Introduction
Toxigenic strains of Vibrio cholerae are a major cause of acute, severe, dehydrating diarrhea in low- and middle-income countries with unsatisfactory hygienic conditions and those affected by natural disasters and humanitarian crises. [1] V cholerae is a gram-negative, comma-shaped bacterium that causes acute, large-volume, watery diarrhea that can result in rapid dehydration and hypovolemia. Cholera is a rapidly progressing illness and is associated with high mortality rates if not treated promptly. The case fatality ratio can exceed 50% without appropriate treatment but decreases to less than 1% when prompt rehydration and antibiotics are administered. [1][2] V cholerae is transmitted through the fecal-oral route by contaminated water or food. Risk factors for acquiring V cholera include poverty, inadequate sanitation, contaminated water, and food.[1][3]
Cases of cholera are commonly underreported for many reasons, including a lack of appropriate surveillance and resources. The World Health Organization (WHO) estimates that annually there are 1.4 to 4.0 million cases and 21,000 to 143,000 deaths worldwide from cholera.[4] Historically, cholera has been endemic in the Asian subcontinent, but it has also established itself in other regions and is now endemic in Latin and Central America and sub-Saharan Africa.[4]
Over the past 2 centuries, V cholerae has caused 7 pandemics. The seventh pandemic, which is still ongoing, was first reported in 1961 in Sulawesi, an island governed by Indonesia, in 1971 in Africa, and in 1991 in the Americas.[5] V cholerae is constantly evolving, with new genotypes and phenotypes emerging, most likely due to antibiotic pressure.
Cholera continues to be a significant public health threat for many reasons: its endemicity is expanding, outbreaks are unpredictable, it has high morbidity and mortality, it is underdetected and underreported, antimicrobial resistance is increasing, and resources for treatment are inadequate. The WHO's Global Task Force for Cholera Control (GTFCC), a global network of partners coordinated by the WHO, established an initiative with a goal to reduce cholera globally by 90% and eradicate the disease in at least 20 countries by 2030.[6]
Appropriate oral and intravenous rehydration therapy and administering antibiotics and electrolytes are the cornerstones for treating cholera. Oral cholera vaccines are a significant component of the treatment and control strategies implemented in endemic zones or during an outbreak. These vaccines have been shown to be effective in preventing and managing cholera. Water, sanitation, and hygiene (WASH) practices are also crucial in preventing the transmission of V cholera.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
V cholerae is a gram-negative, comma-shaped bacterium responsible for causing is the cause of cholera (see Image. Vibrio cholerae). V cholerae species are highly motile bacteria with a single polar flagellum. Transmission of V cholera occurs via the fecal-oral route through contaminated water and food, fomites, and direct contact with infected individuals. This transmission mode is more pronounced in vulnerable communities affected by natural disasters, war, and famines. [1][3] Inter-household transmission has been documented, which can give rise to clustering patterns of cases. [4]
V cholerae are free-living organisms and diverse species, encompassing pathogenic and non-pathogenic strains classified serologically based on the O antigen of their lipopolysaccharide.[1] V cholerae is salt-tolerant, requiring NaCl to grow, and can be found naturally in aquatic coastal habitats and brackish water contaminated with human or animal waste. This condition can explain the occasional infections through shellfish.[7] Freshly shed feces are hyperinfectious for 24 hours after being released into the environment but can survive for a long time outside the gut. [8] More than 200 serogroups of V cholerae have been identified, but only the toxigenic strains of V cholera cause cholera, and of those, only serogroups O1 and O139 cause cholera epidemics. [1] Non–toxin-producing strains of V cholerae non-O1 and non-O139 have been reported as the cause of smaller outbreaks and isolated cases of diarrhea, and V cholerae non–toxin-producing non-O1 and non-O139 strains have been reported as the cause of sporadic gastroenteritis and sepsis.[9]
Serogroup O1 contains serotypes Inaba and Ogawa and 2 biotypes, classical and El Tor, which cause cholera epidemics. The first 6 pandemics were likely caused by the classical biotype belonging to serotype O1, and the ongoing seventh pandemic (7P) that started in 1961 is caused by the O1 El Tor biotype (7 PET). In 1992, the V cholera serotype O139, known as Bengal, emerged in the Indian subcontinent as another epidemic variant of V cholerae, after that, spreading to other areas in Asia and causing large epidemics.[10][7]
Phylogenetic analyses using whole-genome analyses have tracked the worldwide circulation of 7PET lineages, establishing that a single lineage, 7PET, is responsible for the current pandemic and identifying the Bay of Bengal as the original source of V cholerae.[4][11] Spread occurs with human travel, causing local and long-lived regional outbreaks.
The V cholerae genome contains two chromosomes distinct from other bacteria. V cholerae evolves quickly by acquiring mobile genetic elements. Examples of unique features of the V. cholerae genome include the bacteriophage CTXΦ, which is a lysogenic bacteriophage that infects bacteria and inserts genetic material into the genome, and the Vibrio pathogenicity island, which encodes the toxin that allows intestinal colonization.[12]
Epidemiology
The actual global burden of cholera is not known because cases are underdiagnosed and underreported worldwide. Limited resources for the diagnosis and surveillance of cholera and poor access to healthcare in many areas of the world lead to many cases being undetected. The WHO estimates that annually there are 1.4 to 4.0 million cases and 21,000 to 143,000 deaths worldwide from cholera.[4] Cholera has historically been endemic in the Asian subcontinent but has also established itself in other regions and is now endemic in Latin and Central America and sub-Saharan Africa. Over the past two centuries, V cholerae has caused 7 pandemics. The seventh pandemic, which is still ongoing, was first reported in 1961 in Sulawesi, an island governed by Indonesia, in 1971 in Africa, and in 1991 in the Americas.[5] V cholerae is constantly evolving, and new genotypes and phenotypes are being produced due to the pressure of antibiotic use.
Serogroup O1 contains serotypes Inaba and Ogawa and 2 biotypes, classical and El Tor, which cause cholera epidemics. The first 6 pandemics were likely caused by the classical biotype belonging to serotype O1, and the ongoing seventh pandemic (7P) that started in 1961 is caused by the O1 El Tor biotype (7 PET). In 1992, the V cholera serotype O139, known as Bengal, emerged in the Indian subcontinent as another epidemic variant of V cholerae, thereafter spreading to other areas in Asia and causing large epidemics.[10][7]
Phylogenetic analyses using whole-genome sequencing have facilitated our better understanding of the evolution of V cholerae and have tracked the worldwide circulation of 7PET lineages. We now know that a single lineage, 7PET, is responsible for the current pandemic by the expansion of a single bacterial lineage associated with 3 waves of transmission that allowed the acquisition of new genetic material.[4][11] This evolutionary process allowed the bacterium to have improved environmental compatibility, increased antimicrobial resistance, and increased virulence compared to previous strains of V cholerae. These analyses pointed to the Bay of Bengal as the original source of V cholerae, causing 6 pandemics from 1827 through 1923. The seventh pandemic has been ongoing since 1961, reaching South America and most of the Western Hemisphere in 1991.[13]. Recently, cholera has continued to affect vulnerable communities such as post-earthquake Haiti (2010), Iraq, and Yemen, where natural disasters, refugee movements, war, and conflict increase the risk of infection and outbreaks.[10][14] Although safe drinking water and advanced sanitation systems have made cholera a treatable illness in Europe and North America, new V cholerae strains, ease of travel, and constant migration of possibly infected individuals have raised serious public health concerns.[15]
Pathophysiology
V cholerae is a gram-negative aerobic or facultative anaerobic comma-shaped bacillus. The antigenic structure of the bacterium comprises a somatic O antigen and a flagellar H antigen. The latter distinguishes pathogenic from nonpathogenic strains. To reach the small intestine, the organism has to pass through the stomach and survive the low gastric pH. V cholerae is not acid-resistant; therefore, it requires a large inoculum size to survive the gastric acid in the stomach (10^8).[4][16] Once it passes through the stomach, it enters the small intestine colonization. V cholerae then moves to the epithelial cells, where it adheres, multiplies, and secretes cholera toxin, which is eventually endocytosed by the intestinal epithelial cells. This cholera toxin has an A1 subunit, which activates adenylate cyclase to increase cyclic adenosine monophosphate (cAMP). cAMP inhibits the absorption of sodium and chloride from the microvilli and increases the secretion of chloride and water from the crypt cells, causing profuse, isotonic, watery diarrhea.[4] When the toxin enters the epithelial cells, it alters the electrolyte channels, resulting in endoluminal fluid loss rich in chloride, bicarbonate, sodium, and potassium.[17] Once the V cholerae is excreted into the environment, it remains hyperinfectious for the next 24 hours, which may contribute to the explosive nature of cholera epidemics.[18]
In addition to the inoculum size, other factors also influence an individual's susceptibility to V cholerae infection and the severity of illness. Individuals with blood group O are more likely to develop severe cholera compared to those with other blood types, and prior infection or vaccination against cholera has often been found to gain temporary acquired immunity.[19][20][21] Recently, there has been an increase in non-O1 and non-O139 V cholerae infections presenting as self-limited gastroenteritis after bathing in contaminated recreational waters or ingesting raw and undercooked seafood.[9][22]
The O139 Bengal strain of V cholerae acts similarly, except that it has a novel O139 lipopolysaccharide and an immunologically linked O-antigen capsule.[23] These two features increase its virulence, resistance to human serum, and the occasional development of O139 bacteremia.[23]
Histopathology
V cholerae is a noninvasive bacterium that causes secretory diarrhea by colonizing the small intestine and producing cholera toxin. Transmission of V cholera occurs via the fecal-oral route through contaminated water and food, fomites, and direct contact with infected infected individuals. V cholera attaches to the small intestine and produces cholera toxins. These bacteria have specialized adherence factors that allow them to adhere to the hostile microvilli surface. Once attached, V cholera exports 1 of 2 antigenically related but distinct forms of cholera enterotoxin (CT-1 or CT-2) into the intestinal epithelial cell. The cholera toxin causes adenylate cyclase to be locked on the on mode, leading to an excess in cAMP and subsequent hypersecretion of chloride and bicarbonate followed by water.[7]
History and Physical
Cholera is typically characterized by profuse, painless diarrhea and vomiting without a fever, although the diarrhea may be of varied severity. Severe cases may present with hypovolemic shock due to significant fluid and electrolyte loss. Although initial diarrhea may include fecal material, the classic diarrhea presentation consists of watery, foul-smelling mucous described as rice-water stools. The rate of fluid loss and high stool sodium concentration distinguish cholera from other diarrheal diseases.[24]
In severe cases, known as cholera gravis, hypotensive shock can develop within hours of the initial symptoms. If treatment is not started immediately, death may occur. Mortality rates are reported to be as high as 70%.[25] Cholera sicca is a variant of the disease when fluid accumulates in the intestinal lumen, followed by circulatory collapse and death before any diarrheal symptoms arise.[26]
Clinical findings in patients with hypovolemic shock may include decreased urine output, cold, clammy skin, decreased skin turgor, sunken eyes, Kussmaul breathing (acidosis), tachycardia, and hypotension. Electrolyte imbalances can cause muscle cramping and weakness.[27]
Clinical signs of cholera depend on the level of volume contraction. Symptoms and signs associated with the percentages of fluid lost are as follows:
- 3% to 5% loss of body weight - excessive thirst
- 5% to 8% loss of body weight - postural hypotension, weakness, tachycardia, fatigue, dry mucous membranes, or dry mouth
- >10% loss of body weight - oliguria; weak, thready, or absent pulse; sunken eyes (sunken fontanelles in infants); wrinkled washerwoman skin; and hypersomnolence and coma
Evaluation
V cholera is mainly diagnosed clinically in the setting of a diarrheal illness outbreak. Various factors differentiate it from other diarrheal diseases. Given the pathophysiology of cholera and its effects on chloride secretion through apical channels and inhibition of sodium chloride absorption, laboratory results often reveal hypokalemia, hypocalcemia, metabolic acidosis, and isonatremic dehydration.[27][28] In children, severe hypoglycemia may occur, coupled with altered mental status, seizures, and coma.[29] Otherwise, no strict laboratory or radiographic findings are required to diagnose and care for cholera patients.
Confirmatory V cholerae diagnosis involves isolating the bacteria in stool cultures by polymerase chain reaction and rapid tests. Nonetheless, given the morbidity and mortality associated with the disease, treatment is never to be delayed for diagnostic testing, as clinical diagnosis is usually sufficient.[25] Stool culture remains the gold standard for detecting V cholerae, and susceptibilities are performed using selective media. Stool culture requires time, however, and is not the appropriate technique when a rapid diagnosis is necessary.[30]
Rapid diagnostic tests have commanded greater attention compared to stool culture due to their ease of use in field settings, low cost, and rapid turnaround time, which can be important in setting up and performing epidemic and preventive surveillance.[31][32] Most rapid diagnostic tests follow the principles of dipstick tests by applying a fresh stool sample to the stick and adding specific reagents to produce a positive or negative result.[2] Given the importance of the WHO on faster, easier, and less expensive diagnostic tests, new rapid diagnostic tests are developed periodically. Recently, enriched rapid diagnostic tests have shown diagnostic performance equivalent to cultures.[33][34][35] On the downside, recent outbreaks, similar to the one that occurred in Haiti after the 2010 earthquake, have revealed the drawbacks of relying too heavily on rapid diagnostic tests that have significant variations. While these tests are useful for outbreak response and surveillance, they are not optimal for use as point-of-care tests.[36]
Treatment / Management
Oral rehydration therapy is the mainstay treatment for acute cholera infection and has dramatically improved patient outcomes globally when given to patients for cholera and all other dehydrating diarrheal diseases.[37] The level of hypovolemia determines the fluid replacement requirement, correlating it to findings on the physical examination such as ear, nose, throat, skin pinch, pulse, and mental status. There are three levels: none, some, or severe volume depletion.[38] Given the average loss of 20 mL/kg/h in patients affected by cholera, rehydration must be started immediately. After assessing the initial volume deficit, the route of rehydration is determined and started as soon as possible. Patients with severe dehydration are typically in hypovolemic shock. They require emergent intravenous rehydration of as much as 350 mL/kg in the first 24 hours, with complete fluid deficit replaced during the first 3 to 4 hours.[39] Oral rehydration then begins as soon as the patient can drink.
Oral rehydration therapy was originally developed in the 1960s as an equimolar oral rehydration solution rich in sodium and glucose to respond to the small intestine's severe electrolyte and fluid losses.[40] Today, different oral rehydration solutions (ORS) are recommended for treating cholera. Since 2002, the WHO has recommended a reduced osmolar ORS that reduces stool fluid and electrolyte depletion.[41] Other forms of ORS, such as rice-derived, non-absorbed starch, polymer-based, and homemade ORS, have also shown a reduction in stool output and fluid and electrolyte losses.[42][43][44][45](A1)
Other types of oral rehydration therapy reported involve the initial administration of glucose solution without salts for a more gradual correction of dehydration.[46] Nonetheless, all solutions composed of salt, water, and glucose have been shown to increase sodium reabsorption through the sodium-glucose-linked transporter in the intestinal wall and can be life-saving.[47](B3)
Patients experiencing severe vomiting in addition to diarrhea require special attention and additional measures. In these circumstances, ORS is typically not sufficient, and intravenous rehydration must be started to avoid the progression of dehydration. Continuous assessment for ongoing fluid loss is deemed paramount to ensure that fluid replacement is maintained and that volume status is periodically reassessed. For this purpose, cholera cots, where patients defecate directly into a measuring collection bucket, were developed.[15]
The most common mistake when treating patients with cholera is underestimating their correct fluid requirement and not reassessing the patients' ongoing fluid losses.[48]
Antimicrobials are considered an adjunctive treatment for V cholerae and are typically administered once the initial volume deficit is corrected and vomiting has ceased. Antimicrobials result in a substantial improvement in clinical and microbiological outcomes. They shorten the mean duration of diarrhea from 5 days to 1 to 2 days, reduce the total stool volume by 50%, reduce the amount of rehydration fluid required by 40%, and reduce the amount of excretion of hyperinfective bacteria by 3 days.[49] Although the initial empiric therapy is typically based on the availability of antimicrobials and the antimicrobial resistance patterns locally, most strains are, in fact, susceptible to tetracyclines, and doxycycline is the drug of choice. [4] Tetracyclines are the most studied and used antimicrobials for cholera.[50] In comparison studies, tetracyclines and azithromycin may have advantages over other antimicrobials when comparing the duration of diarrhea.[49] Recently, reports of resistance to azithromycin have emerged, requiring caution when administering without knowing the local antimicrobial susceptibility patterns.[4] The development of resistance to antimicrobials is a global concern because treatment options can be limited, and patient outcomes may be worse.[1](A1)
Infants diagnosed with cholera should be treated with ORS while breastfeeding, and zinc supplementation is also recommended.[51] In addition, vitamin A supplementation has been shown to reduce morbidity and mortality in children from 6 months to 5 years.[52](A1)
Antiemetics and antimotility agents have been found to have no benefit and may impede ORS therapy, hence producing worse outcomes.[25][53](A1)
Differential Diagnosis
The clinical presentation of infection with V cholerae may be indistinguishable from infections with other pathogens. Patients with cholera can have varied degrees of acuity ranging from uncomplicated diarrhea to severe, watery, dehydrating diarrhea with vomiting that can cause hypovolemic shock within hours. Many infectious and noninfectious causes can manifest with acute watery diarrhea and can potentially mimic cholera. As cholera needs to be promptly diagnosed and treated, it should always be included in the differential diagnosis of diarrhea when a new patient is being evaluated. A high index of suspicion is required when patients have diarrhea in an outbreak setting or an endemic area. The differential diagnosis is broad, including infectious and noninfectious causes. Obtaining a thorough medical, exposure, and travel history is crucial to assess the patient's risk factors for cholera.[54]
In resource-limited settings where cholera is common, giardia, rotavirus, and cryptosporidium are commonly identified as significant causative pathogens in infants and young children. In contrast, enterotoxigenic Escherichia coli is very common in older children and adults.
The list below contains examples of causes of acute watery diarrhea that may have a similar clinical presentation to cholera and must be considered in patients' differential diagnoses. The list of diseases is not meant to be exhaustive.[54]
- Other infectious causes of acute watery diarrhea can include enterotoxigenic E coli, V cholerae O1 or O139, Campylobacter spp, nontyphoidal Salmonella enterica, Aeromonas spp, enteroaggregative E coli, norovirus, adenovirus, and rotavirus.
- Other infectious causes of acute bloody diarrhea can include Shigella spp, Campylobacter spp, enteroinvasive E coli, enterohemorrhagic E coli, nontyphoidal S enterica, E histolytica, and Schistosoma mansoni.
Pertinent Studies and Ongoing Trials
In 2011, the WHO recognized the first oral cholera vaccine and started stockpiling it in 2013. Two additional oral cholera vaccines were added to the stockpile in 2016. Although found to be a cost-effective short-to-medium term option compared to the high upfront costs of creating water, sanitation, and hygiene infrastructure from scratch, oral cholera vaccines have encountered numerous challenges that continue to be studied.[55]
A 2017 study discussing the 12 most recent oral cholera vaccine campaigns identified 3 main obstacles to more widespread vaccine use: regulatory hurdles (country-specific and time-consuming), cold-chain logistics (challenging to maintain in low-resource settings), and vaccine coverage/uptake.[56] An effective and immediate approach for utilizing oral cholera vaccines from the global stockpile must be developed to overcome the hurdles associated with disease prevention.[57][58][59]
Current and future research areas include whether supplementation with probiotics, thus restoring the gut microbiota, can clinically manage patients with cholera, reduce antimicrobial use, and improve outcomes.[60]
The WHO's GTFCC is a global network of partners coordinated by the WHO. One of the most important goals of GTFCC is the WHO-backed Global Roadmap to 2030, whose goals are to reduce cholera globally by 90% and eradicate it in at least 20 countries by 2030.[6] To achieve these goals, they suggest a 3-pronged approach: early detection of cholera cases and prompt response to curtail outbreaks; implementation of a targeted multisectoral strategy to prevent cholera recurrence; and development of a coordinated mechanism for advocacy, technical support, and partnership at local and global levels.[4][6]
Prognosis
Before the introduction of effective oral rehydration therapy, mortality rates in severe cholera were more than 50%, with even higher rates in pregnant women and children. The average case fatality rates for the United States of America and Europe are estimated to be 1%, which is the WHO-recommended percentage. In Africa, a marked decline in mortality has occurred since 1970. The case fatality rate was 1.9% to 2.9% in 2021 and 1.8% in 2023, exceeding the target level of 1% [61]. Lower case fatality rates are observed in South America, presumably due to the availability of appropriate treatment facilities and trained personnel.
Complications
The most common and life-threatening complication of V cholerae is severe and rapid dehydration within hours, leading to hypovolemic shock and metabolic acidosis, which can potentially be fatal.[10] However, with appropriate and prompt treatment, patients typically recover fully without any long-term sequelae.
Deterrence and Patient Education
Cholera is an infectious disease caused by ingesting food or water contaminated by the bacteria V cholerae. Patients living in endemic areas and travelers to these regions should be educated on the importance of hand hygiene. In addition, boiling water before it is consumed or used to rinse food is imperative. As stated above, some vaccines can also be used in endemic areas. Patients who live or have traveled to endemic countries should be encouraged to seek medical attention immediately if they develop signs of cholera infection.
The key to reducing morbidity and mortality from cholera is patient education about transmission risks, improving environmental living conditions, good hygiene, and implementing all prevention techniques outlined in the WHO. Furthermore, understanding and addressing the social dynamics that lead to cholera risk may be more effective for better targeting efforts and the possibility of eliminating cholera.[62][63]
Cholera infection rates and outbreaks have been closely linked to the socioeconomic characteristics of the region.[64] Cholera reveals how a country's socioeconomic status can propagate a preventable disease. This circumstance has led to increased research and teamwork between public health, government, and medical authorities to develop oral cholera vaccines and improve implementation practices and strategies.[57][59]
Pearls and Other Issues
Key facts to keep in mind regarding V cholerae infection are as follows:
- Cholera is an acute secretory diarrheal illness caused by the toxin of the comma-shaped gram-negative V cholerae bacterium that is known worldwide for its pandemic potential.
- Cholera affects resource-poor and developing countries lacking water, sanitation, and hygiene infrastructure. Historically, it has been responsible for 7 pandemics. Currently, it is endemic in 69 countries, with particular severity in Sub-Saharan Africa and the Middle East.[65]
- Infection begins with the ingestion of food or water contaminated with V cholerae. V cholerae subsequently colonizes the small intestine and produces toxins that induce changes in the electrolyte channels in the small intestine, causing massive fluid and electrolyte loss, clinically manifesting as watery diarrhea.
- The clinical presentation consists of painless, severe, dehydrating watery diarrhea, often resembling rice water, which can rapidly lead to hypovolemic shock in a few hours if not promptly treated.
- A high index of suspicion must be present when managing patients with acute, watery diarrhea, especially in endemic areas or during an outbreak. The diagnosis is clinical, but confirmation using stool cultures on special selective media is recognized as the gold standard. As stool cultures are impractical for use in the field, rapid diagnostic tests such as dipsticks have been favored for outbreak surveillance and control.
- ORS is the mainstay treatment for cholera and consists of aggressive volume repletion depending on the initial level of volume depletion and ongoing fluid losses.
- Patients with cholera must be given fluids by replacing the initial volume deficit in the first 4 to 6 hours and giving 350 mL/kg of fluid in the first 24 hours. Close monitoring of ongoing fluid losses is essential to prevent mortality.
- Isotonic oral fluids are preferred except in severe cases and in patients actively vomiting, where intravenous hydration may be needed.
- Other treatment modalities, such as antibiotics and nutritional supplements, have been proven to help with symptom duration and severity. Tetracyclines and macrolides are considered first- and second-line therapy, respectively.
- Although adequate water, sanitation, and hygiene infrastructure are the main preventive measures, oral cholera vaccines are effective, comparably inexpensive, and safe for outbreak control in high-risk endemic areas.
Enhancing Healthcare Team Outcomes
Improving healthcare outcomes for patients in resource-poor and developing countries infected with V cholerae is an issue at the forefront of public health. Concerted local, regional, and global efforts must be established to prevent, evaluate, diagnose, and treat patients with cholera. The care of patients with cholera necessitates a collaborative approach among healthcare professionals to ensure patient-centered care and improve overall outcomes.
Cholera presents a significant public health challenge requiring the collaboration of an interprofessional healthcare team. This team typically includes an epidemiologist, infectious disease specialist, primary care provider, infection control nurse, social workers, dietitians, emergency department staff, advanced practitioners, nurses, pharmacists, and other health professionals. Each team member should possess the essential clinical skills and knowledge to diagnose and manage suspected and confirmed cholera cases. This includes expertise in recognizing the varied clinical presentations and understanding the nuances of diagnostic techniques. These teams have been shown to improve early diagnosis and prompt treatment with improvement in morbidity and mortality.[1]
Media
(Click Image to Enlarge)
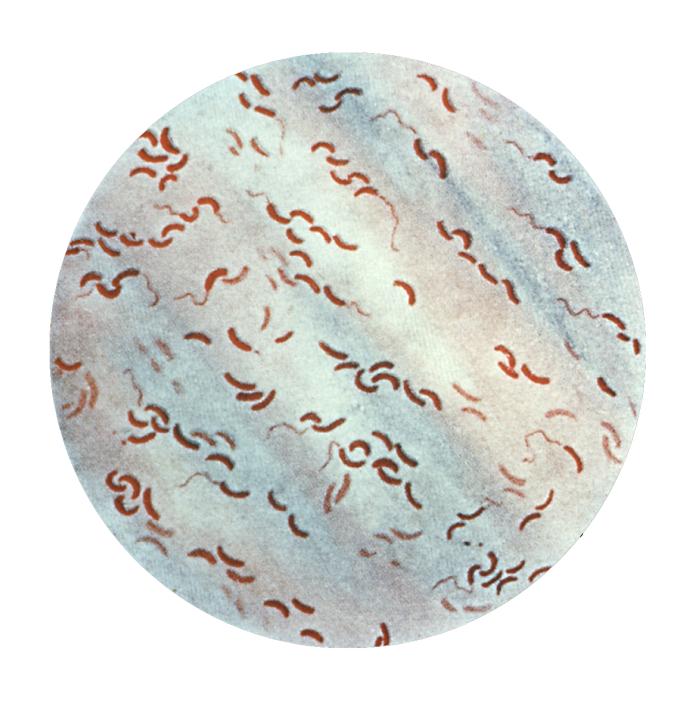
Vibrio cholerae. This illustration depicts a photomicrograph of a Gram-stained specimen, revealing the presence of numerous flagellated, Vibrio cholerae, also known as Vibrio comma bacteria. This particular strain of V. cholerae causes Asiatic cholera. Note the characteristic “comma” shape of these organisms.
CDC. Public Domain Image.
References
Chowdhury F, Ross AG, Islam MT, McMillan NAJ, Qadri F. Diagnosis, Management, and Future Control of Cholera. Clinical microbiology reviews. 2022 Sep 21:35(3):e0021121. doi: 10.1128/cmr.00211-21. Epub 2022 Jun 21 [PubMed PMID: 35726607]
Dick MH, Guillerm M, Moussy F, Chaignat CL. Review of two decades of cholera diagnostics--how far have we really come? PLoS neglected tropical diseases. 2012:6(10):e1845. doi: 10.1371/journal.pntd.0001845. Epub 2012 Oct 11 [PubMed PMID: 23071851]
Schaetti C, Sundaram N, Merten S, Ali SM, Nyambedha EO, Lapika B, Chaignat CL, Hutubessy R, Weiss MG. Comparing sociocultural features of cholera in three endemic African settings. BMC medicine. 2013 Sep 18:11():206. doi: 10.1186/1741-7015-11-206. Epub 2013 Sep 18 [PubMed PMID: 24047241]
Kanungo S, Azman AS, Ramamurthy T, Deen J, Dutta S. Cholera. Lancet (London, England). 2022 Apr 9:399(10333):1429-1440. doi: 10.1016/S0140-6736(22)00330-0. Epub [PubMed PMID: 35397865]
Kaper JB, Morris JG Jr, Levine MM. Cholera. Clinical microbiology reviews. 1995 Jan:8(1):48-86 [PubMed PMID: 7704895]
Legros D, Partners of the Global Task Force on Cholera Control. Global Cholera Epidemiology: Opportunities to Reduce the Burden of Cholera by 2030. The Journal of infectious diseases. 2018 Oct 15:218(suppl_3):S137-S140. doi: 10.1093/infdis/jiy486. Epub [PubMed PMID: 30184102]
Baron S, Finkelstein RA. Cholera, Vibrio cholerae O1 and O139, and Other Pathogenic Vibrios. Medical Microbiology. 1996:(): [PubMed PMID: 21413330]
Merrell DS, Butler SM, Qadri F, Dolganov NA, Alam A, Cohen MB, Calderwood SB, Schoolnik GK, Camilli A. Host-induced epidemic spread of the cholera bacterium. Nature. 2002 Jun 6:417(6889):642-5 [PubMed PMID: 12050664]
Level 3 (low-level) evidenceDutta D, Chowdhury G, Pazhani GP, Guin S, Dutta S, Ghosh S, Rajendran K, Nandy RK, Mukhopadhyay AK, Bhattacharya MK, Mitra U, Takeda Y, Nair GB, Ramamurthy T. Vibrio cholerae non-O1, non-O139 serogroups and cholera-like diarrhea, Kolkata, India. Emerging infectious diseases. 2013 Mar:19(3):464-7. doi: 10.3201/eid1903.121156. Epub [PubMed PMID: 23622872]
Somboonwit C, Menezes LJ, Holt DA, Sinnott JT, Shapshak P. Current views and challenges on clinical cholera. Bioinformation. 2017:13(12):405-409. doi: 10.6026/97320630013405. Epub 2017 Dec 31 [PubMed PMID: 29379258]
Oprea M, Njamkepo E, Cristea D, Zhukova A, Clark CG, Kravetz AN, Monakhova E, Ciontea AS, Cojocaru R, Rauzier J, Damian M, Gascuel O, Quilici ML, Weill FX. The seventh pandemic of cholera in Europe revisited by microbial genomics. Nature communications. 2020 Oct 22:11(1):5347. doi: 10.1038/s41467-020-19185-y. Epub 2020 Oct 22 [PubMed PMID: 33093464]
Waldor MK, Mekalanos JJ. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science (New York, N.Y.). 1996 Jun 28:272(5270):1910-4 [PubMed PMID: 8658163]
Level 3 (low-level) evidenceSiddique AK, Cash R. Cholera outbreaks in the classical biotype era. Current topics in microbiology and immunology. 2014:379():1-16. doi: 10.1007/82_2013_361. Epub [PubMed PMID: 24368696]
Raslan R, El Sayegh S, Chams S, Chams N, Leone A, Hajj Hussein I. Re-Emerging Vaccine-Preventable Diseases in War-Affected Peoples of the Eastern Mediterranean Region-An Update. Frontiers in public health. 2017:5():283. doi: 10.3389/fpubh.2017.00283. Epub 2017 Oct 25 [PubMed PMID: 29119098]
Davies HG, Bowman C, Luby SP. Cholera - management and prevention. The Journal of infection. 2017 Jun:74 Suppl 1():S66-S73. doi: 10.1016/S0163-4453(17)30194-9. Epub [PubMed PMID: 28646965]
Nelson EJ, Harris JB, Morris JG Jr, Calderwood SB, Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nature reviews. Microbiology. 2009 Oct:7(10):693-702. doi: 10.1038/nrmicro2204. Epub [PubMed PMID: 19756008]
Level 3 (low-level) evidenceReidl J, Klose KE. Vibrio cholerae and cholera: out of the water and into the host. FEMS microbiology reviews. 2002 Jun:26(2):125-39 [PubMed PMID: 12069878]
Hartley DM, Morris JG Jr, Smith DL. Hyperinfectivity: a critical element in the ability of V. cholerae to cause epidemics? PLoS medicine. 2006 Jan:3(1):e7 [PubMed PMID: 16318414]
Harris JB, Khan AI, LaRocque RC, Dorer DJ, Chowdhury F, Faruque AS, Sack DA, Ryan ET, Qadri F, Calderwood SB. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infection and immunity. 2005 Nov:73(11):7422-7 [PubMed PMID: 16239542]
Czerwiński M. [Blood groups - minuses and pluses. Do the blood group antigens protect us from infectious diseases?]. Postepy higieny i medycyny doswiadczalnej (Online). 2015 Jun 25:69():703-22. doi: 10.5604/17322693.1158795. Epub 2015 Jun 25 [PubMed PMID: 26206987]
Levine MM, Black RE, Clements ML, Cisneros L, Nalin DR, Young CR. Duration of infection-derived immunity to cholera. The Journal of infectious diseases. 1981 Jun:143(6):818-20 [PubMed PMID: 7252264]
De Keukeleire S, Hoste P, Crivits M, Hammami N, Piette A. Atypical manifestation of Vibrio cholerae: fear the water! Acta clinica Belgica. 2018 Dec:73(6):462-464. doi: 10.1080/17843286.2018.1483563. Epub 2018 Jun 19 [PubMed PMID: 29916306]
Knirel YA, Widmalm G, Senchenkova SN, Jansson PE, Weintraub A. Structural studies on the short-chain lipopolysaccharide of Vibrio cholerae O139 Bengal. European journal of biochemistry. 1997 Jul 1:247(1):402-10 [PubMed PMID: 9249053]
Lippi D, Gotuzzo E, Caini S. Cholera. Microbiology spectrum. 2016 Aug:4(4):. doi: 10.1128/microbiolspec.PoH-0012-2015. Epub [PubMed PMID: 27726771]
Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet (London, England). 2012 Jun 30:379(9835):2466-2476. doi: 10.1016/S0140-6736(12)60436-X. Epub [PubMed PMID: 22748592]
Roy MM. A Case of Cholera Sicca. The Indian medical gazette. 1934 Jul:69(7):394 [PubMed PMID: 29009163]
Wang F, Butler T, Rabbani GH, Jones PK. The acidosis of cholera. Contributions of hyperproteinemia, lactic acidemia, and hyperphosphatemia to an increased serum anion gap. The New England journal of medicine. 1986 Dec 18:315(25):1591-5 [PubMed PMID: 3785323]
Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. The Journal of clinical investigation. 2002 Dec:110(11):1651-8 [PubMed PMID: 12464670]
Level 3 (low-level) evidenceDube A, Moffatt M, Davison C, Bartels S. Health Outcomes for Children in Haiti Since the 2010 Earthquake: A Systematic Review. Prehospital and disaster medicine. 2018 Feb:33(1):77-88. doi: 10.1017/S1049023X17007105. Epub 2017 Dec 18 [PubMed PMID: 29248034]
Barzilay EJ, Schaad N, Magloire R, Mung KS, Boncy J, Dahourou GA, Mintz ED, Steenland MW, Vertefeuille JF, Tappero JW. Cholera surveillance during the Haiti epidemic--the first 2 years. The New England journal of medicine. 2013 Feb 14:368(7):599-609. doi: 10.1056/NEJMoa1204927. Epub 2013 Jan 9 [PubMed PMID: 23301694]
Boncy J, Rossignol E, Dahourou G, Hast M, Buteau J, Stanislas M, Moffett D, Bopp C, Balajee SA. Performance and utility of a rapid diagnostic test for cholera: notes from Haiti. Diagnostic microbiology and infectious disease. 2013 Aug:76(4):521-3. doi: 10.1016/j.diagmicrobio.2013.03.010. Epub [PubMed PMID: 23886437]
Hasan JA, Huq A, Nair GB, Garg S, Mukhopadhyay AK, Loomis L, Bernstein D, Colwell RR. Development and testing of monoclonal antibody-based rapid immunodiagnostic test kits for direct detection of Vibrio cholerae O139 synonym Bengal. Journal of clinical microbiology. 1995 Nov:33(11):2935-9 [PubMed PMID: 8576349]
Ontweka LN, Deng LO, Rauzier J, Debes AK, Tadesse F, Parker LA, Wamala JF, Bior BK, Lasuba M, But AB, Grandesso F, Jamet C, Cohuet S, Ciglenecki I, Serafini M, Sack DA, Quilici ML, Azman AS, Luquero FJ, Page AL. Cholera Rapid Test with Enrichment Step Has Diagnostic Performance Equivalent to Culture. PloS one. 2016:11(12):e0168257. doi: 10.1371/journal.pone.0168257. Epub 2016 Dec 19 [PubMed PMID: 27992488]
Debes AK, Ateudjieu J, Guenou E, Ebile W, Sonkoua IT, Njimbia AC, Steinwald P, Ram M, Sack DA. Clinical and Environmental Surveillance for Vibrio cholerae in Resource Constrained Areas: Application During a 1-Year Surveillance in the Far North Region of Cameroon. The American journal of tropical medicine and hygiene. 2016 Mar:94(3):537-543. doi: 10.4269/ajtmh.15-0496. Epub 2016 Jan 11 [PubMed PMID: 26755564]
Sayeed MA, Islam K, Hossain M, Akter NJ, Alam MN, Sultana N, Khanam F, Kelly M, Charles RC, Kováč P, Xu P, Andrews JR, Calderwood SB, Amin J, Ryan ET, Qadri F. Development of a new dipstick (Cholkit) for rapid detection of Vibrio cholerae O1 in acute watery diarrheal stools. PLoS neglected tropical diseases. 2018 Mar:12(3):e0006286. doi: 10.1371/journal.pntd.0006286. Epub 2018 Mar 14 [PubMed PMID: 29538377]
Matias WR, Julceus FE, Abelard C, Mayo-Smith LM, Franke MF, Harris JB, Ivers LC. Laboratory evaluation of immunochromatographic rapid diagnostic tests for cholera in Haiti. PloS one. 2017:12(11):e0186710. doi: 10.1371/journal.pone.0186710. Epub 2017 Nov 1 [PubMed PMID: 29091945]
Banwell JG. Worldwide impact of oral rehydration therapy. Clinical therapeutics. 1990:12 Suppl A():29-36; discussion 36-7 [PubMed PMID: 2187610]
Liamis G, Filippatos TD, Elisaf MS. Correction of hypovolemia with crystalloid fluids: Individualizing infusion therapy. Postgraduate medicine. 2015 May:127(4):405-12. doi: 10.1080/00325481.2015.1029421. Epub 2015 Mar 26 [PubMed PMID: 25812486]
Mahalanabis D, Wallace CK, Kallen RJ, Mondal A, Pierce NF. Water and electrolyte losses due to cholera in infants and small children: a recovery balance study. Pediatrics. 1970 Mar:45(3):374-85 [PubMed PMID: 5442912]
Nalin DR, Cash RA, Islam R, Molla M, Phillips RA. Oral maintenance therapy for cholera in adults. Lancet (London, England). 1968 Aug 17:2(7564):370-3 [PubMed PMID: 4173788]
Level 1 (high-level) evidenceMurphy C, Hahn S, Volmink J. Reduced osmolarity oral rehydration solution for treating cholera. The Cochrane database of systematic reviews. 2004 Oct 18:(4):CD003754 [PubMed PMID: 15495063]
Level 1 (high-level) evidenceLebenthal E, Khin-Maung-U, Khin-Myat-Tun, Tin-Nu-Swe, Thein-Thein-Myint, Jirapinyo P, Visitsuntorn N, Ismail R, Bakri A, Firmansyah A. High-calorie, rice-derived, short-chain, glucose polymer-based oral rehydration solution in acute watery diarrhea. Acta paediatrica (Oslo, Norway : 1992). 1995 Feb:84(2):165-72 [PubMed PMID: 7538837]
Level 2 (mid-level) evidencePierce NF, Fontaine O, Sack RB. Amylase-resistant starch plus oral rehydration solution for cholera. The New England journal of medicine. 2000 Jun 29:342(26):1995-6 [PubMed PMID: 10877651]
Level 3 (low-level) evidenceGregorio GV, Gonzales ML, Dans LF, Martinez EG. Polymer-based oral rehydration solution for treating acute watery diarrhoea. The Cochrane database of systematic reviews. 2016 Dec 13:12(12):CD006519. doi: 10.1002/14651858.CD006519.pub3. Epub 2016 Dec 13 [PubMed PMID: 27959472]
Level 1 (high-level) evidenceBhattacharyya AK, Hati AK. WHO formula of ORS and home made ORS. Journal of the Indian Medical Association. 1994 Feb:92(2):69-70 [PubMed PMID: 8071565]
Level 3 (low-level) evidenceButler T. Treatment of severe cholera: a review of strategies to reduce stool output and volumes of rehydration fluid. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2017 May 1:111(5):204-210. doi: 10.1093/trstmh/trx041. Epub [PubMed PMID: 28957470]
Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiological reviews. 2011 Apr:91(2):733-94. doi: 10.1152/physrev.00055.2009. Epub [PubMed PMID: 21527736]
Level 3 (low-level) evidenceCenters for Disease Control and Prevention (CDC). Update on cholera --- Haiti, Dominican Republic, and Florida, 2010. MMWR. Morbidity and mortality weekly report. 2010 Dec 24:59(50):1637-41 [PubMed PMID: 21178947]
Leibovici-Weissman Y, Neuberger A, Bitterman R, Sinclair D, Salam MA, Paul M. Antimicrobial drugs for treating cholera. The Cochrane database of systematic reviews. 2014 Jun 19:2014(6):CD008625. doi: 10.1002/14651858.CD008625.pub2. Epub 2014 Jun 19 [PubMed PMID: 24944120]
Level 1 (high-level) evidenceGREENOUGH WB 3rd, GORDON RS Jr, ROSENBERG IS, DAVIES BI, BENENSON AS. TETRACYCLINE IN THE TREATMENT OF CHOLERA. Lancet (London, England). 1964 Feb 15:1(7329):355-7 [PubMed PMID: 14090856]
Level 3 (low-level) evidenceBhandari N, Mazumder S, Taneja S, Dube B, Agarwal RC, Mahalanabis D, Fontaine O, Black RE, Bhan MK. Effectiveness of zinc supplementation plus oral rehydration salts compared with oral rehydration salts alone as a treatment for acute diarrhea in a primary care setting: a cluster randomized trial. Pediatrics. 2008 May:121(5):e1279-85. doi: 10.1542/peds.2007-1939. Epub [PubMed PMID: 18450870]
Level 1 (high-level) evidenceImdad A, Mayo-Wilson E, Herzer K, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. The Cochrane database of systematic reviews. 2017 Mar 11:3(3):CD008524. doi: 10.1002/14651858.CD008524.pub3. Epub 2017 Mar 11 [PubMed PMID: 28282701]
Li ST, Grossman DC, Cummings P. Loperamide therapy for acute diarrhea in children: systematic review and meta-analysis. PLoS medicine. 2007 Mar 27:4(3):e98 [PubMed PMID: 17388664]
Level 1 (high-level) evidenceShane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, Cantey J, Pickering LK. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Nov 29:65(12):e45-e80. doi: 10.1093/cid/cix669. Epub [PubMed PMID: 29053792]
Level 1 (high-level) evidenceHsiao A, Hall AH, Mogasale V, Quentin W. The health economics of cholera: A systematic review. Vaccine. 2018 Jul 16:36(30):4404-4424. doi: 10.1016/j.vaccine.2018.05.120. Epub 2018 Jun 12 [PubMed PMID: 29907482]
Hsiao A, Desai SN, Mogasale V, Excler JL, Digilio L. Lessons learnt from 12 oral cholera vaccine campaigns in resource-poor settings. Bulletin of the World Health Organization. 2017 Apr 1:95(4):303-312. doi: 10.2471/BLT.16.175166. Epub 2017 Feb 21 [PubMed PMID: 28479625]
Schwerdtle P, Onekon CK, Recoche K. A Quantitative Systematic Review and Meta-Analysis of the Effectiveness of Oral Cholera Vaccine as a Reactive Measure in Cholera Outbreaks. Prehospital and disaster medicine. 2018 Feb:33(1):2-6. doi: 10.1017/S1049023X17007166. Epub 2018 Jan 10 [PubMed PMID: 29317005]
Desai SN, Pezzoli L, Alberti KP, Martin S, Costa A, Perea W, Legros D. Achievements and challenges for the use of killed oral cholera vaccines in the global stockpile era. Human vaccines & immunotherapeutics. 2017 Mar 4:13(3):579-587. doi: 10.1080/21645515.2016.1245250. Epub [PubMed PMID: 27813703]
Poncin M, Zulu G, Voute C, Ferreras E, Muleya CM, Malama K, Pezzoli L, Mufunda J, Robert H, Uzzeni F, Luquero FJ, Chizema E, Ciglenecki I. Implementation research: reactive mass vaccination with single-dose oral cholera vaccine, Zambia. Bulletin of the World Health Organization. 2018 Feb 1:96(2):86-93. doi: 10.2471/BLT.16.189241. Epub 2017 Oct 19 [PubMed PMID: 29403111]
Cho JY, Liu R, Macbeth JC, Hsiao A. The Interface of Vibrio cholerae and the Gut Microbiome. Gut microbes. 2021 Jan-Dec:13(1):1937015. doi: 10.1080/19490976.2021.1937015. Epub [PubMed PMID: 34180341]
. Responding to the global cholera pandemic. Bulletin of the World Health Organization. 2023 Apr 1:101(4):234-235. doi: 10.2471/BLT.23.020423. Epub [PubMed PMID: 37008267]
Taylor DL, Kahawita TM, Cairncross S, Ensink JH. The Impact of Water, Sanitation and Hygiene Interventions to Control Cholera: A Systematic Review. PloS one. 2015:10(8):e0135676. doi: 10.1371/journal.pone.0135676. Epub 2015 Aug 18 [PubMed PMID: 26284367]
Lund AJ, Keys HM, Leventhal S, Foster JW, Freeman MC. Prevalence of cholera risk factors between migrant Haitians and Dominicans in the Dominican Republic. Revista panamericana de salud publica = Pan American journal of public health. 2015 Mar:37(3):125-32 [PubMed PMID: 25988248]
Bwire G, Munier A, Ouedraogo I, Heyerdahl L, Komakech H, Kagirita A, Wood R, Mhlanga R, Njanpop-Lafourcade B, Malimbo M, Makumbi I, Wandawa J, Gessner BD, Orach CG, Mengel MA. Epidemiology of cholera outbreaks and socio-economic characteristics of the communities in the fishing villages of Uganda: 2011-2015. PLoS neglected tropical diseases. 2017 Mar:11(3):e0005407. doi: 10.1371/journal.pntd.0005407. Epub 2017 Mar 13 [PubMed PMID: 28288154]
Idoga PE, Toycan M, Zayyad MA. Analysis of Factors Contributing to the Spread of Cholera in Developing Countries. The Eurasian journal of medicine. 2019 Jun:51(2):121-127. doi: 10.5152/eurasianjmed.2019.18334. Epub [PubMed PMID: 31258350]
