Introduction
Arrhythmias originating from the ventricular myocardium or His-Purkinje system are grouped under ventricular arrhythmia (VA). This includes a subset of arrhythmias such as ventricular tachycardia (VT), ventricular fibrillation (VF), premature ventricular contractions (PVC), and ventricular flutter. Wide complex tachycardia (WCT) is used to define all tachyarrhythmia with QRS complex duration greater than 0.12 seconds. VF is a WCT caused by irregular electrical activity and characterized by a ventricular rate of usually greater than 300 with discrete QRS complexes on the electrocardiogram (ECG). QRS morphology in VF varies in shape, amplitude, and duration with a prominent irregular rhythm.[1] VF is an extremely dangerous rhythm significantly compromising cardiac output and ultimately leading to sudden cardiac death (SCD).
VF has been identified in nearly 70% of cardiac arrest patients. Without treatment, the condition is fatal within minutes. The rates of survival for VF patients outside the hospitals have increased slightly but many continue to have residual anoxic brain damage and neurological deficits.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
VF is often linked to underlying structural heart disease. Three percent to 12% of cases of myocardial infarction (MI) develop VF during the acute phase.[2] MI patients with complete coronary occlusion on an angiogram, anterior wall infarction, atrial fibrillation, and pre-infarction angina are more prone to develop VF.[3] Many common conditions associated with VF include electrolyte abnormalities (hypokalemia/hyperkalemia, hypomagnesemia), acidosis, hypothermia, hypoxia, cardiomyopathies, family history of sudden cardiac death, congenital QT abnormalities, Brugada syndrome, and alcohol use. Patients with a history of VA especially sustained monomorphic or polymorphic VT may transition to VF in susceptible patients.[4] Genetic predisposition to VF is now increasingly recognized. The first genome-wide association was reported in the AGNES study identifying susceptibility locus for VF at 21q21.[5]
Epidemiology
In 2017 the American Heart Association (AHA) update estimated the total annual burden of Out of Hospital Cardiac Arrest (OHCA) at 356,500. At least 23% of OHCA treated by Emergency Medical Service (EMS) have VF/VT as the initial rhythm. With more than 60% of cardiovascular deaths resulting from cardiac arrest, it remains the leading cause of death worldwide.[6] Modern advances in assistive devices such as implantable cardioverter-defibrillator (ICD) have had a significant impact on these numbers. Many studies have identified VF as the most common underlying arrhythmia in patients with SCD. Among patients hospitalized with acute MI, 5% to 10% have VF or VT, and another 5% will have VF or VT within 48 hours of admission.[7] The true incidence of VF is underestimated, as individuals who suffer OHCA are not covered in most of these studies. Based on Resuscitation Outcomes Consortium (ROC) data, survival to hospital discharge for VF patients was 31.4%.
Pathophysiology
VF occurs when parts of ventricular myocardium depolarize erratically in an uncoordinated manner. VF results from the following:
Abnormal Impulse Formation
- Increased automaticity: Purkinje cells around ischemic areas during MI can initiate ventricular tachycardia.
- Triggered activity: Early and late after depolarization can overcome refractory threshold and generate triggered potential causing extrasystoles.
Impulse Conduction
- Functional and anatomical reentry circuits help sustain ventricular arrhythmia.
History and Physical
The most common presentation for VF is sudden collapse from cardiac arrest leading to SCD. This results from improper ventricular contraction resulting in low cardiac output. Patients may demonstrate signs of acute MI such as chest pain, shortness of breath, nausea, and vomiting before the event. Patients with a known history of coronary artery disease or congestive heart failure may show a worsening of chronic symptoms such as angina, dyspnea, orthopnea, paroxysmal nocturnal dyspnea, and pedal edema. At the time of presentation, patients are unconscious, unresponsive, and have no palpable pulse. Without prompt action, this leads to death within the next few minutes. Patients with ICD for primary or secondary prevention can experience shock from ICD firing at the time of experiencing VF.
Evaluation
Acute presentation of symptoms and ECG findings lead to the diagnosis.
ECG Findings
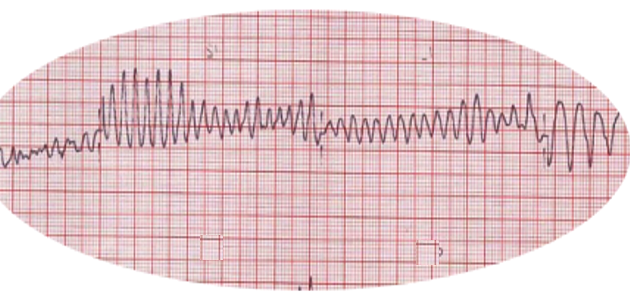
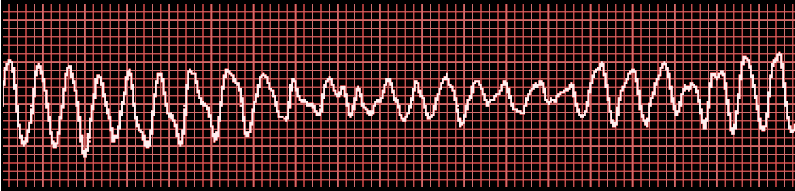
- Fibrillation waves of varying amplitude and shape.
- No identifiable P waves, QRS complexes, or T waves
- Heart rate anywhere between 150 to 500 per minute
VF storm: Identified by 3 or more episodes of VF or appropriate shocks from ICD within 24 hours
Patients surviving VF should have a thorough history and physical examination. A family history of unexplained cardiac death should be noted. Physicians should review c the patient's cardiac history and the medication list for arrhythmogenic drugs closely. They should also look for and correct reversible causes of VF such as electrolyte abnormalities, acidosis, and hypoxia. The healthcare professional should also evaluate patients for underlying ischemic heart disease with an echocardiogram and emergency angiogram. Of all OHCA, more than 50% have significant coronary artery disease on angiogram.[8]
Appropriate laboratory studies include:
- Serum electrolytes
- Arterial blood gas
- Complete blood cell count
- Cardiac enzymes
- Levels of drugs
- Toxicology screen
- BNP levels
The ECG may reveal:
- MI
- Brugada syndrome
- Long or short QT interval
- WPW
- Digitalis toxicity
- Epsilon sign (arrhythmogenic right ventricular cardiomyopathy)
ECHO is usually done to assess the wall motion, ejection fraction and any valvular problems. In addition, Echo will identify any pericardial fluid that may have resulted from CPR.
EPS is done after the patient is stable to differentiate patients with inducible VF from those with noninducible VF. Patients with induced monomorphic ventricular arrhythmias may be candidates for an ICD.
Treatment / Management
Acute Management
Due to the high mortality rate and extreme acuity of the condition, VF patients warrant immediate attention. Healthcare professionals should immediately initiate guideline-directed management as per Advanced Cardiac Life Support (ACLS) protocol. There is a lower likelihood of survival if the healthcare professional deviates from the ACLS guidelines.[9] All patients with cardiac arrest should have an initial assessment while receiving quality CPR. Pulseless VT and VF are both shockable rhythm, and once the staff identifies the rhythm as VF, patients should be shocked immediately with 120 to 200 joules on a biphasic defibrillator or 360 joules using a monophasic. Patients receiving prompt defibrillation have shown improved survival (39.3%) compared to patients in whom defibrillation was delayed by 2 minutes or more (22.2%).[10] Administer epinephrine and amiodarone as per ACLS protocol in patients sustaining VF rhythm regardless of receiving 3 shocks. Amiodarone significantly improves survival to hospital admission without affecting survival to hospital discharge.[11] Identifying and addressing the cause of inciting event is equally important. Professionals should undertake cause-specific measures such as securing the airway, correcting electrolytes, administrating fluids, decompressing pneumothorax, draining tamponade while resuscitating the patient. Once the patient attains return of spontaneous circulation (ROSC), physicians should begin a definitive evaluation for coronary artery disease.(A1)
Preventative Management
Primary prevention has been a significant factor in reducing VF-related SCDs. Most VF transition from VT and other VA and hence identifying such arrhythmias at an early stage can help prevent VF. In patients with symptoms suspected to be related to VA, detection using ambulatory electrocardiography and implanted cardiac monitors is recommended. [7]. Healthcare professionals should offer family members of patients with inherited arrhythmia syndromes genetic testing and counseling for risk stratification.(A1)
Medication Therapy
Amiodarone is the most commonly studied antiarrhythmic for prevention of SCD. The overall effect of amiodarone on survival is controversial. Most studies have failed to show any added benefit when compared to placebo or ICD.[12] Sotalol, on the other hand, is associated with an increased risk of mortality by decreasing the defibrillation threshold. A meta-analysis published in 2007 showed a significant reduction in risk of SCD with statin treatment.[13] Lower SCD incidence has been reported in patients on chronic beta-blocker therapy for heart failure with reduced ejection fraction.(A1)
Defibrillators
Randomized control trials such as MADIT-I (Multicenter Automated Defibrillator Implantation Trial), MADIT-II, SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) have clearly demonstrated mortality benefits with ICD when compared to standard medical therapy. Trials comparing ICD with antiarrhythmic therapy such as AVID (Antiarrhythmic Versus Implantable Defibrillator) have shown similar results.
ICD placement is recommended for primary prevention of SCD in patients at increased risk of life-threatening VF/VT. ICD placement is also indicated for secondary prevention of SCD in patients with prior episodes of VF and sustained VT.
Differential Diagnosis
It is important to differentiate VF from pulseless electrical activity (PEA)/asystole as both these conditions are managed differently as per ACLS protocol. Other causes of sudden collapse such as aortic dissection and pulmonary embolism should be considered. The following conditions can be easily confused with VF on an ECG and should be ruled out.
- Polymorphic ventricular tachycardia
- Torsade de pointes
- Ventricular flutter
- Pulseless electrical activity
- Accelerated idioventricular rhythm
- SVT with aberrancy
Staging
Types of VF on ECG
- Coarse VF: Majority of the waveforms measure 3 mm or greater
- Fine VF: Majority of the waveforms measure less than 3 mm
Prognosis
Prognosis of VF depends on the time from onset to early intervention and defibrillation. Shorter delays are associated with survival rates as high as 50%.[14] In patients with ST-elevation MI, early VF (fewer than 24 hours) is associated with increased mortality compared to late VF (longer than 24 hours).[15]
VF outside the hospital can be reversed as today there are defibrillators available in many places. But the success of reversal declines at a rate of 5-10% for every minute that is delayed. Even under ideal circumstances, 30-40% of patients survive but many also develop residual neurological deficits because of anoxia. Full recovery is rare.
Complications
- Anoxic brain injury
- Post defibrillation arrhythmias
- Injuries from CPR and resuscitation
- Skin burns
- Long terms disabilities
- Myocardial injury
- Death
Consultations
Consultations should include cardiology, electrophysiology, and interventional cardiology.
Enhancing Healthcare Team Outcomes
Cardiac arrest is a near-death experience impacting not only the patient but family members too. A variety of health care professionals should be involved from the onset of arrest up to hospital discharge. Studies have continuously demonstrated the importance of Interprofessional communication and teamwork while resuscitating patients. Successful resuscitation is an outcome of good communication and understanding your responsibilities. (LOE-1) Physicians, nurses, pharmacists, laboratory technicians all come together and commit to the success of the team.
Post-resuscitation care brings further contributions from consultants such as a cardiologist, electrophysiologists, and neurologists. These patients need close monitoring by the nurses as recurrence of VF is high. In addition, the patient should be assessed for complications like aspiration, CPR related injuries, renal failure, and brain anoxic damage
Unfortunately, mortality associated with VF/SCA remains high. Involving family in every step of management is pivotal as it helps in providing a holistic picture. Seeking help from chapel when deciding about goals of care can help the family make difficult decisions. Mild hypothermia has been shown to improve outcomes and should be considered. The patient needs to be seen by the neurologist to assess brain activity, dietitian to assess feeding, nephrologist as renal failure is common, and the pulmonologist to ensure that no aspiration has occurred during resuscitation. The entire team should communicate with each other to ensure that the patient is receiving the optimal standard of care.
Outcomes
Patient outcomes can be improved by spreading awareness about the condition and by identifying patients at risk. The outcome of cardiac arrest and subsequently VF depends on time from the arrest to first intervention. Educating the general population about the basics of resuscitation can help avoid delay in treatment and improve patient outcomes especially in OHCA. Identifying and establishing roles amongst various team members can improve outcomes among In-hospital cardiac arrest (IHCA). Mock drills to assess for preparedness can help enhance the quality of resuscitation efforts.
Media
References
American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology), Buxton AE, Calkins H, Callans DJ, DiMarco JP, Fisher JD, Greene HL, Haines DE, Hayes DL, Heidenreich PA, Miller JM, Poppas A, Prystowsky EN, Schoenfeld MH, Zimetbaum PJ, Goff DC, Grover FL, Malenka DJ, Peterson ED, Radford MJ, Redberg RF. ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (ACC/AHA/HRS Writing Committee to Develop Data Standards on Electrophysiology). Circulation. 2006 Dec 5:114(23):2534-70 [PubMed PMID: 17130345]
Glinge C, Sattler S, Jabbari R, Tfelt-Hansen J. Epidemiology and genetics of ventricular fibrillation during acute myocardial infarction. Journal of geriatric cardiology : JGC. 2016 Sep:13(9):789-797 [PubMed PMID: 27899944]
Jabbari R, Engstrøm T, Glinge C, Risgaard B, Jabbari J, Winkel BG, Terkelsen CJ, Tilsted HH, Jensen LO, Hougaard M, Chiuve SE, Pedersen F, Svendsen JH, Haunsø S, Albert CM, Tfelt-Hansen J. Incidence and risk factors of ventricular fibrillation before primary angioplasty in patients with first ST-elevation myocardial infarction: a nationwide study in Denmark. Journal of the American Heart Association. 2015 Jan 5:4(1):e001399. doi: 10.1161/JAHA.114.001399. Epub 2015 Jan 5 [PubMed PMID: 25559012]
Level 2 (mid-level) evidenceSamie FH,Jalife J, Mechanisms underlying ventricular tachycardia and its transition to ventricular fibrillation in the structurally normal heart. Cardiovascular research. 2001 May [PubMed PMID: 11334828]
Bezzina CR, Pazoki R, Bardai A, Marsman RF, de Jong JSSG, Blom MT, Scicluna BP, Jukema JW, Bindraban NR, Lichtner P, Pfeufer A, Bishopric NH, Roden DM, Meitinger T, Chugh SS, Myerburg RJ, Jouven X, Kääb S, Dekker LRC, Tan HL, Tanck MWT, Wilde AAM. Genome-wide association study identifies a susceptibility locus at 21q21 for ventricular fibrillation in acute myocardial infarction. Nature genetics. 2010 Aug:42(8):688-691. doi: 10.1038/ng.623. Epub 2010 Jul 11 [PubMed PMID: 20622880]
Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nature reviews. Cardiology. 2010 Apr:7(4):216-25. doi: 10.1038/nrcardio.2010.3. Epub 2010 Feb 9 [PubMed PMID: 20142817]
Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2018 Oct 2:72(14):1677-1749. doi: 10.1016/j.jacc.2017.10.053. Epub 2017 Oct 30 [PubMed PMID: 29097294]
Level 1 (high-level) evidenceDumas F, Cariou A, Manzo-Silberman S, Grimaldi D, Vivien B, Rosencher J, Empana JP, Carli P, Mira JP, Jouven X, Spaulding C. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circulation. Cardiovascular interventions. 2010 Jun 1:3(3):200-7. doi: 10.1161/CIRCINTERVENTIONS.109.913665. Epub 2010 May 18 [PubMed PMID: 20484098]
Honarmand K, Mepham C, Ainsworth C, Khalid Z. Adherence to advanced cardiovascular life support (ACLS) guidelines during in-hospital cardiac arrest is associated with improved outcomes. Resuscitation. 2018 Aug:129():76-81. doi: 10.1016/j.resuscitation.2018.06.005. Epub 2018 Jun 6 [PubMed PMID: 29885353]
Chan PS, Krumholz HM, Nichol G, Nallamothu BK, American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to defibrillation after in-hospital cardiac arrest. The New England journal of medicine. 2008 Jan 3:358(1):9-17. doi: 10.1056/NEJMoa0706467. Epub [PubMed PMID: 18172170]
Level 2 (mid-level) evidenceLaina A, Karlis G, Liakos A, Georgiopoulos G, Oikonomou D, Kouskouni E, Chalkias A, Xanthos T. Amiodarone and cardiac arrest: Systematic review and meta-analysis. International journal of cardiology. 2016 Oct 15:221():780-8. doi: 10.1016/j.ijcard.2016.07.138. Epub 2016 Jul 9 [PubMed PMID: 27434349]
Level 1 (high-level) evidenceBardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH, Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. The New England journal of medicine. 2005 Jan 20:352(3):225-37 [PubMed PMID: 15659722]
Level 1 (high-level) evidenceLevantesi G, Scarano M, Marfisi R, Borrelli G, Rutjes AW, Silletta MG, Tognoni G, Marchioli R. Meta-analysis of effect of statin treatment on risk of sudden death. The American journal of cardiology. 2007 Dec 1:100(11):1644-50 [PubMed PMID: 18036362]
Level 1 (high-level) evidenceHolmberg M, Holmberg S, Herlitz J. Incidence, duration and survival of ventricular fibrillation in out-of-hospital cardiac arrest patients in sweden. Resuscitation. 2000 Mar:44(1):7-17 [PubMed PMID: 10699695]
Demidova MM, Smith JG, Höijer CJ, Holmqvist F, Erlinge D, Platonov PG. Prognostic impact of early ventricular fibrillation in patients with ST-elevation myocardial infarction treated with primary PCI. European heart journal. Acute cardiovascular care. 2012 Dec:1(4):302-11. doi: 10.1177/2048872612463553. Epub [PubMed PMID: 24062921]