Introduction
Vestibular dysfunction is a disturbance of the body's balance system. Etiologies of this disorder are broadly categorized into peripheral and central causes based on the anatomy involved. The symptoms of peripheral and central vestibular dysfunction can overlap, and a comprehensive physical examination can often help differentiate the two. Vestibular disorders usually present acutely, and the most common form of acute peripheral vestibular dysfunction is benign paroxysmal positional vertigo (BPPV).[1]
The most common cause of severe central vestibular dysfunction is an ischemic stroke of the posterior fossa, which contains the brainstem and cerebellum. Acute ischemic stroke accounts for up to 25% of patients who present with central vestibular dysfunction. Since acute stroke is treated differently from other causes of disequilibrium, it is essential to recognize this process promptly. Vertebrobasilar artery disease can lead to stroke in 5% of patients, and patients with this condition often present initially with syncopal episodes and/or vestibular dysfunction. The second most common cause of central vestibular dysfunction is a demyelinating disease.[2][3]
Symptoms of vestibular dysfunction include a variety of complaints: vertigo, nausea and vomiting, intolerance to head motion, spontaneous nystagmus, unsteady gait, and postural instability.[4] The prevalence of each of these symptoms varies, and there is no single symptom that is pathognomonic for vestibular dysfunction. The presentation of these symptoms as a cluster should raise the level of clinical suspicion for vestibular dysfunction. A complete history and physical examination is the best way to differentiate peripheral from central vestibular dysfunction.[5][6][7][8]
Identifying which type of vestibular dysfunction a patient has is crucial, as this determines the therapeutic approach and the urgency of initiating treatment.[9] The mainstay of treatment for peripheral vestibular disorders is symptomatic therapy, but the treatment for central vestibular dysfunction caused by an ischemic stroke can include emergent intravenous thrombolytic therapy and interventional clot retrieval. Early identification of demyelinating disorders, such as multiple sclerosis, is essential so that treatment can be initiated to prevent the rapid decline and development of disability.[10]
This article will review the epidemiology, history and physical examination, evaluation, differential diagnosis, treatment, complications, and critical points in diagnosing and managing vestibular dysfunction and differentiating peripheral from central vestibular disorders.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of vestibular dysfunction can be divided into peripheral and central causes, both of which may present acutely or chronically. The term "peripheral" refers to a pathology of the vestibular system itself: the membranous labyrinth and the superior and inferior vestibular nerves. The term "central" refers to a pathology of the central nervous system (CNS) proper. Acute vestibular syndrome is characterized by a constellation of symptoms, including vertigo, nausea, vomiting, head motion intolerance, unsteady gait, and postural instability. The symptoms must persist for at least 24 hours to meet the criteria for an acute vestibular syndrome, except in cases of paroxysmal positional vertigo.
Paroxysmal positional vertigo is a mechanical disorder of the inner ear causing short intervals of transient vertigo, often accompanied by autonomic symptoms. Benign paroxysmal positional vertigo (BPPV) accounts for at least 20% of individuals with moderate to severe dizziness/vertigo and is the most common cause of brief, episodic, peripheral vestibular dysfunction.[2][3] Women and patients over age fifty are among the most commonly affected.[9][11]
The average duration of symptoms is two weeks, although individual episodes typically last less than one minute and may occur several times daily. Symptoms are caused by the stimulation of a semicircular canal, usually the posterior semicircular canal, and typically only affect one side. Anatomically, BPPV is most often caused by "canalithiasis," free-floating otoconia (calcium carbonate-protein crystals that have become detached from the utricle or saccule) that move to the most dependent position within the canal when the head changes position, thereby shifting the endolymph and causing displacement of the cupula with a subsequent spinning sensation. Less commonly, the particles may adhere to the cupula in the ampulla of the semicircular canal and cause similar but longer-lasting symptoms with head movements - this is termed "cupulolithiasis."[12] BPPV symptoms are most obvious with changes in position and are usually worse in the morning.[13]
Another common peripheral vestibular disorder is Ménière disease, characterized by episodes of vertigo lasting minutes to hours that are accompanied by hearing loss and roaring tinnitus. This condition is thought to be due to endolymphatic hydrops with distortion and distention of the endolymph portions of the labyrinthine system. The cause of excess fluid in the inner ear has been difficult to determine, but numerous proposed etiologies exist.
One of the etiologies is the blockage of the endolymphatic sac or duct. The entrance of the endolymphatic sac potentially becomes blocked by saccular otoconia that detach from the membranes and block the flow of endolymph. This is similar to the mechanism proposed for BPPV. The second theory is a hypoplasia of the vestibular aqueduct, leading to a reduction in the ability to regulate the volume of fluid in the endolymphatic space. The third is an immunologic mechanism that has not been clearly defined and may explain the association between Ménière disease and allergies. There also appears to be a genetic predisposition through an autosomal dominant inheritance pattern, which has been demonstrated in 8% to 15% of patients with Ménière disease.[14]
A family history of earlier age at onset is associated with genetic Ménière disease, which tends to produce more severe manifestations in successive generations. However, a specific gene marker has not been identified. There may also be a link between migraines and Ménière disease; as vestibular migraines may often be mistaken for Ménière disease, it may be that a vascular phenomenon that dysregulates blood flow plays a role in the production of vertigo.[15][16][11]
Another theory studied in the 1980s proposes that the rupture of the dilated or distended endolymphatic sac allows potassium-saturated endolymph into the perilymphatic space. The result is that the biochemical gradient depolarizes the cochlear and vestibular hair cells, leading to loss of function. Once the membrane rupture seals and the ion pumps restore the electrical gradient, the hair cells' function is normalized. This process repeats and results in degeneration of the hair cells. Trauma can also cause vestibular damage, whether via a direct blow, such as in the case of a temporal bone fracture, whiplash, or overpressure, as in a blast injury.[17][18][19]
In the vestibular variant of Ménière disease, hearing loss and tinnitus are absent, and differentiating this entity from a vestibular migraine can be very challenging. Bilateral disease may develop in up to 17% of patients, but it is much more likely to develop sequentially (78%) than simultaneously (22%).[20][21][20]
A third common peripheral cause of vertigo is vestibular neuronitis; it is believed to be caused by an acute viral or post-viral inflammatory disorder. The inflammation affects the vestibular branch of the eighth cranial nerve, resulting in hypofunctional vestibulopathy and vertigo that can persist for days at a time.[22] When the inflammation also affects the cochlea and causes hearing loss, the syndrome is referred to as labyrinthitis.[23]
Many other conditions may also result in peripheral vestibulopathy, including autoimmune disorders like Cogan syndrome and autoimmune inner ear disease, neoplasms like vestibular schwannoma, temporal bone fracture, vestibulotoxic medications like gentamicin and streptomycin, perilymph fistula, and semicircular canal dehiscence syndrome. Ultimately, any process that causes inflammation of the membranous labyrinth or vestibular nerves aberrantly stimulates the vestibular apparatus or structurally violates it can cause peripheral vertigo.
The common central causes of vestibulopathy are vertebrobasilar transient ischemic attack (TIA), acute ischemic stroke involving the vestibular nerve tracts, cerebellum, or brainstem; hemorrhagic stroke affecting the brainstem and cerebellum, and demyelinating diseases, such as multiple sclerosis, that affect the vestibular tracts, cerebellum, and brainstem.[24][25][26][27] Strokes affecting the brain stem and cerebellum have similar symptoms to peripheral vestibular dysfunction: vertigo, nystagmus, nausea and vomiting, and gait disturbances, although vital sign instability may also be seen. Another etiology of central vestibular dysfunction is brain injury affecting the cerebellum and brain stem, such as a concussion. The history and physical examination are critical in identifying the causes of vestibular dysfunction, given its broad differential diagnosis.
Epidemiology
Vestibular dysfunction often presents as dizziness. Dizziness is a common complaint of patients 40 years of age and older, leading to 10 million visits to ambulatory care settings per year and accounting for 25% of emergency department visits. Vestibular dysfunction is strongly associated with stroke and demyelinating diseases. Twenty-five percent of patients with vestibular disorders have had a stroke. Patients with vestibular dysfunction are at higher risk for falls due to vertigo and gait imbalance, and falls are a major cause of morbidity and mortality in patients 70 years of age and older.[28][29]
The overall 1-year incidence of vestibular dysfunction is 4.9%, while the lifetime incidence is 7.4%. Risk factors that increase the risk of vestibular dysfunction include female gender, lower educational level, age above 40, cardiovascular disease, and depression.[30]
Pathophysiology
While myriad distinct clinical entities may produce either peripheral vestibulopathy or central vertigo, it is important to understand the relationship between the nystagmus that may be apparent on physical examination and the underlying vestibular pathophysiology in patients with peripheral vertigo. In cases of peripheral vertigo, the lesion almost always causes hypofunction rather than hyperfunction; the main exceptions are "third window" phenomena, such as perilymphatic fistulae and semicircular canal dehiscences, in which sound waves stimulate the vestibular system and cause a Tullio phenomenon. The Tullio phenomenon occurs when nystagmus and/or vertigo are produced as a result of loud sounds; it was named for Pietro Tullio, who described it in 1929.[31][32]
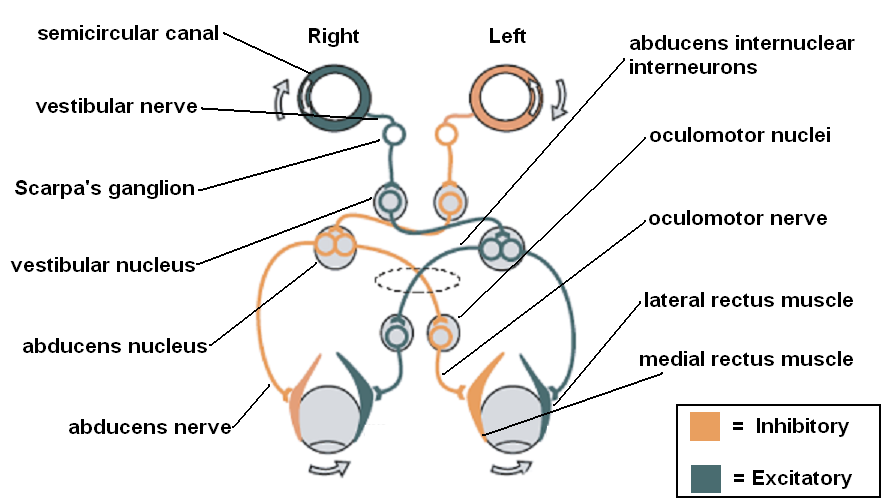
Both vestibular hyperstimulation and hypofunction activate the vestibulo-ocular reflex (VOR) abnormally and cause nystagmus. The VOR is produced by a neural pathway that begins with the vestibular organs, consisting of the semicircular canals, which sense rotational acceleration (superior/anterior, horizontal/lateral, and posterior/inferior semicircular canals, which sense rotation in the sagittal, transverse, and coronal planes, respectively), and the otolith organs - the utricle and saccule - that sense linear acceleration (horizontal and vertical, respectively). These send signals through the vestibular nerves to Scarpa's ganglion and thence to the ipsilateral vestibular nucleus. From there, signals follow different pathways through the pons and medulla, depending on the vestibular organ that was stimulated, via the interstitial nucleus of Cajal, the ventral tegmental tract, the medial longitudinal fasciculus, the ascending tract of Deiters, and the brachium conjunctivum to ultimately stimulate the nucleus of the nerve controlling the relevant extraocular muscle contralaterally and inhibit the nucleus of the nerve controlling the opposing extraocular muscle on the side ipsilateral to the stimulation.[33][34] For lesions producing vestibular hypofunction, the following laws outline how the VOR responds.
Alexander's Law states that:
- After an injury, the fast phase of the resulting nystagmus will be directed toward the uninjured side.
- After an injury, the amplitude of horizontal nystagmus will increase when a patient looks in the direction of the fast phase of the nystagmus and decrease or disappear when directed towards the side of the injury.
- Nystagmus produced by an injury will be magnified by a lack of visual fixation. This is readily apparent on videonystagmography testing, in which the patient's eyes are examined under infrared light while the patient perceives darkness.[35]
Ewald's Laws state that:[36]
- 1. Stimulation of a semicircular canal causes nystagmus in the plane of that canal.
- 2. For the horizontal semicircular canals, the movement toward the ampullated end (ampulopetal movement) causes greater stimulation than movement in the opposite direction (ampullofugal).
- 3. For the superior and posterior semicircular canals, ampullofugal movement causes greater stimulation than ampullopetal movement.
The semicircular canals are filled with endolymph, and at one end of each canal is an ampulla that contains the cupula. This gelatinous bulb rests upon hair cells and shifts with rotation, thereby triggering the hair cells to send impulses via the vestibular nerves. The ampulla of the horizontal canal is located at the anterior end of the canal. The ampullae of the superior and posterior canals are also located anteriorly; their posterior ends join together into a crus commune prior to entering the vestibule. The three semicircular canals are effectively arranged at right angles to one another to ensure that at least one canal is stimulated by every head rotation, thereby keeping the vestibular system informed of head movement and orientation. The otolith organs - the utricle and saccule - are located within the vestibule itself, with the saccule situated more anteriorly, close to the round window of the cochlea. The utricle is oriented roughly horizontally and the saccule roughly vertically.
Similar to the semicircular canals, the otolith organs each have a sheet of hair cells with their stereocilia and kinocilia embedded in a gelatinous layer that moves when the head shifts. The gelatinous layer is weighted down with otoconia that provide the inertia required for the gelatinous layer to move relative to the hair cells during linear acceleration, deflect the kinocilia, and cause a signal to be sent to the vestibular nerves. These otoconia, if dislodged, become the canaliths and cupuloliths that are the primary culprits in BPPV. Even though each hair cell in the semicircular canals and otolith organs has many stereocilia, it is only the kinocilium on each cell that will cause a signal to be sent if it is deflected. The horizontal and superior semicircular canals, as well as the utricle, are all innervated by the superior vestibular nerve, while the posterior semicircular canal and the saccule are innervated by the inferior vestibular nerve.
History and Physical
The history and physical examination are the first steps in identifying the etiology of vestibular dysfunction, narrowing the differential diagnosis based on time course, symptomatology, and clinical signs. The history and physical will also determine the need for diagnostic testing. History taking should focus on clarifying the timing of symptomatic episodes. It is also important to differentiate dizziness or vertigo from syncope or presyncope. Open-ended questions that give the patient the ability to describe their symptoms are helpful, although some patients will require redirection with more specific "yes or no" questions to avoid lengthy but clinically irrelevant monologues. Each symptom revealed by the history should be detailed with respect to time course, severity, and exacerbation or alleviation, with the goal of separating peripheral vestibular from central nervous system pathology.[37][38] The term "dizziness" especially needs to be defined by the patient to differentiate vertigo from presyncope. The classical spinning sensation is not required; the truly vertiginous patient may experience a swaying or a tilting sensation instead. Patients may describe their symptoms in vague terms of imbalance or disorientation as well, but swaying is not to be confused with a woozy or faint feeling that indicates presyncope.[8][38]
Elucidating the progression of symptoms over time is essential as well. Vertigo is usually not continuous and chronic with a vestibular etiology because the CNS compensates and causes the feeling of vertigo to subside over days or weeks. If the patient recounts a history of constant vertigo or dizziness that has lasted for months, the cause is almost certainly not peripheral. On the other hand, a history of tinnitus may indicate a peripheral cause that affects both the hearing and balance organs, such as Ménière disease.[37] The most important part of the clinical history is the length of the symptomatic episodes, determining which will differentiate peripheral from central causes and potentially indicate the most likely peripheral cause. [4]
Recurrent episodes lasting under one minute are usually BPPV. A single episode of vertigo lasting several minutes to hours may be due to a vestibular migraine or a TIA that is related to the vascular areas of the labyrinth or brainstem. Recurrent episodes that last minutes to hours are associated with Ménière disease, particularly when accompanied by roaring tinnitus and hearing loss. Vertiginous episodes that last for days can occur with vestibular neuronitis, labyrinthitis, multiple sclerosis, and infection or infarction of the brainstem or cerebellum.[9][11]
A history of aggravating and provoking factors must be obtained. Vertigo that worsens with head motion implies vestibular causes. Aggravating symptoms of coughing, sneezing, exertion, or loud noises raise the suspicion of perilymphatic fistula or a "third window" effect. Perilymphatic fistula is a defect between the middle ear and the perilymphatic space of the inner ear that may allow changes in middle ear pressure to affect hearing and the vestibular system; these are potentially caused by trauma, iatrogenic injury, or cholesteatoma. A "third window" effect is caused by a defect in the bone surrounding the labyrinth, such as with superior semicircular canal dehiscence, and also causes vertigo with pressure changes in the middle ear or the cerebrospinal fluid space. The condition is called "third window" because the vestibular system only has two natural openings: the round and oval windows. Patients at high risk of perilymphatic fistula include those with a history of a traumatic event from diving or flying, vestibular surgery, or weight lifting. They often note symptoms worsening with straining, heavy lifting, or bowel movements. A history of hyperextension injury of the neck points to vertebral artery dissection, which presents with persistent neck pain. A recent viral illness or symptoms can help identify vestibular neuronitis as the etiology.[39][40]
Symptoms associated with vertigo are also helpful in identifying etiology. Patients presenting with vertigo, diplopia, dysarthria, weakness, or numbness should be evaluated for central nervous system etiologies like acute ischemic stroke and demyelinating disorders like multiple sclerosis. These neurologic symptoms may precede, accompany, or follow the onset of vertigo. Ménière disease is often accompanied by low-frequency sensorineural hearing loss and a tinnitus which is described as a "roaring" or "whooshing" sound in addition to a sensation of pressure in the ear. Headache, photophobia, and phonophobia suggest migraines with vertigo.[41][42]
The patient's past medical history may also provide a clue as to the identity of the pathology—vasculopathy risk factors such as hypertension, diabetes mellitus, and smoking increase the likelihood of stroke. Patients with vertigo and two or more of these risk factors have an 8% 2-year stroke risk. Patients with three or more risk factors and vertigo have a 14% 2-year risk of suffering a stroke. A prior history of migraine headaches increases the chance that vertigo is a result of vestibular migraines, and head trauma can be a cause of BPPV or perilymphatic fistula. Medications such as aminoglycosides and chemotherapeutic agents like cisplatin can cause vestibular toxicity. Long-term use of seizure medications like phenytoin and carbamazepine can affect the cerebellum and also cause dysequilibrium.[43]
Commonly, patients who present with vertigo will also demonstrate nystagmus on physical examination. The severity of nystagmus and the pattern can help differentiate peripheral from central disorders. Some patients will present with tilt illusion, which is a feeling of leaning or even being upside down, related to otolithic organ dysfunction. This peripheral dysfunction may also cause lateral propulsion, which tends to fall to the side of the lesion. Patients with nystagmus may describe oscillopsia, the visual illusion of a to and fro motion with poor visual fixation when the head is moving due to impairment of the VOR, particularly when there is a bilateral vestibular weakness.[4][38][44] Some patients may suffer drop attacks, in which patients fall and often experience a feeling of being pushed or pulled to the ground. During these events, there is no presyncope or loss of consciousness. Drop attacks are caused by loss of tone in postural muscles mediated by an impaired vestibulospinal reflex. Ménière disease and aminoglycoside toxicity are known to produce drop attacks.[45]
There are many types of nystagmus. Spontaneous nystagmus is a slow drift of the eyes away from the target in one direction followed by a fast corrective movement in the reverse direction. By convention, nystagmus is named for its fast component, e.g., a "right beating" nystagmus drifts slowly to the left and then corrects rapidly back to the right. The nystagmus continues until the peripheral vestibular function returns to normal or the CNS adapts to the vestibular lesion. Nystagmus can be classified as either physiologic or pathological. Physiologic nystagmus is a form of involuntary eye movement. It is part of the VOR, characterized by smooth eye movements in one direction alternating with saccadic movement in the other direction, generally horizontally. Pathological nystagmus, on the other hand, is produced by an abnormality of the central or peripheral components of the VOR. It can also be the result of VOR activation from a stimulus other than the motion of the head, such as caloric water stimulation.[46][47]
Any clinically apparent nystagmus should be examined in detail because it can provide important diagnostic information about whether the etiology is a peripheral pathology located in the vestibular apparatus or in the CNS, as well as indicate the laterality. Additionally, specific physical exam maneuvers such as Dix-Hallpike and head impulse testing, skew deviation, HINTS examination, and observation of the ocular tilt reaction and visual fixation should be performed. These tests help to differentiate peripheral from central etiologies. Horizontal nystagmus results from a horizontal semicircular canal lesion, and torsional nystagmus results from superior or posterior semicircular canal involvement (down-beating and up-beating, respectively). A mixed horizontal-torsional nystagmus results if a peripheral lesion affects all three semicircular canals on one side. Visual fixation, however, should suppress nystagmus if it is due to a peripheral lesion but not a central lesion.
The following tests are important, and clinicians must know how to perform and interpret them.
Rinne and Weber tests: The Rinne and Weber tests are used to evaluate conductive and sensorineural hearing using a tuning fork, usually vibrating at 512 HZ, and are performed at the bedside. The Weber test is normal when the sound of the tuning fork placed on the glabella, rhinion, or central maxillary incisors is heard equally in both ears. In the presence of a sensorineural hearing loss, the sound should lateralize to the better-hearing ear. With conductive hearing deficits, the sound lateralizes to the affected ear.[48] The Rinne test differentiates between conductive and sensorineural hearing loss by comparing air and bone conduction. Normally, air conduction is better than bone conduction, and the patient hears the sound better when the tuning fork is located next to the ear than when the tuning fork is placed on the mastoid bone. In sensorineural deficits, both air and bone conduction loss is demonstrated; however, air conduction remains better than bone conduction. With a conductive hearing deficit exceeding 15 to 30 dB, the sound is better heard with the tuning fork on the mastoid than when the tuning fork is held next to the ear. Identifying unilateral sensorineural hearing loss provides a clue that the vestibular lesion is likely due to a peripheral etiology. On the other hand, abnormally good bone conduction may point to a third window phenomenon. Audiometry is the next step to confirm the physical exam findings.[49][50]
Dix-Hallpike-maneuver: The Dix-Hallpike maneuver is the use of a positional change to reproduce vertigo and demonstrate nystagmus in patients with positional dizziness. The test is most appropriate for patients who are not dizzy or vertiginous at rest. It is designed to identify benign paroxysmal positional vertigo by evaluating for posterior semicircular canal dysfunction in the dependent ear. When the patient is laid back with the dysfunctional ear in the dependent position, geotropic nystagmus and vertigo will often appear within 30 to 60 seconds, particularly if Frenzel lenses are worn to inhibit visual fixation and magnify the eyes for the examiner. The nystagmus and vertigo typically disappear after a short period, although it may be intense within that brief interval, and patients may vomit during the examination. After the patient sits up, the nystagmus will reverse direction. The maneuver is repeated on the same side, and each time the intensity and duration of nystagmus diminish. The delayed onset of nystagmus, transient and diminishing response with repetition and the geotropic nature of the nystagmus are indicative of posterior semicircular canal BPPV. If the dysfunction is located in the horizontal canal rather than the posterior canal, nystagmus may not be evoked by the Dix-Hallpike test, and if the debris is located on the cupula rather than within the canal, the nystagmus may rotate in the opposite direction, apogeotropically. The sensitivity of the Dix-Hallpike test for identifying BPPV is in the range of 88%.[49][50][51]
Head impulse test (HIT): HIT is for differentiating vestibular dysfunction from non-vestibular dizziness.[52] The HIT is performed by keeping the patient's eyes focused forward on a target, then turning the patient's head sharply to the side and looking for corrective saccades. If vestibular function is normal, the eyes remain focused on the object despite the head movement because of an intact VOR. If the eyes are dragged off the target in the direction of head rotation and then execute corrective saccades to fixate, the VOR is not functioning correctly, and a peripheral vestibulopathy is likely to present. The test is 82-100% specific and 34-39% sensitive. Sensitivity is increased to between 71% and 84% by flexing the neck 30 degrees during the maneuver, which aligns the plane of the head shakes to that of the horizontal semicircular canal.[49][50] The test helps differentiate between peripheral and central lesions. For example, the HIT should be abnormal with vestibular neuritis but normal with cerebellar infarction. With infarctions of the eighth cranial nerve or inner ear, the test will also likely appear abnormal.[53][54]
Head shaking nystagmus testing is also used to test for unilateral vestibular lesions. The patient is instructed to shake the head from side to side with eyes closed for 15-40 seconds, causing stimulation of the horizontal semicircular canals of both sides. When instructed, the patient stops shaking, opens their eyes, and looks straight ahead. If both sides were equally stimulated, the response is balanced, leaving the eyes still. If there is unilateral or asymmetric vestibular damage, there will be nystagmus that beats away from the damaged side. The test can, however, appear normal in patients with bilateral, symmetric injury. Reported sensitivity and specificity are 46% and 75%, respectively.[55] In one study, a positive head shaking nystagmus test was found in 85-100% of patients with vestibular neuritis.[56] Similarly, the head-shaking visual acuity is a test where the patient reads the eye chart while the head is shaking and then reads the eye chart while not shaking the head. If the shaking acuity is more than four lines worse than the non-shaking acuity, VOR function is poor.
Skew deviation: This is a test designed to identify lesions in the brainstem. A red lens is placed over one eye, and a white point of light is shined at the patient, who is asked to note the relative position of the red spot to the white point of light. The examiner then moves the white point of light to see how the separation changes with a lateral and vertical gaze. With skew deviation due to a central lesion, gaze direction has little effect on the distance between images, known as commitment. If commitment changes with gaze direction, a cranial nerve palsy affecting ocular motility may be to blame.[49][50][52]
A specific type of skew deviation is the ocular tilt reaction, in which the patient has a triad of skew deviation, head tilt in the direction of the lower eye, and bilateral ocular torsion in the direction of the head tilt. The ocular tilt reaction can occur with acute peripheral lesions or central lesions involving the cerebellum or brainstem vestibular pathway. Besides the ocular tilt test and skew, a tilt of the subjective visual vertical plane is a sensitive sign of static vestibular imbalance, which can be due to either peripheral or central pathology.[57]
HINTS examination: The HINTS examination is an exam combining the Head Impulse test (HIT) with the skew test. The presence of normal HIT and skew deviation suggests central rather than peripheral vertigo.
Evaluation
The evaluation of patients with vestibular dysfunction may include magnetic resonance imaging (MRI), computed tomography (CT), electronystagmography (ENG) or videonystagmography (VNG), vestibular-evoked myogenic potentials (VEMP), and brainstem auditory evoked potentials/brainstem auditory evoked responses/auditory brainstem responses (BAEP/BAER/ABR). Not all of these tests are always needed due to their low yield and the expense associated.[58][51][59]
Electronystagmography and videonystagmography (also known as electrooculography and videooculography) are among the more common tests performed to quantify VOR function. ENG uses surface electrodes to measure nystagmus, and VNG uses cameras for the same purpose. During ENG and VNG studies, a patient will typically undergo several different tests: following lights on a light bar, undergoing positional testing, such as the Dix-Hallpike maneuver, and undergoing caloric testing. The latter involves warm and cold water or air irrigation of the external auditory canal to cause a convection current in the horizontal semicircular canal. The amount and symmetry of nystagmus produced will indicate laterality and severity of peripheral vestibular lesions.[60]
VEMP testing may also form a part of the testing battery performed by the audiologist. There are two types of VEMP tests, cervical and ocular. The former involves delivering an impulse sound to one ear and recording relaxation of the ipsilateral sternocleidomastoid muscle to determine at what thresholds the relaxation response occurs.[61] For ocular VEMP testing, the recording electrode is placed over the contralateral inferior oblique muscle, but the principle remains the same. Lower thresholds for these responses may indicate a third window phenomenon.[62]
Magnetic resonance imaging of the brain is indicated when history and physical examination tests like HIT, skew deviation, HINTS, Dix-Hallpike maneuver, and visual fixation identify a central cause. Patients with risk factors for stroke or neurologic signs and symptoms should have neuroimaging to evaluate for acute ischemic stroke. Young patients who have acute sustained vertigo with no risk factors for stroke, no neurologic signs or symptoms, suppression with visual fixation, falling in the opposite direction to the nystagmus, and horizontal or torsional nystagmus do not need immediate imaging unless symptoms persist for 48 hours or longer. Brain MRI can detect acute ischemic stroke with very high sensitivity and specificity. Head CT, however, has a lower sensitivity for infarctions and other lesions in the brainstem but a high sensitivity for intracranial hemorrhage; it is also more readily available in most places and can be performed emergently. For this reason, it is often the initial screening test when evaluating for stroke.[63]
Treatment / Management
Treatment of vestibular dysfunction depends upon the etiology. Symptomatic management is the mainstay for most patients. Options include antiemetics (metoclopramide, ondansetron, prochlorperazine, promethazine), vestibular suppressant medications (diphenhydramine, dimenhydrinate, meclizine), and benzodiazepines (alprazolam, clonazepam, diazepam, and lorazepam). For vestibular migraines, management with prophylactic and/or abortive medications is appropriate.[64][65][66][67]
When autoimmune labyrinthitis or Cogan syndrome is suspected, high-dose oral corticosteroids may be prescribed.[68] Other forms of labyrinthitis and vestibular neuritis are self-limited and may simply be observed.[23][22] However, surgical options should be considered in some cases, particularly for refractory Ménière disease or when a mass lesion, like an acoustic neuroma, is the culprit.
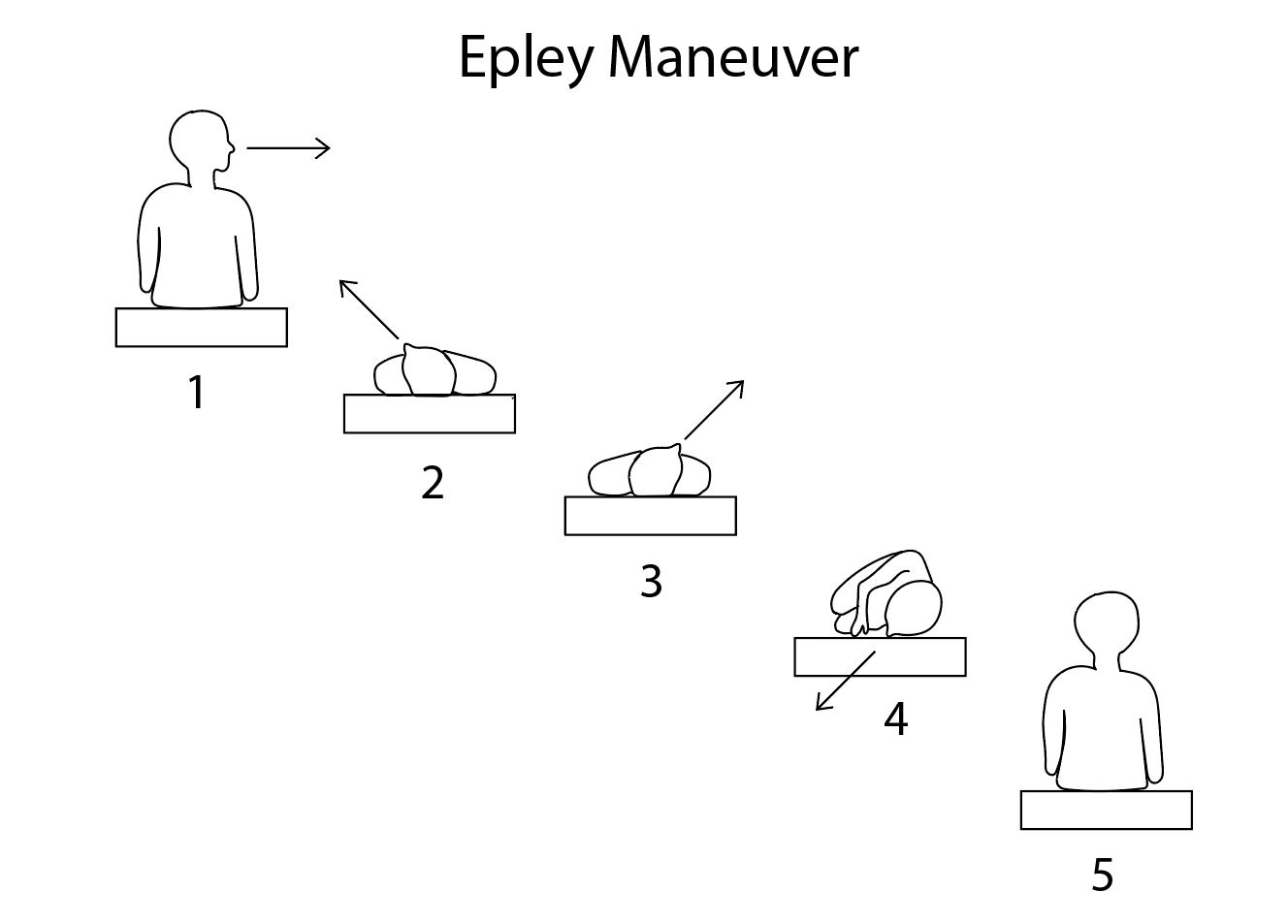
Benign positional vertigo involving the posterior semicircular canal is commonly treated using the Epley maneuver, which involves performing a Dix-Hallpike maneuver while the patient is supine, slowly rotating the head from the vertiginous side through 180 degrees to face the other side while keeping the rest of the body still.[69] Patients will frequently note vertigo about halfway through the head turn, and nystagmus may be apparent. If this occurs, the maneuver should be paused until the symptoms subside; they may recur at the end of the head turn. From there, the patient's head is held still while the body is rotated into a lateral decubitus position. Then the patient is assisted into a sitting position on the side of the examination table, now facing the opposite direction from where the Dix-Hallpike maneuver began.[70] Other interventions may be easier for patients to perform by themselves, such as the Semont and Foster maneuvers and Brandt-Daroff exercises.
The Semont maneuver involves sitting on the edge of a bed and turning the head 45 degrees away from the side of the lesion, then lying down in the lateral decubitus position without moving the head relative to the body; this should result in the head looking upward. The patient stays in this position for 30 to 60 seconds so that any vertigo can subside. The patient then rolls over, again keeping the head still relative to the body; this should result in the face looking downward. The patient maintains this position for 30 to 60 seconds, again waiting for any vertigo to resolve, then slowly rises back to a sitting position.
The Foster, or half-somersault, maneuver is easier for patients with limited mobility than the Semont or Epley maneuvers. It begins with the patient kneeling while looking upward and holding the position for 30-60 seconds. The patient then tucks his chin to his chest and bends over to touch the floor with his head, waiting 30-60 seconds for any vertigo that occurs to stop. The patient then turns his head to look towards the side that has the vestibulopathy and waits for any vertigo to subside. From there, the patient rises onto hands and knees, maintaining the head position relative to the body and waiting for any vertigo that appears to resolve. Then the patient returns to a kneeling position, again maintaining the position of the head relative to the body and waiting for any vertigo to stop.
Brandt-Daroff exercises begin with the patient sitting upright on the edge of a bed, then turning the head all the way to one side. The patient then lies down on their side so that the nose points upward and holds that position for 30 to 60 seconds, or until any visible vertigo subsides. The patient then sits back up and repeats the maneuver, turning the head to the opposite side.
For cases of horizontal canal BPPV, the Gufoni maneuver may be more useful than those described above.[71] When performed for geotropic nystagmus, this maneuver involves the patient sitting upright, looking straight forward on the edge of a bed, and then the patient quickly lies down on the side opposite the nystagmus; the head is then turned immediately 45 degrees downwards, and this position is held for 2-3 minutes before slowly returning to the sitting position. Geotropic nystagmus in horizontal semicircular canal BPPV is due to canalithiasis of the posterior arm of the horizontal canal. Apogeotropic nystagmus indicates canalithiasis of the anterior arm of the horizontal canal and is treated with a Gufoni maneuver involving lying down on the same side as the nystagmus.
These maneuvers, as a group, are used to reposition canaliths such that they are no longer interfering with vestibular function.[2][72][73][38](B3)
The goals of treatment for Ménière disease are the reduction in frequency and severity of vertigo attacks, reduction or elimination of hearing loss and tinnitus associated with attacks, alleviation of chronic symptoms, and minimization of disability. Conservative treatment options include lifestyle changes and avoiding triggers. A low sodium diet of 2 to 3 gm per day, limiting caffeinated beverages, alcohol, nicotine, stress, monosodium glutamate, and allergens can all help.[74] Intratympanic steroid injections, using dexamethasone or hydrocortisone in patients who fail conservative measures, have also been proven useful.[75][76][77] The use of overpressure devices, like the handheld Meniett device, has also proven useful for some patients, though its overall effectiveness remains debatable.[78][79] These devices are designed to provide impulses of air pressure to the round window to help evacuate excess endolymph via the endolymphatic duct and sac, but in order to do this, there has to be a tympanostomy tube in place. While the efficacy of these devices is not consistently better than placebo, they appear safe.[80](A1)
Should these minimally-invasive treatments fail, there are surgical interventions available as well. Like the options above, endolymphatic sac shunting via a transmastoid approach is considered a non-ablative approach because it does not involve a deliberate reduction of function in the vestibular system. The procedure involves identifying the endolymphatic sac between the layers of the posterior cranial fossa dura and placing a silastic shunt to allow endolymph to drain into the mastoid cavity. As with Meniett device use, the efficacy of this option is unclear, although it was the subject of a controversial, blinded study published in 1981 that involved sham surgery as the control arm.[81][82] (A1)
Ablative therapies for Ménière disease include intratympanic injection of the aminoglycoside antibiotic gentamicin, vestibular neurectomy, and labyrinthectomy.[74] Gentamicin is ototoxic but is more selectively vestibulotoxic than cochleotoxic; therefore, careful titration may limit hearing loss acquired during vestibular chemoablation.[83][84] While it may seem counterintuitive to further ablate the vestibular system to produce symptomatic relief, more symptoms occur when one side of the vestibular system is hypofunctioning than if that side is completely non-functional because of the CNS's ability to compensate more easily for a completely non-functional vestibular system than a hypofunctioning one. For this reason, dividing the vestibular nerve on the affected side via a middle fossa craniotomy has been proven helpful for alleviating symptoms after a period of post-operative CNS compensation.[85][86] Similarly, for patients with profound hearing loss, a labyrinthectomy via a transmastoid or transcanal approach, which may or may not include a vestibular neurectomy, may provide long-term symptomatic relief.[87](A1)
Differential Diagnosis
Classification of vestibular dysfunction as peripheral or central is the first step to narrowing the differential diagnosis. Central nervous system etiologies should be considered when patients present with vertigo and dizziness, where the examination shows a pattern of nystagmus that is persistently vertical or purely torsional and does not change with repositioning or visual fixation. A history of neurological disorders or symptoms or risk factors for stroke also shifts the balance of probability in favor of a central etiology of the vertigo. Common examples of CNS pathology include cerebellar, brainstem or medulla infarction, cerebellar tumor, Chiari malformation, multiple sclerosis, vestibular migraine, mal de debarquement syndrome, and degenerative ataxia disorders, such as Parkinson disease.
Peripheral vestibular dysfunction should be suspected in the presence of nystagmus that is responsive to repositioning maneuvers, particularly when it is horizontally oriented. The most common conditions to consider are BPPV, Ménière disease, vestibular neuritis, and labyrinthitis. Other less common causes include cerebellopontine angle tumors, such as acoustic neuromas, perilymphatic fistula, semicircular canal dehiscence, vestibular paroxysmia, Cogan syndrome, vestibulotoxic medications, and otitis media. Associated symptoms and timing will help to differentiate clinical entities. When accompanied by hearing loss, vertigo may be due to Ménière disease, Cogan syndrome, or ototoxic medications, but tinnitus and pressure would indicate Ménière disease, vision loss would suggest Cogan syndrome, and a history of aminoglycoside administration or another drug would point towards toxicity. Similarly, vertigo triggered by pressure changes would raise suspicion for a third window effect, such as perilymphatic fistula or semicircular canal dehiscence; these conditions are often accompanied by increased sensitivity to conducted sounds, such as their own voices, footfalls when walking, and even the sounds of their eyes moving in their orbits with gaze changes.
In patients without true vertigo, lightheadedness or unsteadiness may be described as "dizzyness" without being caused by either a peripheral vestibulopathy or central nervous system pathology. Common etiologies of lightheadedness include hypotension, particularly orthostatic, diabetes (due to peripheral neuropathy or hypoglycemia), dehydration, anxiety, and cardiovascular diseases, such as aortic stenosis or cardiomyopathy. When considered altogether, the differential diagnosis for dizzyness is incredibly broad and far exceeds the scope of practice of any single clinical specialty.
Prognosis
The prognosis of vestibular dysfunction depends greatly upon the etiology. The prognosis for BPPV is good when treated appropriately but often recurs. Ménière disease is characterized by recurrent attacks and progressive disease, which results in both hearing loss and balance issues that lead to disability; over time, the second ear becomes involved in up to 47% of cases, with 78% occurring sequentially rather than simultaneously.[21][88] Symptom control and rehabilitation with lifestyle changes delay progression but may not entirely prevent it. Other processes, such as vestibular neuritis and labyrinthitis, may be self-limited despite their debilitating initial presentations. The prognosis for vertigo caused by central lesions is fair, as patients often respond to rehabilitation over time, but accompanying deficits from infarctions or tumors can be debilitating.[9]
Complications
Complications associated with vestibular disorders, in general, are results of increased fall risk and decreased quality of life. In elderly patients particularly, the sequelae of serious falls can be debilitating or even deadly. Regardless, patients with severe vertigo suffer significant quality of life decrements because of the inability to perform many activities of daily living that other people rely upon regularly, such as driving automobiles, operating machinery, riding bicycles, running, or even walking. Many patients with vertigo lose the ability to work in the fields in which they had previously been employed or even to work at all. When vestibular dysfunction is due to a peripheral pathology, hearing loss may accompany it and exacerbate the disability, just as other cranial neuropathies or neurological symptoms may coexist with CNS etiologies.[42][89]
Consultations
For patients with true vertigo, otolaryngologists and neurologists are the consultants of choice, with audiologists and radiologists playing important roles in diagnosis. Neurosurgeons may be required as well if CNS lesions are involved. For patients with lightheadedness in the absence of true vertigo, primary care providers will serve as the starting point for diagnostic evaluation.[51][58][59][90]
Deterrence and Patient Education
Vestibular dysfunction can contribute to symptoms of varying severity and frequency but can nevertheless be debilitating and anxiogenic. Patient education is an important part of the clinical management of vestibular dysfunction. The initial focus of treatment must be on an accurate diagnosis informed by a detailed history, comprehensive physical examination, and supporting diagnostic testing. Once the diagnosis is established, counseling is provided regarding the diagnosis and prognosis. Creating an individualized care plan empowers the patient to take responsibility and initiative for self-care. Medications for symptom control are often helpful, but patients should be counseled about the side effects and potential drug-drug interactions these medications may produce.
Enhancing Healthcare Team Outcomes
An interprofessional team of otolaryngologists, neurologists, radiologists, audiologists, and vestibular therapists that provides a holistic and integrated approach to vestibular care can help achieve the best possible outcomes. Early recognition of patients presenting with vertigo and dizziness of central nervous system etiology is essential. Clinicians need to be familiar with bedside examination techniques that differentiate peripheral from central causes of vertigo, as well as have an understanding of how time course and associated symptoms help to narrow the diagnosis.[91]
Collaboration, shared decision-making, and communication are key elements for increasing the chances of a good outcome. Interprofessional care provided to the patient must use an integrated pathway combined with an evidence-based approach to evaluating and managing vestibular dysfunction.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
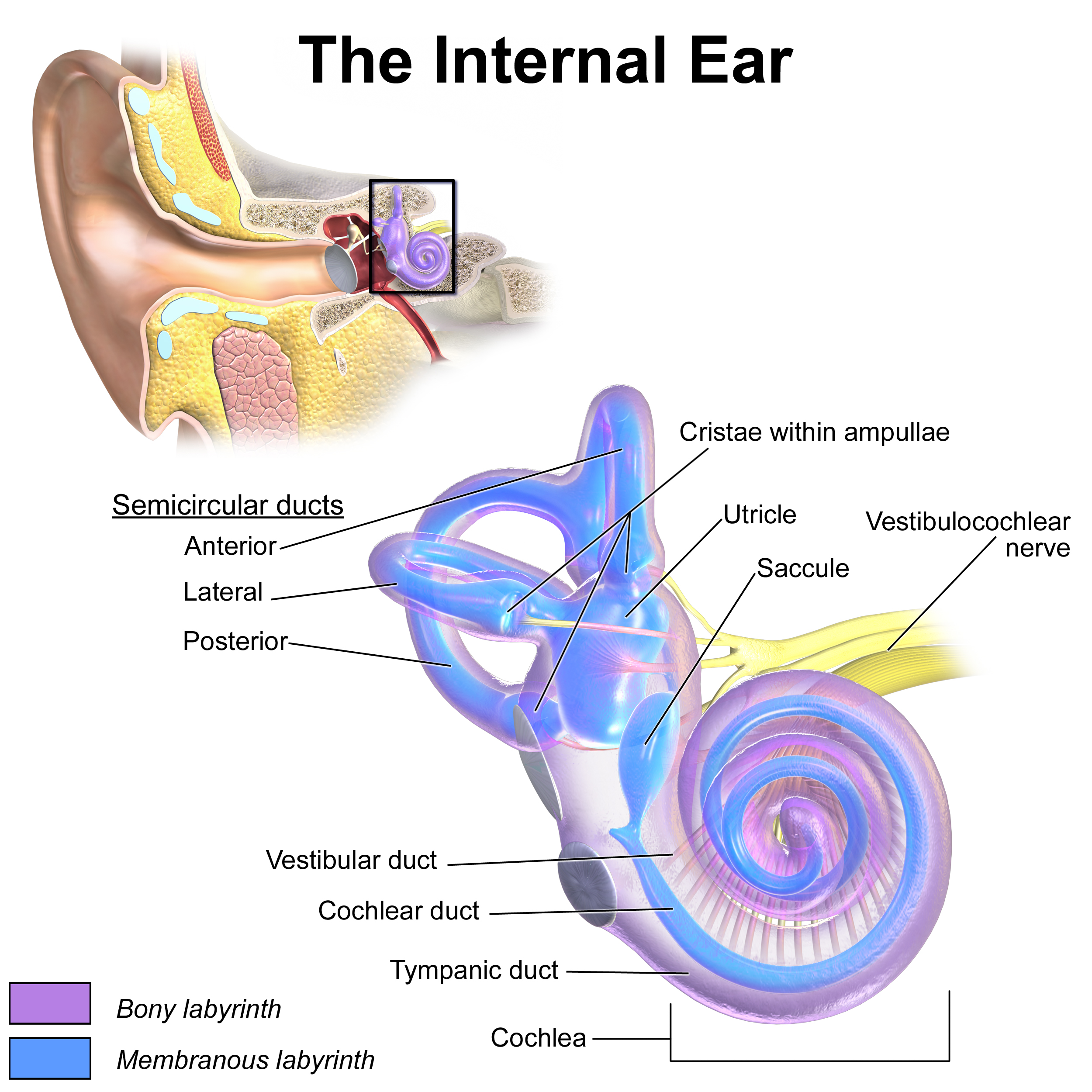
Inner Ear Anatomy. This illustration shows the semicircular ducts and parts of the cochlea.
Blausen.com staff. Medical Gallery of Blausen Medical 2014. WikiJournal of Medicine. doi: 10.15347/wjm/2014.010.
ISSN 2002-4436. [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)] via Wikimedia Commons.
(Click Image to Enlarge)
References
Palmeri R, Kumar A. Benign Paroxysmal Positional Vertigo. StatPearls. 2023 Jan:(): [PubMed PMID: 29261987]
Strupp M, Mandalà M, López-Escámez JA. Peripheral vestibular disorders: an update. Current opinion in neurology. 2019 Feb:32(1):165-173. doi: 10.1097/WCO.0000000000000649. Epub [PubMed PMID: 30562267]
Level 3 (low-level) evidenceTarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2011 Jun 14:183(9):E571-92. doi: 10.1503/cmaj.100174. Epub 2011 May 16 [PubMed PMID: 21576300]
Level 1 (high-level) evidenceNewman-Toker DE, Hsieh YH, Camargo CA Jr, Pelletier AJ, Butchy GT, Edlow JA. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clinic proceedings. 2008 Jul:83(7):765-75. doi: 10.4065/83.7.765. Epub [PubMed PMID: 18613993]
Level 2 (mid-level) evidenceStrupp M, Arbusow V. Acute vestibulopathy. Current opinion in neurology. 2001 Feb:14(1):11-20 [PubMed PMID: 11176212]
Level 3 (low-level) evidenceChoi JY, Kim JS. Nystagmus and central vestibular disorders. Current opinion in neurology. 2017 Feb:30(1):98-106. doi: 10.1097/WCO.0000000000000416. Epub [PubMed PMID: 27941522]
Level 3 (low-level) evidenceSeemungal BM. Neuro-otological emergencies. Current opinion in neurology. 2007 Feb:20(1):32-9 [PubMed PMID: 17215686]
Level 3 (low-level) evidenceMorrison AW, Anticipation in Menière's disease. The Journal of laryngology and otology. 1995 Jun; [PubMed PMID: 7642988]
Tusa RJ, Gore R. Dizziness and vertigo: emergencies and management. Neurologic clinics. 2012 Feb:30(1):61-74, vii-viii. doi: 10.1016/j.ncl.2011.09.006. Epub [PubMed PMID: 22284055]
Choi JY, Lee SH, Kim JS. Central vertigo. Current opinion in neurology. 2018 Feb:31(1):81-89. doi: 10.1097/WCO.0000000000000511. Epub [PubMed PMID: 29084063]
Level 3 (low-level) evidenceKlockars T, Kentala E. Inheritance of Meniere's disease in the Finnish population. Archives of otolaryngology--head & neck surgery. 2007 Jan:133(1):73-7 [PubMed PMID: 17224529]
Aw ST, Todd MJ, Aw GE, McGarvie LA, Halmagyi GM. Benign positional nystagmus: a study of its three-dimensional spatio-temporal characteristics. Neurology. 2005 Jun 14:64(11):1897-905 [PubMed PMID: 15955941]
Ismail HM. Vertigo. A neurobiological review. Neurosciences (Riyadh, Saudi Arabia). 2004 Oct:9(4):243-6 [PubMed PMID: 23377241]
Drachman DA, Hart CW. An approach to the dizzy patient. Neurology. 1972 Apr:22(4):323-34 [PubMed PMID: 4401538]
Hornibrook J, Bird P. A New Theory for Ménière's Disease: Detached Saccular Otoconia. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2017 Feb:156(2):350-352. doi: 10.1177/0194599816675843. Epub 2016 Nov 14 [PubMed PMID: 28145833]
Kimura RS, Schuknecht HF, Ota CY, Jones DD. Experimental study of sacculotomy in endolymphatic hydrops. Archives of oto-rhino-laryngology. 1977 Jul 29:217(2):123-37 [PubMed PMID: 578728]
Level 3 (low-level) evidenceParker W. Menière's disease. Etiologic considerations. Archives of otolaryngology--head & neck surgery. 1995 Apr:121(4):377-82 [PubMed PMID: 7702810]
Level 3 (low-level) evidenceSchuknecht HF, The pathophysiology of Meniere's disease. The American journal of otology. 1984 Oct; [PubMed PMID: 6393773]
Level 3 (low-level) evidenceKolev OI, Sergeeva M. Vestibular disorders following different types of head and neck trauma. Functional neurology. 2016 Apr-Jun:31(2):75-80 [PubMed PMID: 27358219]
Rosenberg S, Silverstein H, Flanzer J, Wanamaker H. Bilateral Menière's disease in surgical versus nonsurgical patients. The American journal of otology. 1991 Sep:12(5):336-40 [PubMed PMID: 1789301]
Frejo L, Soto-Varela A, Santos-Perez S, Aran I, Batuecas-Caletrio A, Perez-Guillen V, Perez-Garrigues H, Fraile J, Martin-Sanz E, Tapia MC, Trinidad G, García-Arumi AM, González-Aguado R, Espinosa-Sanchez JM, Marques P, Perez P, Benitez J, Lopez-Escamez JA. Clinical Subgroups in Bilateral Meniere Disease. Frontiers in neurology. 2016:7():182 [PubMed PMID: 27822199]
Smith T, Rider J, Cen S, Borger J. Vestibular Neuronitis. StatPearls. 2023 Jan:(): [PubMed PMID: 31751056]
Barkwill D, Arora R. Labyrinthitis. StatPearls. 2023 Jan:(): [PubMed PMID: 32809341]
Inui H, Kitaoku Y, Yoneyama K, Nakane M, Ohue S, Yamanaka T, Ueda T, Fujita N, Miyahara H, Matsunaga T. MR-angiographic findings of patients with central vestibular disorders. Acta oto-laryngologica. Supplementum. 1998:533():51-6 [PubMed PMID: 9657312]
Albuquerque FC, Hu YC, Dashti SR, Abla AA, Clark JC, Alkire B, Theodore N, McDougall CG. Craniocervical arterial dissections as sequelae of chiropractic manipulation: patterns of injury and management. Journal of neurosurgery. 2011 Dec:115(6):1197-205. doi: 10.3171/2011.8.JNS111212. Epub 2011 Sep 16 [PubMed PMID: 21923248]
Level 3 (low-level) evidenceDi Stadio A, Dipietro L, Ralli M, Meneghello F, Minni A, Greco A, Stabile MR, Bernitsas E. Sudden hearing loss as an early detector of multiple sclerosis: a systematic review. European review for medical and pharmacological sciences. 2018 Jul:22(14):4611-4624. doi: 10.26355/eurrev_201807_15520. Epub [PubMed PMID: 30058696]
Level 1 (high-level) evidenceTamás TL, Garai T, Tompos T, Szirmai Á. [Vertigo in the Emergency Department: new bedside tests]. Orvosi hetilap. 2016 Mar 13:157(11):403-9. doi: 10.1556/650.2016.30388. Epub [PubMed PMID: 26947088]
Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. The Laryngoscope. 2012 Aug:122(8):1858-61. doi: 10.1002/lary.23376. Epub 2012 May 29 [PubMed PMID: 22645067]
Level 2 (mid-level) evidenceFurman JM, Raz Y, Whitney SL. Geriatric vestibulopathy assessment and management. Current opinion in otolaryngology & head and neck surgery. 2010 Oct:18(5):386-91. doi: 10.1097/MOO.0b013e32833ce5a6. Epub [PubMed PMID: 20613528]
Level 3 (low-level) evidenceNeuhauser HK, von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, Lempert T. Epidemiology of vestibular vertigo: a neurotologic survey of the general population. Neurology. 2005 Sep 27:65(6):898-904 [PubMed PMID: 16186531]
Level 2 (mid-level) evidenceLehmkuhl B, Andaloro C. Tullio Phenomenon. StatPearls. 2023 Jan:(): [PubMed PMID: 30020601]
Addams-Williams J, Wu K, Ray J. The experiments behind the Tullio phenomenon. The Journal of laryngology and otology. 2014 Mar:128(3):223-7. doi: 10.1017/S0022215114000280. Epub 2014 Feb 19 [PubMed PMID: 24548750]
Bronstein AM, Patel M, Arshad Q. A brief review of the clinical anatomy of the vestibular-ocular connections-how much do we know? Eye (London, England). 2015 Feb:29(2):163-70. doi: 10.1038/eye.2014.262. Epub 2014 Nov 21 [PubMed PMID: 25412719]
Somisetty S, M Das J. Neuroanatomy, Vestibulo-ocular Reflex. StatPearls. 2024 Jan:(): [PubMed PMID: 31424881]
Jacobson GP, McCaslin DL, Kaylie DM. Alexander's law revisited. Journal of the American Academy of Audiology. 2008 Sep:19(8):630-8 [PubMed PMID: 19323354]
Level 3 (low-level) evidenceZhang X, Bai Y, Chen T, Wang W, Han X, Li S, Liu Q, Wen C. A Show of Ewald's Law: I Horizontal Semicircular Canal Benign Paroxysmal Positional Vertigo. Frontiers in neurology. 2021:12():632489. doi: 10.3389/fneur.2021.632489. Epub 2021 Feb 3 [PubMed PMID: 33613438]
Kerber KA, Baloh RW. The evaluation of a patient with dizziness. Neurology. Clinical practice. 2011 Dec:1(1):24-33 [PubMed PMID: 23634356]
Young P, Castillo-Bustamante M, Almirón CJ, Bruetman JE, Finn BC, Ricardo MA, Binetti AC. [Approach to patients with vertigo]. Medicina. 2018:78(6):410-416 [PubMed PMID: 30504108]
Fife TD. Dizziness in the Outpatient Care Setting. Continuum (Minneapolis, Minn.). 2017 Apr:23(2, Selected Topics in Outpatient Neurology):359-395. doi: 10.1212/CON.0000000000000450. Epub [PubMed PMID: 28375910]
Bisdorff A. Vestibular symptoms and history taking. Handbook of clinical neurology. 2016:137():83-90. doi: 10.1016/B978-0-444-63437-5.00006-6. Epub [PubMed PMID: 27638064]
Xu KX, Chen TS, Wang W, Li SS, Wen C, Liu Q, Han X, Lin P. [The characteristic of vestibular ocular reflex in patients with vestibular migraine]. Lin chuang er bi yan hou tou jing wai ke za zhi = Journal of clinical otorhinolaryngology, head, and neck surgery. 2017 Jul 20:31(14):1075-1077. doi: 10.13201/j.issn.1001-1781.2017.14.005. Epub [PubMed PMID: 29798243]
Ghavami Y, Haidar YM, Moshtaghi O, Lin HW, Djalilian HR. Evaluating Quality of Life in Patients With Meniere's Disease Treated as Migraine. The Annals of otology, rhinology, and laryngology. 2018 Dec:127(12):877-887. doi: 10.1177/0003489418799107. Epub 2018 Sep 9 [PubMed PMID: 30198300]
Level 2 (mid-level) evidenceFife TD, Blum D, Fisher RS. Measuring the effects of antiepileptic medications on balance in older people. Epilepsy research. 2006 Aug:70(2-3):103-9 [PubMed PMID: 16675199]
Katsarkas A. Benign paroxysmal positional vertigo (BPPV): idiopathic versus post-traumatic. Acta oto-laryngologica. 1999:119(7):745-9 [PubMed PMID: 10687929]
Level 2 (mid-level) evidenceGleich O, Strutz J, Schmid K. [Endolymph homeostasis and Menière's disease: fundamentals, pathological changes, aminoglycosides]. HNO. 2008 Dec:56(12):1243-52. doi: 10.1007/s00106-008-1841-8. Epub [PubMed PMID: 19020845]
Level 3 (low-level) evidencePal'chun VT, Makoeva AA, Guseva AL. [Dizziness and vertigo associated with vestibular neuronitis: the approaches to the diagnostics and treatment]. Vestnik otorinolaringologii. 2018:83(3):4-10. doi: 10.17116/otorino20188334. Epub [PubMed PMID: 29953046]
Baloh RW. Clinical practice. Vestibular neuritis. The New England journal of medicine. 2003 Mar 13:348(11):1027-32 [PubMed PMID: 12637613]
Level 3 (low-level) evidenceWatson SR, Halmagyi GM, Colebatch JG. Vestibular hypersensitivity to sound (Tullio phenomenon): structural and functional assessment. Neurology. 2000 Feb 8:54(3):722-8 [PubMed PMID: 10680810]
Level 3 (low-level) evidenceTraccis S, Zoroddu GF, Zecca MT, Cau T, Solinas MA, Masuri R. Evaluating patients with vertigo: bedside examination. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2004 Mar:25 Suppl 1():S16-9 [PubMed PMID: 15045614]
Huh YE, Kim JS. Bedside evaluation of dizzy patients. Journal of clinical neurology (Seoul, Korea). 2013 Oct:9(4):203-13. doi: 10.3988/jcn.2013.9.4.203. Epub 2013 Oct 31 [PubMed PMID: 24285961]
Hoffman RM, Einstadter D, Kroenke K. Evaluating dizziness. The American journal of medicine. 1999 Nov:107(5):468-78 [PubMed PMID: 10569302]
Level 1 (high-level) evidenceKattah JC. Use of HINTS in the acute vestibular syndrome. An Overview. Stroke and vascular neurology. 2018 Dec:3(4):190-196. doi: 10.1136/svn-2018-000160. Epub 2018 Jun 23 [PubMed PMID: 30637123]
Level 3 (low-level) evidenceGrill E, Heuberger M, Strobl R, Saglam M, Holle R, Linkohr B, Ladwig KH, Peters A, Schneider E, Jahn K, Lehnen N. Prevalence, Determinants, and Consequences of Vestibular Hypofunction. Results From the KORA-FF4 Survey. Frontiers in neurology. 2018:9():1076. doi: 10.3389/fneur.2018.01076. Epub 2018 Dec 7 [PubMed PMID: 30581415]
Level 3 (low-level) evidenceTsang BKT,Chen ASK,Paine M, Acute evaluation of the acute vestibular syndrome: differentiating posterior circulation stroke from acute peripheral vestibulopathies. Internal medicine journal. 2017 Dec; [PubMed PMID: 28696571]
Amari K, Kudo Y, Watanabe K, Yamamoto M, Takahashi K, Tanaka O, Johkura K. Spontaneous, headshaking, and positional nystagmus in post-lateral medullary infarction dizziness. Journal of the neurological sciences. 2016 Sep 15:368():249-53. doi: 10.1016/j.jns.2016.07.019. Epub 2016 Jul 15 [PubMed PMID: 27538643]
Choi KD, Kim JS. Head-shaking nystagmus in central vestibulopathies. Annals of the New York Academy of Sciences. 2009 May:1164():338-43. doi: 10.1111/j.1749-6632.2008.03737.x. Epub [PubMed PMID: 19645923]
Newman-Toker DE, Kerber KA, Hsieh YH, Pula JH, Omron R, Saber Tehrani AS, Mantokoudis G, Hanley DF, Zee DS, Kattah JC. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2013 Oct:20(10):986-96. doi: 10.1111/acem.12223. Epub [PubMed PMID: 24127701]
Level 2 (mid-level) evidenceBösner S,Schwarm S,Grevenrath P,Schmidt L,Hörner K,Beidatsch D,Bergmann M,Viniol A,Becker A,Haasenritter J, Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC family practice. 2018 Feb 20; [PubMed PMID: 29458336]
Level 1 (high-level) evidenceSuo LM, Si NN, Jin L, Zhang L, Sun SF, Song YH, Yang J, Li QF, Zhao CQ. [Evaluation of curative effect of VNG and VEMP in patients with severe sudden hearing loss]. Lin chuang er bi yan hou tou jing wai ke za zhi = Journal of clinical otorhinolaryngology, head, and neck surgery. 2018 Jul:32(14):1102-1105. doi: 10.13201/j.issn.1001-1781.2018.14.015. Epub [PubMed PMID: 30550157]
Munakomi S, Lui F. Caloric Reflex Test. StatPearls. 2023 Jan:(): [PubMed PMID: 32491413]
Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. Journal of neurology, neurosurgery, and psychiatry. 1994 Feb:57(2):190-7 [PubMed PMID: 8126503]
Fife TD,Satya-Murti S,Burkard RF,Carey JP, Vestibular evoked myogenic potential testing: Payment policy review for clinicians and payers. Neurology. Clinical practice. 2018 Apr [PubMed PMID: 29708189]
Ahsan SF, Syamal MN, Yaremchuk K, Peterson E, Seidman M. The costs and utility of imaging in evaluating dizzy patients in the emergency room. The Laryngoscope. 2013 Sep:123(9):2250-3. doi: 10.1002/lary.23798. Epub 2013 Jul 2 [PubMed PMID: 23821602]
Level 2 (mid-level) evidencePescador Ruschel MA, De Jesus O. Migraine Headache. StatPearls. 2024 Jan:(): [PubMed PMID: 32809622]
Kumar A, Kadian R. Migraine Prophylaxis. StatPearls. 2023 Jan:(): [PubMed PMID: 29939650]
Hilton DB,Shermetaro C, Migraine-Associated Vertigo StatPearls. 2022 Jan [PubMed PMID: 29939636]
Lew C, Punnapuzha S. Migraine Medications. StatPearls. 2023 Jan:(): [PubMed PMID: 31985952]
Salman EJ, Tripathy K. Cogans Syndrome. StatPearls. 2023 Jan:(): [PubMed PMID: 35593853]
Epley JM. Particle repositioning for benign paroxysmal positional vertigo. Otolaryngologic clinics of North America. 1996 Apr:29(2):323-31 [PubMed PMID: 8860930]
Nguyen CT, Basso M. Epley Maneuver. StatPearls. 2023 Jan:(): [PubMed PMID: 33085434]
Riggio F, Dispenza F, Gallina S, Kulamarva G, Gargano R, Speciale R. Management of benign paroxysmal positional vertigo of lateral semicircular canal by Gufoni's manoeuvre. American journal of otolaryngology. 2009 Mar-Apr:30(2):106-11. doi: 10.1016/j.amjoto.2008.03.001. Epub 2008 Jul 22 [PubMed PMID: 19239952]
Sienko KH, Whitney SL, Carender WJ, Wall C. The role of sensory augmentation for people with vestibular deficits: Real-time balance aid and/or rehabilitation device? Journal of vestibular research : equilibrium & orientation. 2017:27(1):63-76. doi: 10.3233/VES-170606. Epub [PubMed PMID: 28387692]
Antonenko LM, Parfenov VA. [Non-drug therapy of vertigo]. Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova. 2018:118(8):38-42. doi: 10.17116/jnevro201811808138. Epub [PubMed PMID: 30251976]
Koenen L, Andaloro C. Meniere Disease. StatPearls. 2023 Jan:(): [PubMed PMID: 30725640]
de Cates C, Winters R. Intratympanic Steroid Injection. StatPearls. 2023 Jan:(): [PubMed PMID: 33620785]
Beyea JA, Instrum RS, Agrawal SK, Parnes LS. Intratympanic Dexamethasone in the Treatment of Ménière's Disease: A Comparison of Two Techniques. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2017 Jul:38(6):e173-e178. doi: 10.1097/MAO.0000000000001437. Epub [PubMed PMID: 28437363]
Jumaily M, Faraji F, Mikulec AA. Intratympanic Triamcinolone and Dexamethasone in the Treatment of Ménière's Syndrome. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2017 Mar:38(3):386-391. doi: 10.1097/MAO.0000000000001311. Epub [PubMed PMID: 28192380]
van Sonsbeek S, Pullens B, van Benthem PP. Positive pressure therapy for Ménière's disease or syndrome. The Cochrane database of systematic reviews. 2015 Mar 10:(3):CD008419. doi: 10.1002/14651858.CD008419.pub2. Epub 2015 Mar 10 [PubMed PMID: 25756795]
Level 1 (high-level) evidenceRusso FY, Nguyen Y, De Seta D, Bouccara D, Sterkers O, Ferrary E, Bernardeschi D. Meniett device in meniere disease: Randomized, double-blind, placebo-controlled multicenter trial. The Laryngoscope. 2017 Feb:127(2):470-475. doi: 10.1002/lary.26197. Epub 2016 Aug 12 [PubMed PMID: 27515294]
Level 1 (high-level) evidenceAhsan SF, Standring R, Wang Y. Systematic review and meta-analysis of Meniett therapy for Meniere's disease. The Laryngoscope. 2015 Jan:125(1):203-8. doi: 10.1002/lary.24773. Epub 2014 Jun 10 [PubMed PMID: 24913022]
Level 2 (mid-level) evidenceThomsen J, Bretlau P, Tos M, Johnsen NJ. Placebo effect in surgery for Ménière's disease. A double-blind, placebo-controlled study on endolymphatic sac shunt surgery. Archives of otolaryngology (Chicago, Ill. : 1960). 1981 May:107(5):271-7 [PubMed PMID: 7013741]
Level 1 (high-level) evidenceWelling DB, Nagaraja HN. Endolymphatic mastoid shunt: a reevaluation of efficacy. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2000 Mar:122(3):340-5 [PubMed PMID: 10699806]
Level 2 (mid-level) evidenceHuth ME, Ricci AJ, Cheng AG. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. International journal of otolaryngology. 2011:2011():937861. doi: 10.1155/2011/937861. Epub 2011 Oct 25 [PubMed PMID: 22121370]
Postema RJ, Kingma CM, Wit HP, Albers FW, Van Der Laan BF. Intratympanic gentamicin therapy for control of vertigo in unilateral Menire's disease: a prospective, double-blind, randomized, placebo-controlled trial. Acta oto-laryngologica. 2008 Aug:128(8):876-80. doi: 10.1080/00016480701762458. Epub [PubMed PMID: 18607963]
Level 1 (high-level) evidencePorto E, Revuelta Barbero JM, Medina E, Garzon-Muvdi T, Mattox DE, Solares CA, Vivas EX, Pradilla G. Retrosigmoid Vestibular Neurectomy for Meniere Disease: A Technical Note. World neurosurgery. 2022 Apr:160():71-75. doi: 10.1016/j.wneu.2022.01.027. Epub 2022 Jan 13 [PubMed PMID: 35032712]
Marchioni D, Caiazza N, Calabrese C, Soloperto D. Transcanal Transvestibular Endoscopic Neurectomy: First Experience. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2022 Feb 1:43(2):263-267. doi: 10.1097/MAO.0000000000003397. Epub [PubMed PMID: 35015752]
Lin JW, Winters R. Labyrinthectomy. StatPearls. 2023 Jan:(): [PubMed PMID: 32809635]
Huppert D, Strupp M, Brandt T. Long-term course of Menière's disease revisited. Acta oto-laryngologica. 2010 Jun:130(6):644-51. doi: 10.3109/00016480903382808. Epub [PubMed PMID: 20001444]
Level 2 (mid-level) evidenceBalatsouras DG, Koukoutsis G, Fassolis A, Moukos A, Apris A. Benign paroxysmal positional vertigo in the elderly: current insights. Clinical interventions in aging. 2018:13():2251-2266. doi: 10.2147/CIA.S144134. Epub 2018 Nov 5 [PubMed PMID: 30464434]
Köping M, Shehata-Dieler W, Schneider D, Cebulla M, Oder D, Müntze J, Nordbeck P, Wanner C, Hagen R, Schraven SP. Characterization of vertigo and hearing loss in patients with Fabry disease. Orphanet journal of rare diseases. 2018 Aug 15:13(1):137. doi: 10.1186/s13023-018-0882-7. Epub 2018 Aug 15 [PubMed PMID: 30111353]
Kerber KA, Brown DL, Lisabeth LD, Smith MA, Morgenstern LB. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: a population-based study. Stroke. 2006 Oct:37(10):2484-7 [PubMed PMID: 16946161]