Introduction
Contact urticaria (CU) is a transient wheal and flare reaction that occurs within 10 to 60 minutes at the site of contact of the offending agent and completely resolves within 24 hours. It was first described in 1973 by Alexander Fischer. It is a form of acute urticaria (symptoms last for less than six weeks).
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
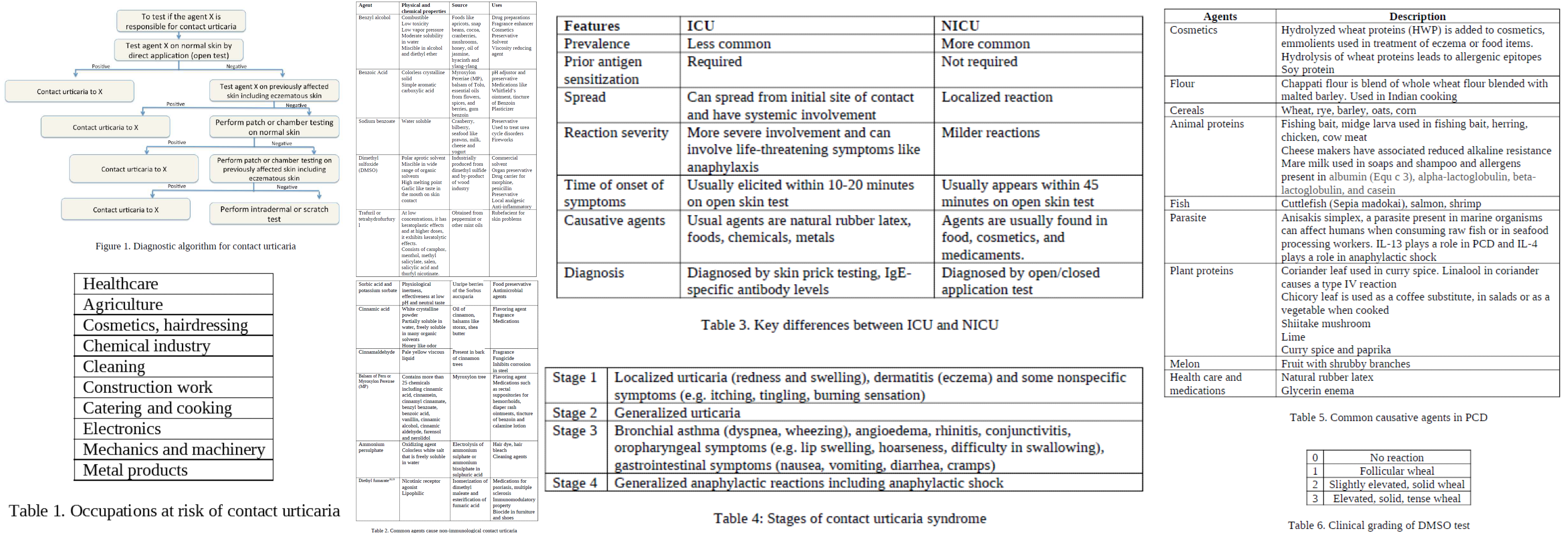
The risk for developing CU increases when there is an interruption of the stratum corneum due to filaggrin gene mutations or skin irritants. The common agents associated with the development of CU appear in the following sections. The occupations at the most risk of developing CU are healthcare, lab work, agriculture, hairdressers, cosmetic industry, chemical industry, cleaning, construction, catering, cooking, electronics, machinery, and metal products.[1][2][3]
Epidemiology
The prevalence in the general population is unrecognized due to lower disease diagnosis rates. The prevalence of occupational CU is 0.4% and accounts for 30% of all occupational skin diseases. Research indicates a prevalence of 5% to 10% for latex-related CU.[3] The prevalence of sensitization to natural rubber latex (NRL) among healthcare workers is between 5% to 13%.[4][5][6] NRL, cosmetics, skin creams, and sorbic acid are some of the most common causative agents identified.[7][8][4][9] The Finnish Registry indicated that the incidence of CU has more than doubled between 1989 and 1994 with cow dander, NRL, flour, and grains as common triggers.[7]
Pathophysiology
CU is classified based on the pathophysiology into:
- Immunologic CU (ICU)
- Non-immunologic CU (NICU)
- CU due to unknown mechanisms
1. Immunologic CU (ICU)
ICU involves antigen binding to IgE-specific antibodies (type I hypersensitivity reaction) on the dermal mast cells. After antigen-antibody binding, there is a release of histamine and vasoactive mediators like prostaglandins and leukotrienes, resulting in disturbance of skin microcirculation. A stronger allergic response than non-immunologic CU (NICU) gets elicited due to the ready absorption of the proteins by the skin[10]. Prior sensitization to the agent is necessary for the reaction to develop. Repeated exposure to the antigen can lead to progressive worsening of symptoms. Concomitant history of other atopic disorders like personal or family history of asthma, eczema, and hay fever is a risk factor.[11] The reaction spreads beyond the site of initial contact and progresses to generalized urticaria, even leading to anaphylactic shock. This progression of symptoms in a step-wise manner has been described in four stages as 'contact urticaria syndrome.'
- Stage 1- Localised urticaria (redness and swelling), dermatitis and some non-specific symptoms like burning, itching, tingling, etc
- Stage 2- Generalized urticaria
- Stage 3- Systemic manifestations like bronchial asthma, angioedema, rhinitis, conjunctivitis, oropharyngeal symptoms (Ex. lip swelling, hoarseness, difficulty in swallowing), gastrointestinal symptoms (Ex.nausea, vomiting, diarrhea, abdominal cramps)
- Stage 4- Generalized anaphylactic reaction including anaphylactic shock
There are two broad categories of agents causing ICU.[11]
- High molecular weight proteins with molecular weight more than 10000 kD. Ex. dietary proteins.
- Hapten chemicals with molecular weight less than 1000 kD: They conjugate with carrier proteins like albumin and form the hapten-carrier protein complex. Ex. Di-isocyanate used in polyurethane products and ammonium persulphate.
The agents causing ICU can divide into four groups.[8][2]
- Group I - Plant-derived proteins (Ex.vegetables, fruits, spices)
- Group II- Animal-derived proteins (Ex.meat, dairy products, seafood)
- Group III- Grains (Ex. wheat, barley, rye)
- Group IV- Enzymes (Ex.alpha-amylase, cellulase, xylanase)
2. Non-immunologic CU (NICU)
NICU is less severe and more common than ICU. There are no specific antibodies against the causative agents. Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit the response showing that prostaglandins (Ex. prostaglandin D2) may play a role in the pathogenesis.[12][10][13][14][15] Histamine has not shown to play a role since antihistamines did not have any inhibitory effect. Other proposed mechanisms include direct capillary damage at the site of contact leading to the non-IgE mediated release of vasoactive amines, prostaglandins, leukotrienes, and acetylcholine.[8][12][10][13][14] Unlike ICU, there are no systemic manifestations, and the reaction remains localized. Prior exposure to the antigen is not necessary, and it can present on initial skin contact with the allergen. The symptoms vary depending on the site of exposure, the concentration of the substance, and the mode of exposure.[16]
NICU agents can classify into the following types:
- Animals (Ex. arthropods, caterpillars, corals)
- Foods (Ex. pepper, mustard, thyme)
- Fragrances and flavorings (Ex. balsam of Peru, cinnamic acid, cinnamic aldehyde)
- Medications (Ex. benzocaine, camphor, witch-hazel)
- Metals (Ex. cobalt)
- Plants (Ex. nettles, seaweed)
- Preservatives and disinfectants (Ex. benzoic acid, formaldehyde)
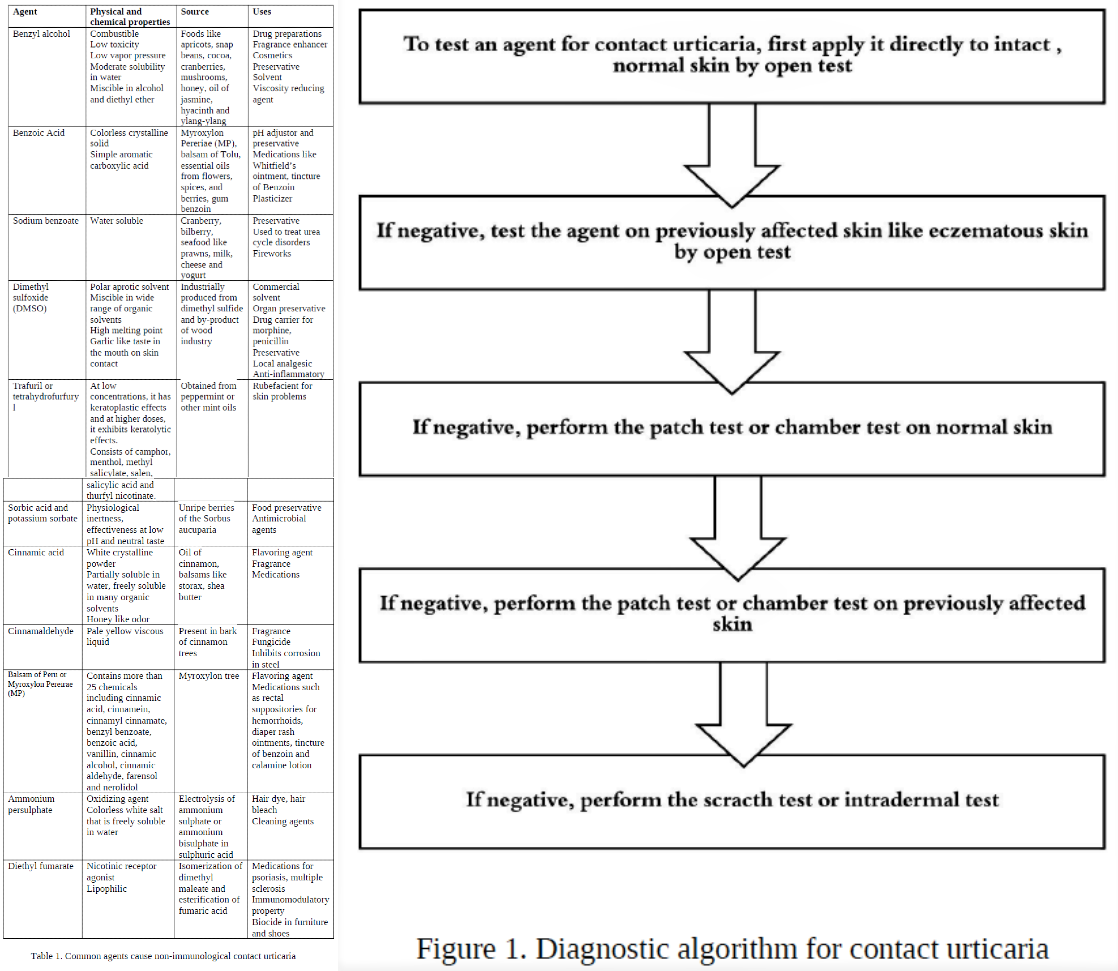
Described in Table 1 are some of the common causative agents for NICU. There is an overlap in the causative agents between ICU and NICU.
3. CU due to an unknown mechanism
Some reactions of CU have unknown mechanisms that remain unclassified. Ammonium persulphate is a classic example of this type of response. CU due to ammonium persulphate can be mediated by type 1 (IgE-mediated) or by classical pathway (IgG or IgM-mediated).
Protein contact dermatitis
Protein contact dermatitis (PCD) was first described in kitchen workers who had localized dermatitis of the hands and forearm. PCD is chronic hand eczema that occurs in contact with proteins, with or without a positive prick test.[17] The pathophysiology involves repeated microtrauma to the skin, leading to the penetration of the large protein molecules. It involves a type I, type IV, or a delayed phase-type I hypersensitivity reaction. The reaction occurs within minutes of contact, but only 15% reported an immediate-type hypersensitivity reaction.[17][18] Those who react to specific dietary proteins rarely present with systemic symptoms on oral ingestion.[19] The proteins can spread from the hands to the sites of contact, including the face or abdomen.[19][18][20]
History and Physical
When a patient presents to the office with symptoms and signs suggestive of CU, a good history and clinical examination aids in the accurate diagnosis of CU.
Clinical history:
In addition to general history-taking, the clinician should focus particular attention on the history of exposure to potential causative agents in food, home/work environment, cosmetics, and personal care products. Temporal relationship from the time of exposure to the development of symptoms and time for resolution of symptoms is necessary information. Also include aggravating factors (i.e., temperature changes, physical pressure, etc.) and relieving factors like medications in history. Obtain details on the specific skin symptoms (Ex. wheals, tingling, burning, itching) and progression of symptoms. A personal and family history of atopy helps make the diagnosis of IgE-mediated ICU. Volatile proteins can cause signs and symptoms of conjunctivitis, rhinitis, or asthma when they come in contact with the mucosa of the conjunctiva or respiratory tract (Ex. flour). Other systemic symptoms like abdominal pain, itching in the mouth on oral ingestion (oral allergy syndrome), and diarrhea may develop in contact with the mucosa of the gastrointestinal tract.
Physical examination:
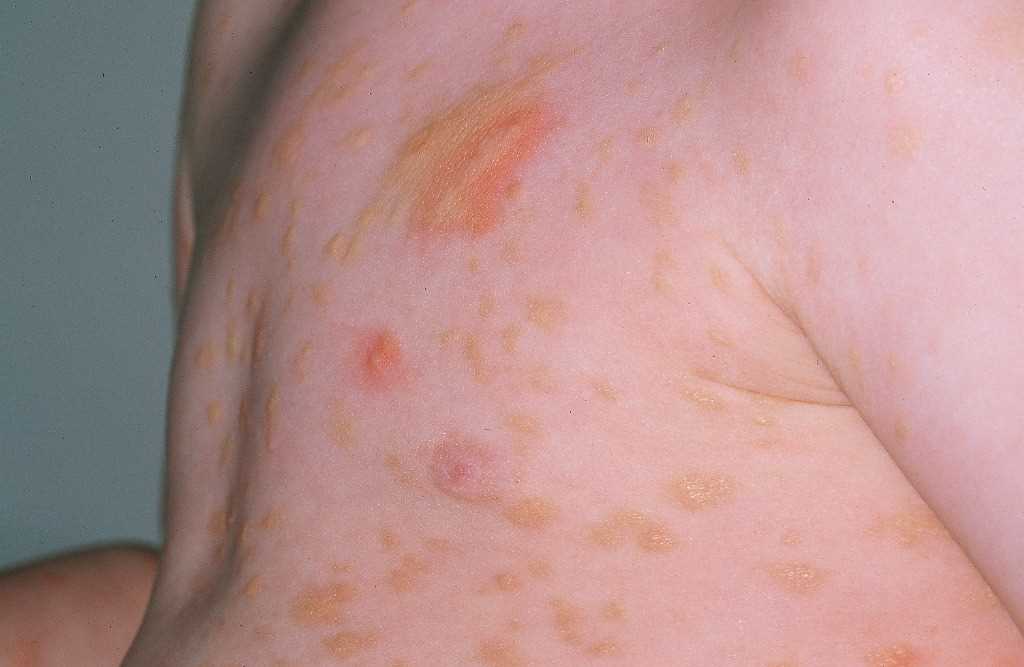
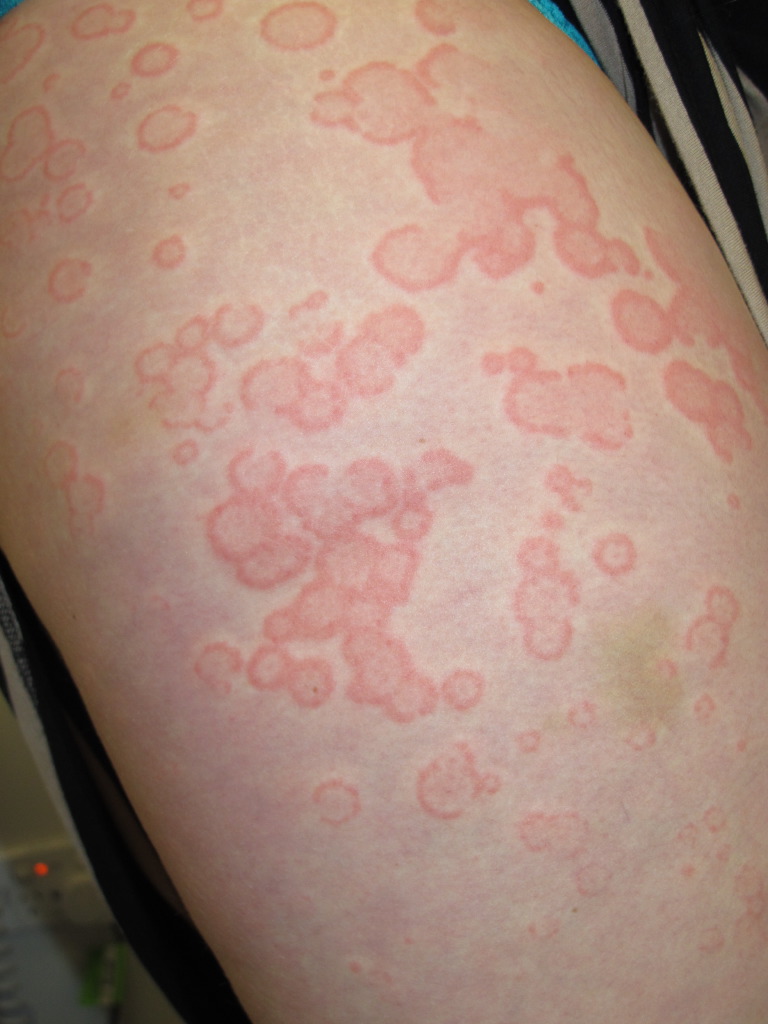
Clinical presentation is commonly a wheal and flare response and urticarial swelling. Patients affected by CU can present with hives (urticaria) or dermatitis (eczema). The urticarial response appears as an erythematous swelling with surrounding pallor. In a NICU reaction, the typical wheal and flare response may be absent. The skin examination should focus on the site of involvement, the pattern of distribution, size, shape, and confluency of lesions, angioedema, erythema, pallor, dermographism, and urticarial vasculitis. CU wheals do not blister, and scaling is generally not seen. They remain transient without any residual skin changes or pigmentation.[21] The diagnostic signs are different during an active flare vs. a period of quiescence.
PCD presents as recurrent dermatitis with urticarial plaques or vesicles. The vesicles can develop rapidly and progress to erythematous-squamous or erythematous-vesicular lesions.[22] Paronychia with periungual edema and erythema can be a sign of PCD.[23]
Evaluation
In-vivo and in-vitro methods are useful in diagnostic evaluation, in addition to history and physical examination. Skin tests are quick to perform and considered relatively safe.
In-vivo test
In-vivo tests are most common for the diagnosis of CU. Clinical supervision with the provision to handle an impending anaphylactic shock is necessary. Both ICU and NICU reactions occur within 15 to 20 minutes or can be delayed. If testing on intact skin is negative, then previously affected skin sites are used. Positive test results require careful correlation with clinical symptoms. Medications like antihistamines, NSAIDs, and exposure to ultra-violet light can cause false-negative results. The patient should be off antihistamines for 48 hours before testing. See figure 1 for the diagnostic algorithm to test for immediate contact reactions.[6][24]
- Prick test: It is considered the test of choice for the diagnosis of ICU. The inner aspect of the forearm is the preferred site, and multiple allergens can undergo testing simultaneously, using positive control with histamine and negative control with normal saline with results interpreted in 30 minutes. False-positive results occur due to a positive reaction from one test site spreading to the neighboring site or irritant reaction. PCD is diagnosable with the prick test. If skin prick tests and serum allergen-specific IgE tests are negative, a provocation or direct challenge test may be needed.
- Open test/ skin provocation test: The open test is used for the diagnosis of NICU. The test consists of gently rubbing 0.1ml of the test substance over a 3 cm x 3 cm area of intact skin on the volar aspect of the forearm. The site requires an examination at 20, 40, and 60 minutes. The best vehicle for testing the agents is an alcohol-water or alcohol propylene glycol mixture.
- Use test: The test agent in the same vehicle of delivery is used to observe for the reaction. The serial incremental dosing of the agent is. The usual sites of skin testing are the upper back, flexor aspect of the upper arm, and forearm.
- Chamber/ patch test: The test substance is applied to small aluminum containers for 15 minutes and attached to the skin via porous tape. The results are recorded at 20, 40, and 60 minutes for an immediate reaction. A delayed-type response gets assessed at 48 to 96 hours after patch application to the back. The sensitivity of this test is higher due to increased absorption by the skin. A smaller area of skin is used than the open test. A false-positive response called 'angry back' due to active dermatitis is noted in some. A patch test is not routinely recommended because many common allergens may cause a false positive response (ex. balsam of Peru, paraben, sorbic acid, formaldehyde, cinnamaldehyde, etc.).
- Scratch test: A deep dermal scratch with the test substance is performed using the blunt bottom of a lancet after the allergen is applied to the skin; this is useful for diagnosing CU to non-standard allergens.[12] Due to the risk of anaphylaxis, it should always take place under medical supervision.
- Intradermal testing: The test uncommon due to the risk of anaphylaxis. When testing with non-standardized substances, the test controls should be assessed on at least 20 people to rule out false-positive results.
- Dimethylsulfoxide (DMSO) test: The test is fast, reliable, and used to test the integrity of the skin barrier function. A wheal response occurs on the application of DMSO to the skin in susceptible individuals. A clinical grading is available to quantify the response (0-no reaction, 1-follicular wheal, 2-slight elevated solid wheal, 3- elevated solid, tense wheal).
In-vitro test
For the diagnosis of ICU, the presence of IgE-specific antibodies against the causative agent is used[10]. Based on the patient’s history, lab workup typically includes complete blood count (CBC), blood culture, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), basic metabolic panel (BMP), thyroid auto-antibodies, anti-nuclear antibody level (ANA) and complement levels (C3, C4) to rule out differential diagnosis like systemic illness, chronic idiopathic urticaria, autoimmune diseases, infection, etc[25].
Treatment / Management
Avoidance of the offending substance is the cornerstone of management. Patients should be educated on cross-reactive proteins and advised avoidance if they develop symptoms on exposure.
Protective gear like gloves, skin conditioning creams or emollients, and cotton liners are necessary when the exposure at work is unavoidable.[26][27](A1)
For patients with allergy to NRL, alternatives such as nitrile, neoprene, and polyvinyl chloride gloves are recommended. Some of the healthcare institutions around the world have banned powdered NRL gloves at work resulting in the decline of incidence of CU.[28][29] For patients with CU to NRL, avoidance of all latex-containing products is recommended.
Second-generation H1-receptor blockers (Ex. diphenhydramine, hydroxyzine, loratadine, desloratadine) are the first-line treatment in ICU. Leukotriene inhibitors like montelukast, zafirlukast, and zileuton inhibit the inflammatory component.[12]
Aspirin and NSAIDs are the first-line treatment options for the management of NICU.
Self-administered subcutaneous epinephrine pens should be available at all times to the patient.
Steroids are second-line treatment options and help to prevent the delayed phase of an anaphylactic reaction.
Immunosuppressive drugs like cyclosporine and methotrexate are options in severe cases.
Ultraviolet A and B light inhibit NICU reaction, and the effect can last for up to 2 weeks after treatment. It has the advantage of inhibiting skin sites that are not directly irradiated.[30](A1)
Subcutaneous immunotherapy in the management of CU is an object of research. A study on 41 bakers sensitized to wheat protein who received subcutaneous immunotherapy, has shown promise as an intervention in the management of CU.[31] Immunotherapy with standardized latex extract in workers sensitized to NRL has shown improvement in the skin reactivity.[32](A1)
A rush (2 or 4-day) sublingual desensitization to NRL has shown significant improvement in symptom scores.[33][34](B3)
Differential Diagnosis
- Urticaria and angioedema can be a part of systemic diseases like autoimmune disorders, endocrinopathies, malignancy, and hemolytic disease. Chronic idiopathic urticaria is usually associated with autoimmune disease.
- Anaphylaxis can present with generalized urticaria with other systemic symptoms.
- CU has overlapping features with allergic contact dermatitis, acquired angioedema, dermatographism, and irritant contact dermatitis.
- Glove related hand urticaria is a type of physical urticaria due to the shearing forces during the application and removal of gloves and requires differentiation from CU to NRL.
- Physical urticarial forms like aquagenic, cold, and solar urticaria can present similar to CU.
- Acute urticaria requires differentiation from erythema multiforme (with typical target lesions), toxic erythema (fixed and symmetric lesions), acute contact dermatitis, polymorphic eruption of pregnancy, and scombroid fish poisoning.[25]
Prognosis
Most of the patients affected by CU experience relief of symptoms with avoidance of triggers and medications. The disease duration is shorter for infants and children and prolonged when associated with other atopic diseases or with other systemic manifestations as seen in ICU.[25] Repeated exposure to the same agents may lead to a progressively severe response in ICU.
Complications
ICU has the potential to lead to life-threatening symptoms like anaphylaxis; hence, it is important to diagnose and manage these patients appropriately. Other complications include the risk of secondary bacterial infection from skin breakdown from repeated scratching.
Consultations
Referral to allergist or dermatologist is necessary in severe cases.
Deterrence and Patient Education
- Avoidance of the offending agent is the key-stone to disease management. For patients with allergy to NRL, avoidance or use of alternative glove materials like bamboo viscose gloves, neoprene, nitrile, or polyvinyl chloride gloves is necessary.
- Primary prevention strategies like avoidance of triggers, pre-employment counseling, and the implementation of personal protective equipment are prudent measures.
- Organizational precautions at the workplace, early disease diagnosis, and screening are other measures.
- Patients with CU should carry subcutaneous epinephrine pens, anti-histamine, and NSAIDs for management.
- Avoid scratching the skin due to the risk of introduction of extraneous bacteria leading to the risk of cellulitis or skin abscess.
Pearls and Other Issues
CU is widely un-recognized, and the provider's knowledge in diagnosing and management of CU will aid in the proper patient management.
Enhancing Healthcare Team Outcomes
Contact urticaria is an often underdiagnosed dermatological condition that is recognized as occupational dermatoses by the World Health Organisation (WHO). It requires the efforts of an interprofessional healthcare team. The general physician is usually the first point of contact for patients seeking help and knowledge of disease presentation, testing, and management can help improve the quality of life of the patients and reduce disease burden. Due to the short time taken for symptom resolution, patients usually first present after the symptoms have disappeared, making diagnosis a challenge. Referral to allergist or dermatologist is integral while managing CU. Specialists can aid in the diagnosis of atypical presentations and management of poorly-controlled cases. Nursing plays a vital role during the diagnosis procedure due to the need to monitor patients closely for anaphylaxis. CU requires an inter-disciplinary approach for optimal results. The pharmacist should review all medications and discuss potential interactions with the prescribing/treating clinician, as well as verifying all dosing. The examples of interprofessional collaboration show how this approach can improve patient outcomes. [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Orb Q, Millsop JW, Harris K, Powell D. Prevalence and interest in the practice of scratch testing for contact urticaria: a survey of the American contact dermatitis society members. Dermatitis : contact, atopic, occupational, drug. 2014 Nov-Dec:25(6):366-9. doi: 10.1097/DER.0000000000000086. Epub [PubMed PMID: 25384226]
Level 3 (low-level) evidenceAmaro C, Goossens A. Immunological occupational contact urticaria and contact dermatitis from proteins: a review. Contact dermatitis. 2008 Feb:58(2):67-75. doi: 10.1111/j.1600-0536.2007.01267.x. Epub [PubMed PMID: 18186738]
Level 3 (low-level) evidenceDoutre MS. Occupational contact urticaria and protein contact dermatitis. European journal of dermatology : EJD. 2005 Nov-Dec:15(6):419-24 [PubMed PMID: 16280292]
Suneja T, Belsito DV. Occupational dermatoses in health care workers evaluated for suspected allergic contact dermatitis. Contact dermatitis. 2008 May:58(5):285-90. doi: 10.1111/j.1600-0536.2007.01315.x. Epub [PubMed PMID: 18416759]
Level 2 (mid-level) evidenceValsecchi R, Leghissa P, Cortinovis R, Cologni L, Pomesano A. Contact urticaria from latex in healthcare workers. Dermatology (Basel, Switzerland). 2000:201(2):127-31 [PubMed PMID: 11053915]
Gimenez-Arnau A, Maurer M, De La Cuadra J, Maibach H. Immediate contact skin reactions, an update of Contact Urticaria, Contact Urticaria Syndrome and Protein Contact Dermatitis -- "A Never Ending Story". European journal of dermatology : EJD. 2010 Sep-Oct:20(5):552-62. doi: 10.1684/ejd.2010.1049. Epub 2010 Aug 23 [PubMed PMID: 20732848]
Kanerva L, Toikkanen J, Jolanki R, Estlander T. Statistical data on occupational contact urticaria. Contact dermatitis. 1996 Oct:35(4):229-33 [PubMed PMID: 8957643]
Level 3 (low-level) evidenceWakelin SH. Contact urticaria. Clinical and experimental dermatology. 2001 Mar:26(2):132-6 [PubMed PMID: 11298101]
Süß H, Dölle-Bierke S, Geier J, Kreft B, Oppel E, Pföhler C, Skudlik C, Worm M, Mahler V. Contact urticaria: Frequency, elicitors and cofactors in three cohorts (Information Network of Departments of Dermatology; Network of Anaphylaxis; and Department of Dermatology, University Hospital Erlangen, Germany). Contact dermatitis. 2019 Nov:81(5):341-353. doi: 10.1111/cod.13331. Epub 2019 Jul 3 [PubMed PMID: 31173644]
Harvell J, Bason M, Maibach HI. Contact urticaria (immediate reaction syndrome). Clinical reviews in allergy. 1992 Winter:10(4):303-23 [PubMed PMID: 1295692]
Wang CY, Maibach HI. Immunologic contact urticaria--the human touch. Cutaneous and ocular toxicology. 2013 Jun:32(2):154-60. doi: 10.3109/15569527.2012.727519. Epub 2012 Oct 9 [PubMed PMID: 23046149]
Bhatia R, Alikhan A, Maibach HI. Contact urticaria: present scenario. Indian journal of dermatology. 2009 Jul:54(3):264-8. doi: 10.4103/0019-5154.55639. Epub [PubMed PMID: 20161861]
Novembre E, Cianferoni A, Mori F, Barni S, Calogero C, Bernardini R, Di Grande L, Pucci N, Azzari C, Vierucci A. Urticaria and urticaria related skin condition/disease in children. European annals of allergy and clinical immunology. 2008 May:40(1):5-13 [PubMed PMID: 18700329]
Davari P, Maibach HI. Contact urticaria to cosmetic and industrial dyes. Clinical and experimental dermatology. 2011 Jan:36(1):1-5. doi: 10.1111/j.1365-2230.2010.03854.x. Epub [PubMed PMID: 20456377]
McFadden J. Immunologic contact urticaria. Immunology and allergy clinics of North America. 2014 Feb:34(1):157-67. doi: 10.1016/j.iac.2013.09.005. Epub [PubMed PMID: 24262696]
Level 3 (low-level) evidenceZhai H, Zheng Y, Fautz R, Fuchs A, Maibach HI. Reactions of non-immunologic contact urticaria on scalp, face, and back. Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI). 2012 Nov:18(4):436-41. doi: 10.1111/j.1600-0846.2011.00590.x. Epub 2011 Nov 9 [PubMed PMID: 22093067]
Level 1 (high-level) evidenceVester L, Thyssen JP, Menné T, Johansen JD. Occupational food-related hand dermatoses seen over a 10-year period. Contact dermatitis. 2012 May:66(5):264-70. doi: 10.1111/j.1600-0536.2011.02048.x. Epub [PubMed PMID: 22486568]
Level 2 (mid-level) evidenceBrancaccio RR, Alvarez MS. Contact allergy to food. Dermatologic therapy. 2004:17(4):302-13 [PubMed PMID: 15327475]
Barbaud A, Poreaux C, Penven E, Waton J. Occupational protein contact dermatitis. European journal of dermatology : EJD. 2015 Nov-Dec:25(6):527-34. doi: 10.1684/ejd.2015.2593. Epub [PubMed PMID: 26242922]
Hernández-Bel P, de la Cuadra J, García R, Alegre V. [Protein contact dermatitis: review of 27 cases]. Actas dermo-sifiliograficas. 2011 Jun:102(5):336-43. doi: 10.1016/j.ad.2011.02.005. Epub 2011 Apr 15 [PubMed PMID: 21497331]
Level 2 (mid-level) evidenceGiménez-Arnau A. Contact urticaria and the environment. Reviews on environmental health. 2014:29(3):207-15. doi: 10.1515/reveh-2014-0042. Epub [PubMed PMID: 25153542]
Lynde C, Guenther L, Diepgen TL, Sasseville D, Poulin Y, Gulliver W, Agner T, Barber K, Bissonnette R, Ho V, Shear NH, Toole J. Canadian hand dermatitis management guidelines. Journal of cutaneous medicine and surgery. 2010 Nov-Dec:14(6):267-84. doi: 10.2310/7750.2010.09094. Epub [PubMed PMID: 21084020]
Kanerva L. Occupational protein contact dermatitis and paronychia from natural rubber latex. Journal of the European Academy of Dermatology and Venereology : JEADV. 2000 Nov:14(6):504-6 [PubMed PMID: 11444276]
Level 3 (low-level) evidencevon Krogh G, Maibach HI. The contact urticaria syndrome--an updated review. Journal of the American Academy of Dermatology. 1981 Sep:5(3):328-42 [PubMed PMID: 6455450]
Sabroe RA. Acute urticaria. Immunology and allergy clinics of North America. 2014 Feb:34(1):11-21. doi: 10.1016/j.iac.2013.07.010. Epub 2013 Aug 24 [PubMed PMID: 24262686]
Adisesh A, Robinson E, Nicholson PJ, Sen D, Wilkinson M, Standards of Care Working Group. U.K. standards of care for occupational contact dermatitis and occupational contact urticaria. The British journal of dermatology. 2013 Jun:168(6):1167-75. doi: 10.1111/bjd.12256. Epub [PubMed PMID: 23374107]
Level 1 (high-level) evidenceNicholson PJ. Evidence-based guidelines: occupational contact dermatitis and urticaria. Occupational medicine (Oxford, England). 2010 Oct:60(7):502-4. doi: 10.1093/occmed/kqq075. Epub [PubMed PMID: 20871017]
Level 1 (high-level) evidenceAllmers H, Schmengler J, John SM. Decreasing incidence of occupational contact urticaria caused by natural rubber latex allergy in German health care workers. The Journal of allergy and clinical immunology. 2004 Aug:114(2):347-51 [PubMed PMID: 15316514]
Bensefa-Colas L, Telle-Lamberton M, Faye S, Bourrain JL, Crépy MN, Lasfargues G, Choudat D, RNV3P members, Momas I. Occupational contact urticaria: lessons from the French National Network for Occupational Disease Vigilance and Prevention (RNV3P). The British journal of dermatology. 2015 Dec:173(6):1453-61. doi: 10.1111/bjd.14050. Epub 2015 Oct 26 [PubMed PMID: 26212252]
Larmi E. PUVA treatment inhibits nonimmunologic immediate contact reactions to benzoic acid and methyl nicotinate. International journal of dermatology. 1989 Nov:28(9):609-11 [PubMed PMID: 2583908]
Level 1 (high-level) evidenceCirla AM, Lorenzini RA, Cirla PE. [Specific immunotherapy and relocation in occupational allergic bakers]. Giornale italiano di medicina del lavoro ed ergonomia. 2007 Jul-Sep:29(3 Suppl):443-5 [PubMed PMID: 18409768]
Sastre J, Fernández-Nieto M, Rico P, Martín S, Barber D, Cuesta J, de las Heras M, Quirce S. Specific immunotherapy with a standardized latex extract in allergic workers: a double-blind, placebo-controlled study. The Journal of allergy and clinical immunology. 2003 May:111(5):985-94 [PubMed PMID: 12743562]
Level 1 (high-level) evidencePatriarca G, Nucera E, Pollastrini E, Roncallo C, Buonomo A, Bartolozzi F, De Pasquale T, Gasbarrini G, Schiavino D. Sublingual desensitization: a new approach to latex allergy problem. Anesthesia and analgesia. 2002 Oct:95(4):956-60, table of contents [PubMed PMID: 12351276]
Tabar AI, Gómez B, Arroabarren E, Rodríguez M, Lázaro I, Anda M. [Perspectives in the treatment of allergy to latex: immunotherapy]. Anales del sistema sanitario de Navarra. 2003:26 Suppl 2():97-102 [PubMed PMID: 13679968]
Level 3 (low-level) evidence