminal ileum perforation occurs in about 1.3% of hospitalized confirmed cases of enteric fever due to hypertrophy and necrosis of Peyer patches.[10] Intestinal perforation occurs approximately 2 times more frequently in men than women, mainly in people older than 15.[10] Intestinal perforation is more common in sub-Saharan Africa (7.6%) than in Asia (0.7%). The rate of intestinal perforation is increasing in areas with increasing AMR typhoid. Gastrointestinal bleeding can also occur, although it is rarely severe.
Surgical repair and peritoneal lavage is indicated for intestinal perforation. Resultant peritonitis and septic shock require treatment with broad-spectrum antibiotics, inotropes, and fluid replacement in an intensive care unit setting. Blood replacement may be necessary for gastrointestinal hemorrhage.[11] The average hospital stay for surgically treated perforation is 18 days.[2]
Even with treatment, the case fatality rate for intestinal perforation or hemorrhage can be high. For intestinal perforation, higher rates occur in confirmed cases in Africa, ranging from 13.7% to 28.0% across sites, than in Asia, where the range is 0% to 8.4%.[10] The median case mortality for intestinal perforation in Asia is 4.6% and 19.7% in Africa, likely owing to more difficult access to quality medical care services in Africa.[10] A hallmark of severe typhoid, intestinal perforations in endemic countries should be considered probable enteric fever cases. While a late marker of disease, reporting these can help identify outbreaks in countries with underdeveloped enteric fever surveillance systems.[3]
Neuropsychiatric Complications
Delirium is a common complication of enteric fever, occurring in a quarter of confirmed hospitalized cases. Neurological manifestations are otherwise not common in adults, manifesting with meningitis, encephalopathy, sleep irregularities, acute psychosis, myelitis, ataxia, muscle rigidity, and focal neurologic deficits.[2][12] Delirium, encephalopathy, and febrile seizures occur more often in school-aged children.[10] Clinical symptoms are thought to be due to cortical irritation by typhoid toxin. Corticosteroids have been suggested for severe cases of encephalopathy.[13]
Other Complications
Anemia is one of the most common complications of enteric fever, affecting up to one-fifth of hospitalized cases. Anemia is more common in South Asia than anywhere else in the world.[2] One study showed a miscarriage rate of 1 in 6 in pregnant women with confirmed typhoid.[10] Widespread dissemination of bacteria can cause multiorgan failure due to disseminated intravascular coagulation and septicemia.[14] Focal abscesses are uncommon. Lung complications, including bronchitis and pneumonia, occur primarily in children and patients with lung cancer, glucocorticoid use, and other structural lung diseases.[15]
Relapse
Relapse is defined as a recurrence of symptoms with laboratory confirmation of S Typhi from a normally sterile site within 1 month of completing an appropriate course of antimicrobial treatment and resolution of symptoms. Relapse occurs in less than 10% of patients, usually 1 to 3 weeks after clinical recovery. Further antibiotic treatment is required.
Chronic Carriage
Convalescent carriage of S Typhi and S Paratyphi can continue for a few weeks to months after treatment of enteric fever. Usually, this clears without further treatment. However, 1% to 4% of patients with typhoid fever become chronic carriers, demonstrating shedding for at least 12 months after finishing an appropriate course of antibiotics.[CDC. Yellow Book. 2024] Chronic carriage is less common with adequate antibiotic treatment and S Paratyphi infections.
Confirmation of chronic carriage is difficult due to intermittent shedding and low organism levels in the stool. A stool culture or polymerase chain reaction is used to determine persistent carriage. Sufficient time must elapse between finishing antibiotics and testing to ensure antibiotic clearance. PCR testing can also detect bacterial DNA well after viable bacteria are cleared. An anti-Vi antibody-based test has been developed for use in nonendemic areas but is unreliable in medium- to high-prevalence areas where regular infection or exposure results in higher baseline titers.[2]
Underlying biliary structural abnormalities and cholelithiasis are underlying risk factors for chronic carriage, which are more commonly seen in women and older individuals. Chronic carriage of S Typhi and S Paratyphi for gallstones is associated with biofilm development.[2] Chronic carriers of typhoid have 4.28 times the risk of developing biliary cancer compared to the general population.[12] Kidney stones and, in Africa, S haematobium infections increase the odds of urinary tract infection and chronic urinary carriage.
Current evidence for treating chronic carriage is lacking. The 2003 WHO guidelines and a 2022 systematic review are based on evidence that precedes 1990.[6][12] Cholecystectomy cures chronic carriage in 70% to 90% of cases.[12] However, because it carries significant anesthetic and surgical risk, cholecystectomy is only recommended if other indications for surgery exist.[2][6] Household members of chronic typhoid carriers may be advised to be vaccinated.
Deterrence and Patient Education
Patients with Enteric Fever
Patients treated on an outpatient basis or who return home after hospitalization and their caretakers need to be aware of methods to prevent transmission within the household. Convalescent carriage of S Typhi and S Paratyphi may last for weeks or even months after acute illness. Hand hygiene with soap and water is of critical importance. Caretakers must be given instructions about caring for the ill person, including the need for gloves, strict hygiene, and safe disposal of potentially contaminated feces or urine. Recently ill people should not prepare food or engage in other activities that may result in transmission within the household. Patients should be informed that previous enteric fever does not provide long-lasting protection. Vaccination should be recommended if future or ongoing risk exists.
Individual Prevention Measures
Washing hands frequently with soap and water and drying hands thoroughly after washing are basic measures for avoiding enteric illnesses. This includes before preparing food, after using the bathroom or changing diapers, and after caring for an ill person. Alochol-based sanitizers containing at least 60% alcohol can be used where water and soap are not available but are not as effective as soap and water for some pathogens.[CDC. Yellow Book. 2024] Hands should be thoroughly washed immediately after using sanitizers.
Maintaining food and water precautions is also essential for travelers and people living in endemic areas. Raw or undercooked meat, fish, shellfish, and produce carry the greatest risk of contamination. Unpasteurized fruit juices, salads, unpeeled fruits, and unpasteurized milk and cheeses should be avoided. All cooked foods should be fully cooked and served hot. Patients should be advised to avoid foods in restaurants that may have been cooked ahead and reheated before serving, such as lasagna, and those sold by street vendors. Buffets should be avoided. Where drinking water safety is uncertain, water should be adequately treated, or only bottled water should be consumed—this includes drinking, preparing food and beverages, brushing teeth, making ice, and cooking. Hot drinks such as tea or coffee are generally considered safe.
For bottle-fed infants, either prepared liquid formula should be used, or particular care should be taken when preparing formula. Water for reconstitution should be hot (≥70 °C).[CDC. Yellow Book. 2024] The formula should be used within 2 hours, and any remaining after feeding should be discarded. Counsel patients about when to seek medical if febrile. VFR travelers are most likely to become infected with enteric fever, as they are less likely to maintain precautions regarding food and water and are less likely than other travelers to obtain pretravel advice.[CDC. Yellow Book. 2024]
Vaccination
Typhoid vaccination is recommended for travelers to endemic areas, particularly those traveling to South Asia and those with health- or travel-related risks traveling to other endemic areas. This includes children, VFR, prolonged travel abroad, anatomic or functional asplenia, the use of acid suppressants, or personal preference. Vaccination does not preclude the need for hand hygiene and food and water precautions. The vaccines currently available in nonendemic areas are not fully effective, and the risk of other illnesses transmitted by the fecal-oral route remains. In endemic countries where typhoid vaccination is not integrated into routine vaccination schedules, patients at higher risk of severe disease may obtain typhoid vaccination for private pay.
Unconjugated Vaccines
There are 2 licensed, unconjugated vaccines in the United States: an inactivated Vi capsular polysaccharide (ViPS) vaccine and a live vaccine of an attenuated Ty21a S Typhi strain.[CDC. Yellow Book. 2024] These are about 50% to 60% effective in preventing typhoid fever.[16][CDC. Yellow Book. 2024] The intramuscular Vi capsular polysaccharide vaccine is appropriate for those 2 years and older; this should be given 2 weeks or more before travel, and a booster should be provided every 2 to 3 years, depending on national recommendations.[23] The CDC recommends every 2 years.[20][CDC. Yellow Book. 2024]
The Ty21a vaccine is indicated for people 6 years or older. One capsule every other day for a total of 4 doses is taken with liquid no warmer than body temperature at least 1 hour before a meal. The series should be completed at least 1 week before exposure, and a booster is indicated every 5 to 7 years.[17][CDC. Yellow Book. 2024]
The oral vaccine is contraindicated in people who are immunocompromised, acutely ill with a gastrointestinal condition, or breastfeeding. It should only be used in pregnant women if the injectable attenuated vaccine is unavailable and a careful risk assessment is done. Though not licensed for this indication, the oral Ty21a vaccine may offer some protection against Salmonella Paratyphi B.[CDC. Yellow Book. 2024]
Typhoid Conjugate Vaccines
The WHO has prequalified 2 typhoid conjugate vaccines (TCVs): Typbar-TCV and TYPHIBEV. By conjugating the Vi capsule to a protein carrier (eg, tetanus toxoid), these vaccines induce a more robust and enduring T-cell-mediated immune response than previous vaccines; these can be used in children under 5 and infants as young as 6 months.[2] Given as a single-dose intramuscular dose, TCVs were found to be 79% to 95% effective in the first 2 years after vaccination in studies covering more than 100,000 children in diverse locations. The antibody response can persist for up to 7 years.[8] Endemic countries are implementing TCV vaccines as part of routine immunization programs. However, they are not licensed in Europe or North America, precluding their use for travel. (Refer to the Enhancing Healthcare Team Outcomes section for more information on the use of typhoid vaccines in endemic countries.)
Enhancing Healthcare Team Outcomes
Despite public health efforts, enteric fever is still a significant cause of morbidity and mortality worldwide. In the United States and other developed nations, it is a source of life-threatening illness, mainly in travelers. An interprofessional healthcare team is essential to all aspects of enteric fever prevention and treatment.
Access to Quality Health Care
Primary care and public health providers are important resources for travelers and people living in endemic areas to emphasize individual prevention measures to decrease the risk of enteric fever and other food and water-borne diseases. For example, in nonendemic countries, healthcare providers can advise patients of their risk of typhoid before travel and advise vaccine. In areas with poor sanitation in endemic countries, campaigns promoting the use of soap and water have been shown to decrease the risk of gastrointestinal illnesses.
Healthcare providers in primary care and emergency settings are usually the first to encounter febrile travelers. A broad differential diagnosis and high index of suspicion in these settings allow the timely diagnosis of enteric fever. Using established regional, national, or international guidelines developed by infectious disease and public health colleagues can improve empiric treatment and avoid unnecessary complications. Reporting enteric fever is usually required within 24 hours in most developed countries to minimize community spread. For patients treated at home, community nursing can provide follow-up and recommend hygiene measures to avoid illness in other family members. For patients requiring hospitalization, timely referral to surgical, infectious disease, or critical care specialists may be required.
The provision of quality care includes the need for increased diagnostic and surveillance capacity in low- to middle-income countries.[3] Community health education about the mode of transmission, prevention, signs and symptoms, and the importance of early treatment can also reduce the incidence and burden of disease. Intersectoral coordination by public health and nonmedical organizations and authorities in sanitation management, awareness of individual prevention measures, and nutritional programs boost disease control and prevention.
WASH
The importance of improved global access to and use of infrastructure supporting safe washing, sanitation, and hygiene cannot be understated. However, coverage for basic sanitation services was less than 50% in sub-Saharan Africa, India, Bangladesh, and Nepal in 2015.[8] Improving clean drinking water access is necessary but insufficient to decrease enteric fever's burden. Human waste must also be disposed of safely away from populations, water supplies, and crops. Legislation may be required to address agricultural practices. Point-of-collection disinfection methods can provide lower-cost and easier-to-use options when governmental resources are insufficient to develop and maintain complicated water treatment infrastructure.[2] WASH is critical to overall population health improvement, decreasing the incidence not only of enteric fever but also other food and water-borne diseases. WASH improvement is the 6th sustainable development goal and a building block of the WHO Global Action Plan on antimicrobial resistance.[8]
Population Approaches to Vaccination
Vaccination is an important adjunct to WASH efforts to control typhoid globally. The current (2018) WHO guidelines on the use of typhoid vaccines recommend the programmatic use of TCVs in countries with endemic typhoid in combination with health education, WASH improvements, and HCW training on typhoid fever diagnosis and treatment.[16] Integration into routine vaccination schedules improves coverage. Prioritization of those countries with the highest incidences of disease or high rates of AMR is advised. Co-administration with other vaccines has been proven to not interfere with immune responses.[17] TCV use in outbreaks in endemic countries is also recommended and has been effectively used in outbreaks in Zimbabwe and Pakistan.
Integration into routine childhood immunization programs is estimated to be cost-effective or highly cost-effective in all countries with high to very high typhoid fever incidence.[16] In some Asian countries, the programmatic use of TCV could be cost-saving. In all countries, programmatic decisions such as immunization age should be based on the local epidemiology of typhoid fever, antimicrobial resistance patterns, cost analyses, and consideration of the routine childhood immunization program.
Catch-up vaccination campaigns targeting children aged up to 14 are also recommended where feasible and supported by data to maximize impact.[18] Single-age vaccine programs may be more cost-saving in some settings. For example, providing 1 dose at 9 months of age with a parallel catch-up program at 15 could avert up to 67 million cases in the 73 low- to middle-income countries eligible for Gavi the Vaccine Alliance funding over 10 years.[18] Depending on the age structure and vaccination coverage, 46% to 74% of cases in individual countries could be avoided.
Six countries now have a childhood vaccination program. Starting in 2019, Pakistan was the first country to implement a campaign through a phased approach. Since then, Liberia (2021), Nepal (2022), Zimbabwe (2021), Samoa (phased, starting 2021), and Malawi (2023) have implemented programs.[18] In Nepal, Pakistan, and Zimbabwe, integrated campaigns were conducted, including various other interventions such as identifying un- or under-vaccinated children, administering other vaccines, supplementing vitamin A, and promoting hygiene. Post-campaign coverage in these countries ranged from 63% to 95%.[18] The COVID-19 pandemic and other competing health priorities have likely delayed the introduction of immunization programs in some countries. Insufficient disease burden data has also limited national vaccine decisions. However, inadequate surveillance data to monitor vaccine impact should not preclude introducing TCVs.[18]
(Refer to "Surveillance and Reporting" for more information on challenges with enteric fever surveillance).
Public Health Measures and Outbreak Control
In countries where enteric fever is a reportable disease, once a report is received, public health assesses whether the source of infection (ie, travel versus locally acquired) poses an ongoing risk of transmission to the local population and ensures that the affected individual does not transmit the illness to others. The discovery of local transmission triggers an investigation, which can include searching for additional cases, food testing, interviewing coworkers, and public communications to advise others who may be at risk, for example, if a person worked while ill or was a chronic carrier.
Local, state, or national codes or guidelines determine specific requirements for exclusion. Public health authorities may restrict recently recovered individuals from daycare attendance or participating in certain high-risk occupations for transmission until 2 to 3 stool cultures taken at least 48 hours apart are negative for S Typhi and S Paratyphi.[17][WA Gov. Typhoid. 2018][FL Gov. Typhoid. 2013] High-risk occupations include food handlers, healthcare and childcare workers, and water park employees. The collection of stool samples must allow antibiotics to be used in treatment to clear them first. For example, many local state codes stipulate that samples must be taken at least 1 week after treatment [CA Gov. Typhoid. 2015][WA Gov. Typhoid. 2018][FL Gov. Typhoid. 2013] while other jurisdictions allow the collection to occur 2 days after ciprofloxacin and 2 weeks after ceftriaxone or azithromycin.[BCCDC. Enteric Exclusions. 2023] People who have ever traveled or lived in a country with schistosomiasis may be required to have at least 1 negative urine sample. People who traveled with the ill person may also be excluded from work in sensitive settings until stool cultures are clear.
Public health commonly addresses outbreaks even where individual case follow-up does not occur. Outbreaks may be identified by increased hospitalizations or incidence of nontraumatic intestinal perforations. In these instances, coordinated public health education campaigns, swiftly administering vaccines, and addressing sanitation concerns can decrease the burden of typhoid fever illness.[20]
Antimicrobial Stewardship
Increasing antimicrobial resistance is one of the most pressing global public health issues. While new antimicrobials continue to be developed, bacteria develop resistance far faster. Although S Typhi and S Paratyphi are exclusively human pathogens, antimicrobial resistance factors mediated by plasmids can transfer between Salmonella and other bacteria. Thus, the lack of access to health care and widespread use of antibiotics for undifferentiated fevers, particularly in typhoid-endemic areas, contributes to the global burden of AMR. The occasional azithromycin-resistant Salmonella strain and the discovery of plasmid-mediated carbapenem-resistant Escherichia coli co-circulating with MDR typhoid are particularly problematic, with the concern for strains resistant to all current antibiotics developing.
In addition to WASH measures, improved diagnostic modalities and vaccination programs can effectively decrease AMR due to enteric disease. Due to the high rate of treatment for enteric fever, with an estimated 3 to 25 infections treated per every culture-confirmed case, typhoid vaccinations integrated into childhood vaccination programs would also decrease AMR due to typhoid by 16% and reduce resistance in bystander organisms.[8]
Surveillance and Reporting
Accurate surveillance and reporting are critical to enteric fever control to assess disease burden, rapidly detect outbreaks, determine emerging AMR patterns, and evaluate vaccine impact. However, the capacity for surveillance and the precision and accuracy of estimates vary significantly between nations. For example, reporting positive S Typhi and S Paratyphi results by laboratories to local public health authorities must occur within 24 hours in the United States. From there, clinical data on laboratory-confirmed S Typhi and S Paratyphi cases is reported to the National Typhoid and Paratyphoid Surveillance System; laboratory-confirmed and clinically compatible cases linked to a laboratory-confirmed case of enteric fever are entered into the National Notifiable Diseases Surveillance System (NNDSS). Highly accurate totals of clinical disease are generally obtained, notwithstanding the likelihood that NNDSS data is incomplete due to incomplete reporting and the inability to reconcile data sets during the COVID-19 pandemic.[CDC. Typhoid and Paratyphoid Surveillance. 2020] However, even robust surveillance systems cannot capture the true incidence due to subclinical infections.
The WHO recommends facility-based surveillance in all endemic countries, with laboratory confirmation of the infection. The recommended minimum parameters for monitoring the disease burden are mortality, morbidity, and economic impact. The United Nations rolled out an electronic Joint Reporting Form in 2018, with reporting to the WHO and UNICEF.[19] The WHO Global Antimicrobial Surveillance System (GLASS) additionally collates AMR patterns across all Salmonella species, with suggestions from some authors that typhoidal Salmonellae be separated from NTS in the future.
In recent years, substantial investments in surveillance systems in less developed endemic countries have been made. However, significant regional surveillance gaps remain. In many endemic countries, the number of lab-confirmed cases dramatically underestimates the true incidence of enteric fever due to the following:
- Similarity between clinical presentation and those of other acute febrile illnesses in areas where typhoid is endemic
- Low sensitivity or specificity of available diagnostic technologies
- High use of antibiotic use before presentation to healthcare
- Low availability of blood culture
- Lack of well-developed surveillance systems in many jurisdictions [19]
Population studies are required to estimate the true incidence of disease more accurately.[19] These are costly, time-consuming, technically challenging, and unavailable in most countries. Three ongoing surveillance projects covering 10 countries are essential for population estimates (Bangladesh, Nepal, Malawi, Pakistan, Burkina Faso, the Democratic Republic of the Congo, Ethiopia, Ghana, Madagascar, and Nigeria). The Global Burden of Disease Study, most recently published in 2019, estimates rates and case counts from published studies, publically available data sets, and contributed data across 369 diseases and injuries across 205 countries and territories.[21]
More recently, global collaborations, improved surveillance systems and distribution, and improved methods to estimate the incidence of enteric fever and determine the prevalence of AMR have improved the availability of more granular AMR patterns, including in areas with little or no data.[3][8] For example, a 2024 spatiotemporal mathematical modeling study estimated phenotypic AMR prevalence in 75 endemic countries using 601 data sources for S Typhi antibiotic resistance patterns from 45 countries over 30 years. A 2023 meta-analysis used all available published and unpublished Typhi genomes from the previous 21 years (12,965 high-quality genomes from 110 countries).[3] This study analyzed AMR patterns using whole genome sequencing to determine phenotype. The study also used specimens from travel-related cases from national reference laboratories in high-income countries to provide some data in countries and regions with low capacity for performing blood cultures.
This need to maximize information recovery from other sources is well-recognized. Countries without robust surveillance can improve disease estimates using data from various sources, including neighboring countries or regions, population-based studies, modeling data, lab-confirmed cases, AMR testing studies, outbreak reports, and non-traumatic intestinal perforation case reports. The recent use of pooled genomic surveillance may allow for early identification of shifts in AMR patterns or evidence of clonal spread. For example, the identification of the extensively drug-resistant strain identified in Pakistan in 2016 was found to have emerged in 2015.[22]
Research and innovation
Improving diagnostic technologies for enteric fever is a priority for research and innovation. Limitations contribute to under- and over-diagnosis, poorer clinical outcomes, and increased antimicrobial resistance. Improved diagnostic techniques will also enhance surveillance. (Refer to the Evaluation section for further information on recent advances in diagnostic technologies).
Another priority is the development of a paratyphoid vaccine. While the Ty21a vaccine may provide some cross-coverage against S Paratyphi B, no vaccine is currently sufficiently effective for controlling S Paratyphi infection; this is particularly concerning in South and Southeast Asia, where S Paratyphi is most prevalent. Paratyphi may also increase proportionally as Typhoid vaccination increases.
With very few options available, new antimicrobial therapies are also needed.[8] While resistance to former first-line antibiotics is decreasing in some areas due to reduced use, high resistance in other regions and the constant threat of re-emergence continue to limit widespread utility.
Expanded and improved surveillance and epidemiological methodologies are also a priority in improving enteric fever control. For example, environmental surveillance using a nucleic acid amplification test is hoped to enhance understanding of the incidence of infection, prevalence of AMR, and other critical surveillance issues.[8] By identifying countries at the highest risk of AMR S Typhi and S Paratyphi, phylogeographic studies can help prioritize the implementation of WASH measures, vaccination programs, and improvements to public health capacity.[23]
The expansion of genomic surveillance is predicted to improve the measurement of the impact of TCVs on local S Typhi populations and decisions about future combination Typhi vaccines, using techniques already used for pneumococcal conjugate vaccines.[3][17] Genomic surveillance will be important in monitoring changes in clinically important resistances in S Typhi and S Paratyphi. Combined with environmental surveillance, early evidence of clonal spread of known resistant organisms could provide early warning of a likely increase in prevalence. Emerging mutations in chromosomal areas that code for resistance can likewise flag the need to watch for emerging resistance in clinical settings.[8]
Media
(Click Image to Enlarge)
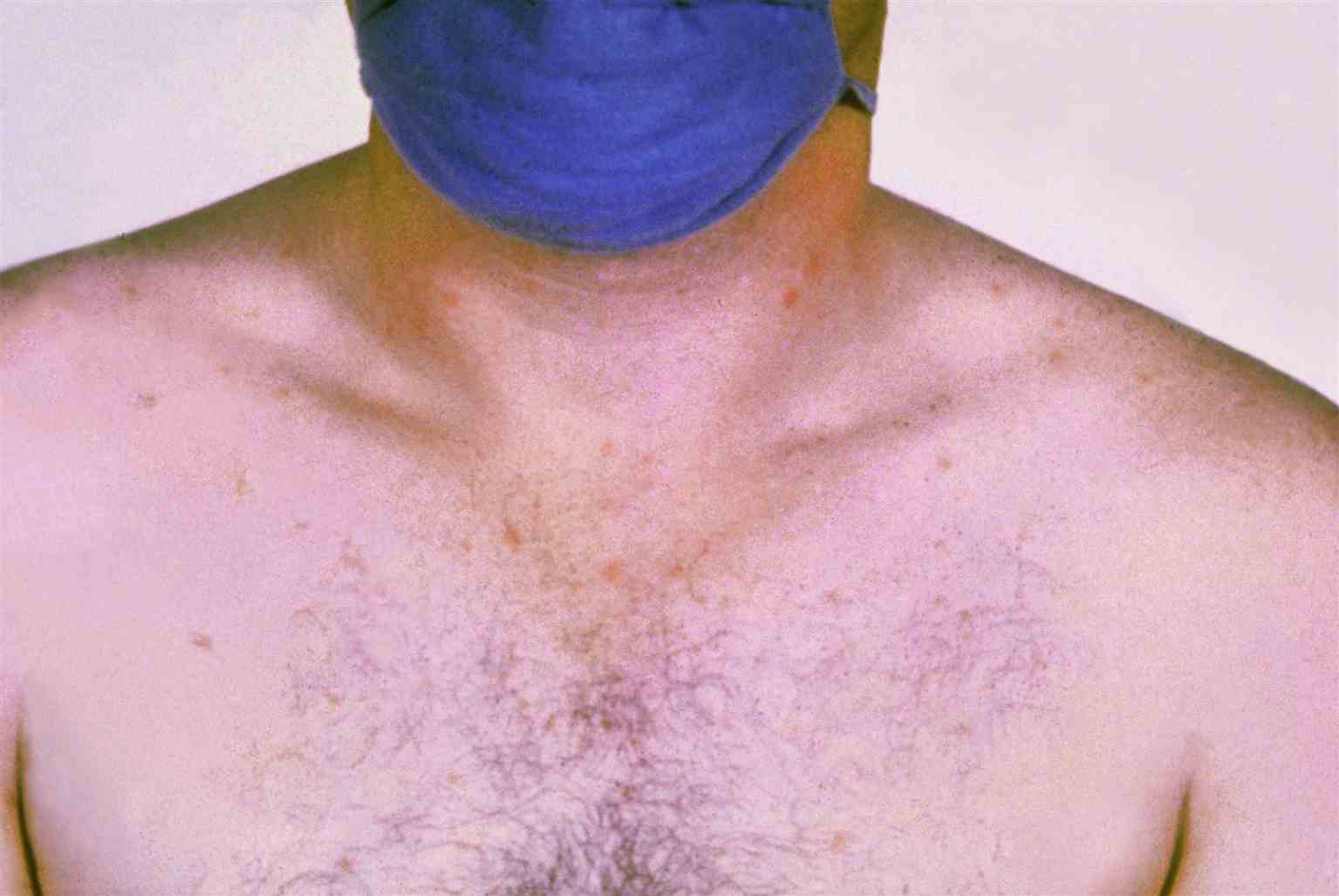
Rose Spots on Chest, Patient With Typhoid Fever. This condition is due to the bacterium Salmonella Typhi. Symptoms of typhoid fever may include a sustained fever as high as 103 to 104 °F (39 to 40 °F), weakness, stomach pains, headache, and loss of appetite. In some cases, patients have a rash of flat, rose-colored spots.
Centers for Disease Control and Prevention
(Click Image to Enlarge)
References
Chattaway MA, Langridge GC, Wain J. Salmonella nomenclature in the genomic era: a time for change. Scientific reports. 2021 Apr 5:11(1):7494. doi: 10.1038/s41598-021-86243-w. Epub 2021 Apr 5 [PubMed PMID: 33820940]
Meiring JE, Khanam F, Basnyat B, Charles RC, Crump JA, Debellut F, Holt KE, Kariuki S, Mugisha E, Neuzil KM, Parry CM, Pitzer VE, Pollard AJ, Qadri F, Gordon MA. Typhoid fever. Nature reviews. Disease primers. 2023 Dec 14:9(1):71. doi: 10.1038/s41572-023-00480-z. Epub 2023 Dec 14 [PubMed PMID: 38097589]
Carey ME, Dyson ZA, Ingle DJ, Amir A, Aworh MK, Chattaway MA, Chew KL, Crump JA, Feasey NA, Howden BP, Keddy KH, Maes M, Parry CM, Van Puyvelde S, Webb HE, Afolayan AO, Alexander AP, Anandan S, Andrews JR, Ashton PM, Basnyat B, Bavdekar A, Bogoch II, Clemens JD, da Silva KE, De A, de Ligt J, Diaz Guevara PL, Dolecek C, Dutta S, Ehlers MM, Francois Watkins L, Garrett DO, Godbole G, Gordon MA, Greenhill AR, Griffin C, Gupta M, Hendriksen RS, Heyderman RS, Hooda Y, Hormazabal JC, Ikhimiukor OO, Iqbal J, Jacob JJ, Jenkins C, Jinka DR, John J, Kang G, Kanteh A, Kapil A, Karkey A, Kariuki S, Kingsley RA, Koshy RM, Lauer AC, Levine MM, Lingegowda RK, Luby SP, Mackenzie GA, Mashe T, Msefula C, Mutreja A, Nagaraj G, Nagaraj S, Nair S, Naseri TK, Nimarota-Brown S, Njamkepo E, Okeke IN, Perumal SPB, Pollard AJ, Pragasam AK, Qadri F, Qamar FN, Rahman SIA, Rambocus SD, Rasko DA, Ray P, Robins-Browne R, Rongsen-Chandola T, Rutanga JP, Saha SK, Saha S, Saigal K, Sajib MSI, Seidman JC, Shakya J, Shamanna V, Shastri J, Shrestha R, Sia S, Sikorski MJ, Singh A, Smith AM, Tagg KA, Tamrakar D, Tanmoy AM, Thomas M, Thomas MS, Thomsen R, Thomson NR, Tupua S, Vaidya K, Valcanis M, Veeraraghavan B, Weill FX, Wright J, Dougan G, Argimón S, Keane JA, Aanensen DM, Baker S, Holt KE, Global Typhoid Genomics Consortium Group Authorship. Global diversity and antimicrobial resistance of typhoid fever pathogens: Insights from a meta-analysis of 13,000 Salmonella Typhi genomes. eLife. 2023 Sep 12:12():. doi: 10.7554/eLife.85867. Epub 2023 Sep 12 [PubMed PMID: 37697804]
Level 1 (high-level) evidenceKhan M, Shamim S. Understanding the Mechanism of Antimicrobial Resistance and Pathogenesis of Salmonella enterica Serovar Typhi. Microorganisms. 2022 Oct 11:10(10):. doi: 10.3390/microorganisms10102006. Epub 2022 Oct 11 [PubMed PMID: 36296282]
Level 3 (low-level) evidenceMoudgil KD, Narang BS. Pathogenesis of typhoid fever. Indian journal of pediatrics. 1985 Jul-Aug:52(417):371-8 [PubMed PMID: 4093170]
Parry CM, Qamar FN, Rijal S, McCann N, Baker S, Basnyat B. What Should We Be Recommending for the Treatment of Enteric Fever? Open forum infectious diseases. 2023 May:10(Suppl 1):S26-S31. doi: 10.1093/ofid/ofad179. Epub 2023 Jun 2 [PubMed PMID: 37274536]
Wen SC, Best E, Nourse C. Non-typhoidal Salmonella infections in children: Review of literature and recommendations for management. Journal of paediatrics and child health. 2017 Oct:53(10):936-941. doi: 10.1111/jpc.13585. Epub 2017 May 29 [PubMed PMID: 28556448]
GRAM Typhoid Collaborators. Estimating the subnational prevalence of antimicrobial resistant Salmonella enterica serovars Typhi and Paratyphi A infections in 75 endemic countries, 1990-2019: a modelling study. The Lancet. Global health. 2024 Mar:12(3):e406-e418. doi: 10.1016/S2214-109X(23)00585-5. Epub [PubMed PMID: 38365414]
Shakoor S, Dittrich S. Better diagnostic tools needed to distinguish typhoid from other causes of acute febrile illness. Bulletin of the World Health Organization. 2023 Nov 1:101(11):683-683A. doi: 10.2471/BLT.23.290678. Epub [PubMed PMID: 37961063]
Marchello CS, Birkhold M, Crump JA. Complications and mortality of typhoid fever: A global systematic review and meta-analysis. The Journal of infection. 2020 Dec:81(6):902-910. doi: 10.1016/j.jinf.2020.10.030. Epub 2020 Nov 2 [PubMed PMID: 33144193]
Level 1 (high-level) evidenceKambire JL, Ouedraogo S, Ouedraogo S, Ouangre E, Traore SS. [Results after surgical management of ileal perforation due to typhoid fever, about 29 cases in Ouahigouya (Burkina Faso)]. Bulletin de la Societe de pathologie exotique (1990). 2017 Dec:110(5):298-299. doi: 10.1007/s13149-017-0579-5. Epub 2017 Nov 10 [PubMed PMID: 29127649]
Level 3 (low-level) evidenceMcCann N, Scott P, Parry CM, Brown M. Antimicrobial agents for the treatment of enteric fever chronic carriage: A systematic review. PloS one. 2022:17(7):e0272043. doi: 10.1371/journal.pone.0272043. Epub 2022 Jul 29 [PubMed PMID: 35905082]
Level 1 (high-level) evidenceMellon G, Eme AL, Rohaut B, Brossier F, Epelboin L, Caumes E. Encephalitis in a traveller with typhoid fever: efficacy of corticosteroids. Journal of travel medicine. 2017 Sep 1:24(6):. doi: 10.1093/jtm/tax063. Epub [PubMed PMID: 29088483]
Kumwenda M, Iroh Tam PY. An adolescent with multi-organ involvement from typhoid fever. Malawi medical journal : the journal of Medical Association of Malawi. 2019 Jun:31(2):159-160. doi: 10.4314/mmj.v31i2.10. Epub [PubMed PMID: 31452851]
Esmailpour N, Rasoolinejad M, Abdolbaghi MH. Cardiopulmonary manifestations of typhoid fever: a prospective analysis of 65 cases in Iran. Tropical doctor. 2006 Apr:36(2):118-9 [PubMed PMID: 16611453]
Level 3 (low-level) evidenceWorld Health Organization. Typhoid vaccines: WHO position paper, March 2018 - Recommendations. Vaccine. 2019 Jan 7:37(2):214-216. doi: 10.1016/j.vaccine.2018.04.022. Epub 2018 Apr 13 [PubMed PMID: 29661581]
Basnyat B, Qamar FN, Rupali P, Ahmed T, Parry CM. Enteric fever. BMJ (Clinical research ed.). 2021 Feb 26:372():n437. doi: 10.1136/bmj.n437. Epub 2021 Feb 26 [PubMed PMID: 33637488]
Hancuh M, Walldorf J, Minta AA, Tevi-Benissan C, Christian KA, Nedelec Y, Heitzinger K, Mikoleit M, Tiffany A, Bentsi-Enchill AD, Breakwell L. Typhoid Fever Surveillance, Incidence Estimates, and Progress Toward Typhoid Conjugate Vaccine Introduction - Worldwide, 2018-2022. MMWR. Morbidity and mortality weekly report. 2023 Feb 17:72(7):171-176. doi: 10.15585/mmwr.mm7207a2. Epub 2023 Feb 17 [PubMed PMID: 36795626]