Introduction
The placenta is a vital organ with multiple functions, such as endocrine, immune, and physiological. The placenta is formed gradually during the first three months of pregnancy, while, after the fourth month, it grows parallel to the development of the uterus. Once completed, it resembles a spongy disc 20 cm in diameter and 3 cm thick. It is a temporary organ, whose genetic characteristics are identical to those of the developing child. The placenta interacts with the environment in which it is present and vice versa. Proper development of the placenta is essential for a successful pregnancy. There are several layers of tissue that make up this delicate organ that need to develop normally for proper function during gestation. Without proper function, there can be devastating consequences to the pregnancy.
Development
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Development
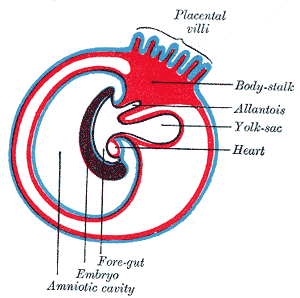
The placenta is a fetal organ made up of its parenchyma, chorion, amnion, and umbilical cord. The fetal structures form from the zygote and therefore separate the fetus from the endometrium. The fetal tissues form from the chorionic sac - which includes the amnion, chorion, yolk sac, and allantois. These tissues get delivered after birth. The maternal part comes from the endometrium and is called the decidua. There are three parts to the decidua - the decidua basalis (deep at the implantation site), the decidua capsularis (covers the implantation site), and the decidua parietalis (everything else).[1]
After fertilization, the fertilized ovum evolves into a morula, which will develop into the embryo and fetal placenta. The inner cell mass develops into the embryoblast, and the outer cell mass is the trophoblast. The morula then takes in fluid and forms a blastocyst with the trophoblast surrounding the inner cell mass and fluid. The blastocyst implants into the uterus approximately six days after fertilization. The contact of the trophoblast with the endometrium causes the development of the syncytiotrophoblast, which secretes human chorionic gonadotrophic hormone (hCG) and the cytotrophoblast which secretes enzymes that break down the bond between endometrial cells so the syncytiotrophoblast can invade the endometrial wall. Both the cytotrophoblast and the syncytiotrophoblast are part of the chorion, which develops into the placenta along with the extraembryonic mesoderm.[2][3][4]
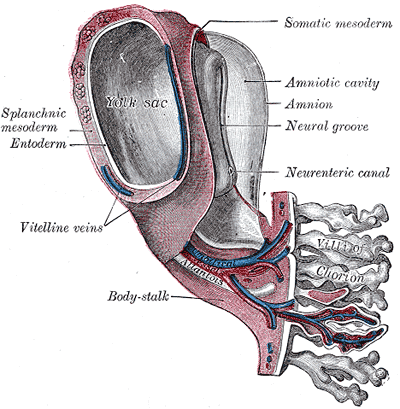
The chorion forms the placenta and consists of the syncytiotrophoblast, cytotrophoblast, and extraembryonic mesoderm. The cytotrophoblast grows into the syncytiotrophoblast as finger-like projections, which are called the primary chorionic villi. The extraembryonic mesoderm splits into somatic and splanchnic mesoderm, and the somatic mesoderm grows into the primary villi creating the secondary villi. The mesenchyme gives rise to blood cells and vessels, which designates tertiary villi when formed. Capillary beds grow from the villi, which connect to the embryo heart. Maternal blood flowing through the embryonic capillaries provide oxygen and nutrients to the fetus. The villi continue to grow and branch into the villus chorion, which is the fetal placenta.[5]
As development continues, cells from the cytotrophoblast continue to extend through the syncytiotrophoblast to eventually form a cytotrophoblastic shell. As progesterone increases, the decidua connective tissue develops into “decidua cells,” which help protect the uterus from an invasion of the syncytiotrophoblast. As the sac continues to grow, the decidua capsularis villi degenerate and eventually disappear as they fuse with the decidua parietalis.
The amniotic sac enlarges faster than the chorionic sac, which causes them eventually to come into contact and fuse into the amniochorionic membrane. The amniochorionic membrane then fuses to the decidua capsularis and, ultimately, the decidua parietalis for stability. The amniochorionic membrane ruptures during labor. The amniochorionic membrane with the fetal vessels makes up the chorionic plate. Parts of the decidua basalis grow into the chorionic plate dividing it into separated septa called cotyledons, in which each contains stem villi.[6]
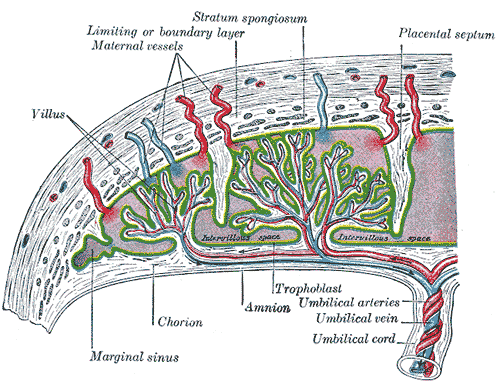
The fetomaternal junction provides stability for the chorion. The chorionic villi that attach to the decidua basalis are an anchor for the fetal chorionic sac to the endometrium. Endometrial vessels, called spiral arteries, make their way through openings in the cytotrophoblastic shell and reside inside the villi where they release maternal blood to bath the chorionic villi in each cotyledon; this allows for maternal blood to provide oxygen and nutrients to the fetus across the placental membrane. Endometrial veins then drain the blood. Although the fetal vessels are bathed in maternal blood, there is normally no mixing between maternal and fetal red blood cells.[7]
The placental membrane is where the mother and fetus exchange gases, nutrients, etc. The membrane forms by the syncytiotrophoblast, cytotrophoblast, embryonic connective tissue (Wharton’s jelly), and the endothelium of fetal blood vessels.
The umbilical cord serves to attach the fetus to the placenta and consists of two umbilical arteries and one umbilical vein.
Cellular
The placenta has several cellular structures to protect the health of the fetus. We can find substances that are part of the superfamily of ATP binding cassettes (ABC), such as multidrug resistance protein type 1, breast cancer resistance protein, multidrug-resistance like protein type 2 and 5.
The placenta can be considered an immune and endocrine organ. It produces many hormones and growth factors in autocrine and paracrine modalities, such as progesterone, corticotropin-releasing hormone, the human chorionic gonadotropin, the human placental lactogen, fibroblast growth factor, and many others.
Biochemical
The placenta has several metabolic functions that are vital for the healthy growth of a fetus. The placenta can make its own glycogen and cholesterol from mom’s glucose and fatty acids, respectively. The glycogen gets stored as energy for the fetus, and the cholesterol is used to make hormones such as progesterone, estrogen, and glucocorticoids. It also synthesizes peptide hormones such as human chorionic gonadotropin (hCG) and human placental lactogen (hPL), growth hormone (GH), vascular endothelial growth factor (VEGF), corticotropin-releasing hormone (CRH), insulin-like growth factor (IGF), placental growth factor (PIGF), and cytokines.[8][9]
Molecular Level
Several molecules interact with the placenta. For example, leptin ensures the health of the placenta, thanks to its actions as a homeostatic agent, proliferation, and protein synthesis, as an anti-apoptotic molecule. In turn, leptin is under the control of other substances, such as insulin, many growth factors, human chorionic gonadotropin, steroids, and hypoxia.
Function
The placenta is the means of communication between mom and fetus. The placental membrane is where the exchange of substances happens between mother and fetus. This exchange is essential for the transfer of gases, electrolytes, hormones, maternal antibodies, fetal waste, and nutrition such as water, amino acids, glucose, vitamins, and free fatty acids. Fetal waste includes urea, uric acid, and bilirubin. Alpha-fetoprotein and other proteins also get exchanged. These transfers are beneficial to the fetus, but many harmful substances can pass through the placental membrane such as certain drugs, live vaccines, carbon monoxide, anti-Rh antibodies, and several infectious agents (“ToRCHes” infections).[10][11] Solvent drag is the bulk flow of water, which brings in nutrients across the placental membrane into each cotyledon to be absorbed. The higher the pressure, the more nutrients that will be absorbed.[12] Solutes and gases are absorbed by simple diffusion depending on their molecular makeup and properties. Oxygen and carbon dioxide are highly permeable across the placental tissues due to their lipophilicity. Their exchange is perfusion limited, which can cause fetal growth restriction if there is tissue hypoxia.[13] The placenta also uses channels for ion transport down their electrochemical gradient, facilitated diffusion for glucose using carrier proteins, and active transport for several solutes.[14]
Due to its multiple nutritional properties, many mammals besides humans, have the habit of eating the expelled placenta after giving birth.
Mechanism
Fetal circulation flows from the fetus to the two umbilical arteries (deoxygenated), then to chorionic arteries in the cotyledons, through the capillary beds to exchange gases with maternal blood, then back to the fetus via a single umbilical vein (oxygenated).
Testing
Chorionic villus sampling (CVS) is either a transabdominal or transcervical procedure where clinicians take samples of the placenta at 10 to 13 weeks of gestation for genetic testing. This test is beneficial because it can be done much earlier than amniocentesis and therefore receive screening results earlier. If the results lead to termination of the pregnancy, there are fewer risks than later termination. Earlier gestation also causes less accurate results. The procedure complications can include infection, bleeding, fetal injury, and fetal death.[15][16]
Amniocentesis is a similar transabdominal procedure where amniotic fluid gets sampled for genetic testing. This procedure can take place at any time after 15 weeks. Amniotic fluid contains cells and substances that are created by the fetus, which can be measured to assess for disorders such as Down syndrome, infection, neural tube defects, fetal blood type determination, and lung development. The risks are similar to CVS, including infection, injury to the fetus or fetal death, and leakage of amniotic fluid.[17]
Pathophysiology
Normally, there is no mixing of fetal and maternal blood. Although, this is possible with specific infectious agents or small breaks in the placental membrane that can happen during parturition. If there are breaks in the membrane, maternal red blood cells are able to cross into the fetal circulation and vice versa. Treponema pallidum (syphilis infection) can cross the barrier without breaks in the membrane. Toxoplasma gondii (toxoplasma infection) can create its own breaks in the membrane to get across into fetal circulation and infect the fetus.
Erythroblastosis fetalis, also referred to as hemolytic disease of the newborn, happens when the mother makes antibodies to the fetus’s Rh factor after an Rh-negative mother becomes exposed to the fetus’s Rh-positive blood. The mom makes IgG anti-Rh antibodies, which can cross the placenta. The first pregnancy is not an issue because it takes time for mom to elicit this response, but can cause problems in future pregnancies with an Rh-positive fetus. The antibodies can cross the placenta and attack the fetal red blood cells, causing fetal hydrops (anemia and edema).[18]
Placenta accreta is when the placenta grows too far into the myometrium due to a lack of decidua, which allows the villi to anchor to the myometrium. In placenta increta, the villi bury even deeper into the myometrium. Placenta percreta is when the placenta grows through the full thickness of the myometrium and reaches the serosa of the uterus. This invasion of myometrium can cause bleeding during pregnancy and bleeding complications postpartum.[19][20]
A healthy umbilical cord should contain two umbilical arteries and one umbilical vein. If there is only one artery, there may be other defects present in the fetus, such as growth restriction or genetic abnormalities.[21] There can also be multiple arteries, which may also be associated with genetic abnormalities or congenital defects.[22] There can also be cysts in the umbilical cord filled with fluid. They are visible on ultrasound throughout the pregnancy.[23] Cysts may resolve on their own, or if there are multiple cysts, there may also be chromosomal abnormalities.[24] A rare complication from a cyst could be torsion or hematoma that could potentially cause fetal death.[25] True and false knots are also an abnormality of the umbilical cord. A false knot is when the vessels inside the cord become tortuous, but there is no knot in the cord itself. There is no adverse outcome associated with this type of knot. A true knot, however, can be dangerous to the fetus. A true knot is where the cord actually twists into a knot, which could potentially cause fetal demise if it is too tight.[26][27]
The umbilical cord normally attaches to the center of the placenta. The umbilical cord can implant abnormally both in the fetus and the placenta. In the placenta, there can be a velamentous insertion or a marginal insertion. A velamentous insertion is when the vessels separate as they develop between the amnion and chorion as they grow toward the placenta. As they get closer to the placenta, they are exposed and not protected by Wharton’s jelly; this can be dangerous, especially in vasa previa where the vessels are especially prone to rupture.[28] Marginal insertion is where the cord inserts on the edge of the placenta. There is still an increased risk of placenta previa and abruption but not quite as high as a velamentous insertion.[29][30]
Many pathologies of the placenta are visible on histological examination, but some pathologies are visible grossly.[31] Gross pathologies include meconium myonecrosis green discoloration, vasculitis yellow-green discoloration, abscesses, placental infarction orange discoloration, masses, cysts, thrombi, and incomplete tissue collection indicating retained placenta. The placenta may be stored fresh in a refrigerator or placed in fixative if needed for further examination after parturition.[11][32]
The detachment of the placenta is a very dangerous circumstance: if it happens, in fact, the placenta detaches from the uterus causing a strong haemorrhage, putting not only the life of the fetus at risk, which does not receive more oxygen and nutrients needed to survive but also of the expectant mother. Placental detachment may be due to: hypertension, overdistention of uterine walls due to excess of amniotic fluid (polyhydramnios), multi-twin pregnancy, diabetes, drug use.
Clinical Significance
The placenta is a vital fetal organ necessary for a healthy pregnancy for both mother and fetus. Incomplete or disordered development of the placenta may cause fetal abnormalities or adverse events in the mother as well. An early gestational sampling of the placenta can be done to test for fetal anomalies, but these procedures do not come without risk to the wellbeing of the fetus. In the case of an abnormal delivery or fetal defects, the placenta can undergo examination for gross and histological analysis.
Most placental diseases can have treatment by preserving the pregnant woman and the fetus from serious consequences. Placenta previa: often, a low placenta returns to its normal position after a few weeks, thanks to the push of the uterus. Where the condition of the low placenta remains throughout the pregnancy, a cesarean section is a strong recommendation. Placenta accreta: to prevent the risk of hemorrhage, an early cesarean section of a few weeks is recommended. In these cases, a hysterectomy, or removal of the uterus without precluding future pregnancies, cannot be excluded. Aged placenta: a diet based on vitamins and calcium and good habits (for example, not smoking) prevents the calcification of the placenta. Placental insufficiency: there is no specific treatment for this type of problem. It is good to undergo periodic checks close to each other to monitor the evolution of the fetus, as well as where it is possible, treat the behavior or pathology of the pregnant woman. Placental detachment: depending on the type of detachment, the hypothesis of monitoring the patient by asking for absolute rest or, as in most cases, proceeding with cesarean section, depending on the time of pregnancy, will be evaluated.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
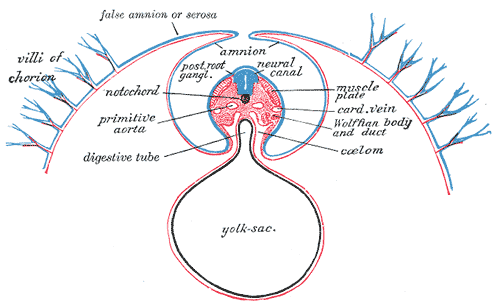
Development of the Fetal membrane and the Placenta, Diagram of a transverse section, showing the mode of formation of the amnion in the chick. The amniotic folds have nearly united in the middle line, Ectoderm is blue is the mesoderm; red is the entoderm and notochord is black
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Burton GJ, Jauniaux E. What is the placenta? American journal of obstetrics and gynecology. 2015 Oct:213(4 Suppl):S6.e1, S6-8. doi: 10.1016/j.ajog.2015.07.050. Epub [PubMed PMID: 26428504]
Guttmacher AE, Maddox YT, Spong CY. The Human Placenta Project: placental structure, development, and function in real time. Placenta. 2014 May:35(5):303-4. doi: 10.1016/j.placenta.2014.02.012. Epub 2014 Mar 6 [PubMed PMID: 24661567]
Wamaitha SE, Niakan KK. Human Pre-gastrulation Development. Current topics in developmental biology. 2018:128():295-338. doi: 10.1016/bs.ctdb.2017.11.004. Epub 2018 Feb 13 [PubMed PMID: 29477167]
Maître JL. Mechanics of blastocyst morphogenesis. Biology of the cell. 2017 Sep:109(9):323-338. doi: 10.1111/boc.201700029. Epub 2017 Aug 4 [PubMed PMID: 28681376]
Solnica-Krezel L, Sepich DS. Gastrulation: making and shaping germ layers. Annual review of cell and developmental biology. 2012:28():687-717. doi: 10.1146/annurev-cellbio-092910-154043. Epub 2012 Jul 9 [PubMed PMID: 22804578]
Level 3 (low-level) evidenceFavaron PO, Carvalho RC, Borghesi J, Anunciação AR, Miglino MA. The Amniotic Membrane: Development and Potential Applications - A Review. Reproduction in domestic animals = Zuchthygiene. 2015 Dec:50(6):881-92. doi: 10.1111/rda.12633. Epub 2015 Oct 29 [PubMed PMID: 26510939]
Level 3 (low-level) evidenceLabarrere CA, DiCarlo HL, Bammerlin E, Hardin JW, Kim YM, Chaemsaithong P, Haas DM, Kassab GS, Romero R. Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. American journal of obstetrics and gynecology. 2017 Mar:216(3):287.e1-287.e16. doi: 10.1016/j.ajog.2016.12.029. Epub 2016 Dec 27 [PubMed PMID: 28034657]
Hahn D, Blaschitz A, Korgun ET, Lang I, Desoye G, Skofitsch G, Dohr G. From maternal glucose to fetal glycogen: expression of key regulators in the human placenta. Molecular human reproduction. 2001 Dec:7(12):1173-8 [PubMed PMID: 11719595]
Herrera E, Amusquivar E, López-Soldado I, Ortega H. Maternal lipid metabolism and placental lipid transfer. Hormone research. 2006:65 Suppl 3():59-64 [PubMed PMID: 16612115]
Level 3 (low-level) evidenceNeu N, Duchon J, Zachariah P. TORCH infections. Clinics in perinatology. 2015 Mar:42(1):77-103, viii. doi: 10.1016/j.clp.2014.11.001. Epub 2014 Dec 20 [PubMed PMID: 25677998]
Kaplan C. Gross Examination of the Placenta. Surgical pathology clinics. 2013 Mar:6(1):1-26. doi: 10.1016/j.path.2012.11.001. Epub 2013 Jan 17 [PubMed PMID: 26838700]
Brownbill P, Mahendran D, Owen D, Swanson P, Thornburg KL, Nelson DM, Sibley CP. Denudations as paracellular routes for alphafetoprotein and creatinine across the human syncytiotrophoblast. American journal of physiology. Regulatory, integrative and comparative physiology. 2000 Mar:278(3):R677-83 [PubMed PMID: 10712288]
Level 2 (mid-level) evidenceCarter AM. Factors affecting gas transfer across the placenta and the oxygen supply to the fetus. Journal of developmental physiology. 1989 Dec:12(6):305-22 [PubMed PMID: 2701106]
Level 3 (low-level) evidenceSibley CP, Brownbill P, Glazier JD, Greenwood SL. Knowledge needed about the exchange physiology of the placenta. Placenta. 2018 Apr:64 Suppl 1():S9-S15. doi: 10.1016/j.placenta.2018.01.006. Epub 2018 Jan 19 [PubMed PMID: 29370939]
Wapner RJ. Chorionic villus sampling. Obstetrics and gynecology clinics of North America. 1997 Mar:24(1):83-110 [PubMed PMID: 9086520]
Sileo FG,Curado J,Bhide A, A survey of current clinical practice of chorionic villus sampling. Prenatal diagnosis. 2019 Mar; [PubMed PMID: 30682214]
Level 3 (low-level) evidenceBaird PA,Yee IM,Sadovnick AD, Population-based study of long-term outcomes after amniocentesis. Lancet (London, England). 1994 Oct 22; [PubMed PMID: 7934498]
ALLEN FH Jr, DIAMOND LK. Erythroblastosis fetalis. The New England journal of medicine. 1957 Oct 10:257(15):705-12 contd [PubMed PMID: 13477375]
Khong TY, The pathology of placenta accreta, a worldwide epidemic. Journal of clinical pathology. 2008 Dec [PubMed PMID: 18641410]
Piñas Carrillo A, Chandraharan E. Placenta accreta spectrum: Risk factors, diagnosis and management with special reference to the Triple P procedure. Women's health (London, England). 2019 Jan-Dec:15():1745506519878081. doi: 10.1177/1745506519878081. Epub [PubMed PMID: 31578123]
Ghezzi F, Raio L, Di Naro E, Franchi M, Cromi A, Dürig P. Single and multiple umbilical cord cysts in early gestation: two different entities. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2003 Mar:21(3):215-9 [PubMed PMID: 12666213]
Beck R, Naulty CM. A human umbilical cord with four arteries. Clinical pediatrics. 1985 Feb:24(2):118-9 [PubMed PMID: 3967448]
Level 3 (low-level) evidenceAoki S, Hata T, Ariyuki Y, Makihara K, Hata K, Kitao M. Antenatal diagnosis of aberrant umbilical vessels. Gynecologic and obstetric investigation. 1997:43(4):232-5 [PubMed PMID: 9194620]
Sepulveda W, Reyes M, Gonçalves LF. Two uncommon umbilical vessel anomalies in a fetus with trisomy 18. Prenatal diagnosis. 1998 Oct:18(10):1098-9 [PubMed PMID: 9826906]
Level 3 (low-level) evidenceSepulveda W,Wong AE,Gonzalez R,Vasquez P,Gutierrez J, Fetal death due to umbilical cord hematoma: a rare complication of umbilical cord cyst. The journal of maternal-fetal [PubMed PMID: 16390804]
Level 3 (low-level) evidenceGembruch U, Baschat AA. True knot of the umbilical cord: transient constrictive effect to umbilical venous blood flow demonstrated by Doppler sonography. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 1996 Jul:8(1):53-6 [PubMed PMID: 8843621]
Level 3 (low-level) evidenceHertzberg BS, Bowie JD, Bradford WD, Bolick D. False knot of the umbilical cord: sonographic appearance and differential diagnosis. Journal of clinical ultrasound : JCU. 1988 Oct:16(8):599-602 [PubMed PMID: 3152409]
Rocha J,Carvalho J,Costa F,Meireles I,do Carmo O, Velamentous cord insertion in a singleton pregnancy: an obscure cause of emergency cesarean-a case report. Case reports in obstetrics and gynecology. 2012; [PubMed PMID: 23243528]
Level 3 (low-level) evidenceNkwabong E, Njikam F, Kalla G. Outcome of pregnancies with marginal umbilical cord insertion. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2021 Apr:34(7):1133-1137. doi: 10.1080/14767058.2019.1628206. Epub 2019 Jun 17 [PubMed PMID: 31164018]
Ebbing C, Kiserud T, Johnsen SL, Albrechtsen S, Rasmussen S. Prevalence, risk factors and outcomes of velamentous and marginal cord insertions: a population-based study of 634,741 pregnancies. PloS one. 2013:8(7):e70380. doi: 10.1371/journal.pone.0070380. Epub 2013 Jul 30 [PubMed PMID: 23936197]
Kaplan CG. Gross pathology of the placenta: weight, shape, size, colour. Journal of clinical pathology. 2008 Dec:61(12):1285-95. doi: 10.1136/jcp.2008.055269. Epub 2008 Aug 15 [PubMed PMID: 18708423]
Roberts DJ. Placental pathology, a survival guide. Archives of pathology & laboratory medicine. 2008 Apr:132(4):641-51 [PubMed PMID: 18384216]