Neuroanatomy, Edinger–Westphal Nucleus (Accessory Oculomotor Nucleus)
Neuroanatomy, Edinger–Westphal Nucleus (Accessory Oculomotor Nucleus)
Introduction
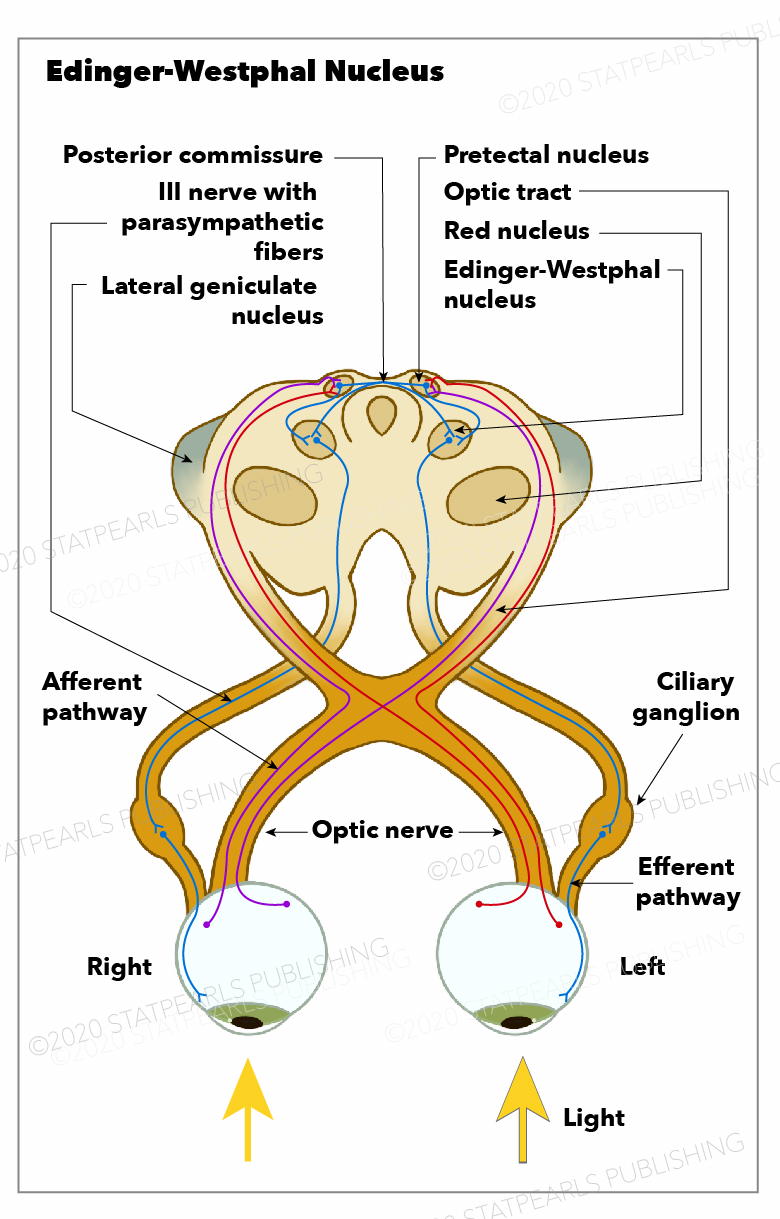
The Edinger-Westphal (EW) nucleus, which is part of the oculomotor nuclear complex (ONC), was first described in the literature in the 17th century (see Image. Edinger-Westphal Nucleus).[1] Recently, it has been discovered that 2 different cell populations within the EW nucleus[2] – subdivide into the EW preganglionic (EWpg) population and the EW nucleus centrally projecting (EWcp) population. However, the accepted nomenclature for these 2 groups varies.[1][2]
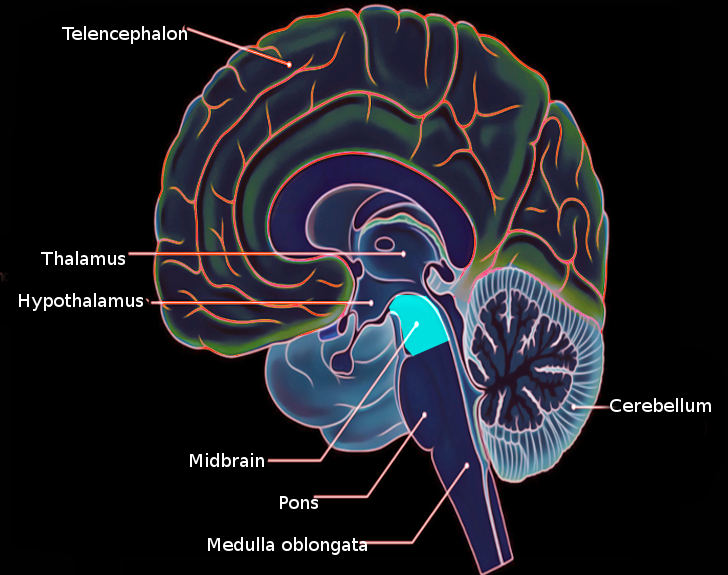
The EWpg is what is thought of as the classic ONC—sending parasympathetic nerve fibers toward the eye. It is located in the midbrain immediately dorsal to the oculomotor nucleus near the level of the superior colliculus, which is why it is often included in the overarching term oculomotor complex (see Image. Midbrain Anatomy).[3] The EWcp population of cells (the non-preganglionic EW cells), also referred to as the subgriseal paramedian midbrain neuronal stream to reflect their actual path, differ in function from the EWpg nucleus.[4][5] However, the name subgriseal paramedian midbrain neuronal stream has not been found favorable in the literature due to the complexity of the name and the belief that the EW nucleus consisted of only 1 cell type. These 2 bodies of cells, referred to as the EW nucleus and intermingled from a structural standpoint in the midbrain, have fundamentally different roles in function.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The EWpg nucleus houses choline acetyltransferase-positive cell bodies responsible for the parasympathetic innervation of the eye.[6] The EWpg nucleus comprises preganglionic cell bodies that track along the course of the oculomotor nerve (cranial nerve III) toward the postganglionic ciliary bodies.[6] These cell bodies participate in the light reflex pathway that leads to pupillary constriction when the retina is exposed to light.[6][7] The EWpg nucleus receives input from the locus ceruleus in response to the light hitting the retina, which then prompts the nucleus to send a forward signal that synapses at the postganglionic cells of the ciliary ganglion (CG).[6] This synapse between the EWpg nerve fibers and the ciliary ganglion postganglionic cell bodies is a nicotinic synapse with acetylcholine as the neurotransmitter.[6]
In response to the signal from the EWpg, the postganglionic ciliary bodies relay the signal along their axons through the ciliary nerves toward the eye. This relay leads to the innervation of the sphincter pupillae (causing miosis) and ciliary muscles (ocular accommodation). The constriction of the pupil moderates the amount of light the retina is exposed to, which is the efferent limb of the pupillary light reflex. Additionally, the contraction of the ciliary muscles leads to the relaxation of the zonular fibers, allowing for increased lens convexity and, subsequently, an increase in refractive power and accommodation.[6]
Since the discovery of the 2 cell populations of the EW nucleus, the function of the EWcp has been a subject of ongoing research. The EWcp is medial and dorsal to the OCN in the midbrain and comprises a collection of peptidergic neuron cell bodies.[6][2][8] A large population of these cells is known to be urocortinergic neurons, which are positive for the neuropeptide urocortin-1 (part of the corticotropin-releasing factor family) and negative for choline acetyltransferase.[4] These differ immunohistochemically from the cholinergic parasympathetic neurons of the EWpg, which are choline acetyltransferase positive. The EWcp nucleus significantly contributes to the amount of urocortin-1 neuropeptide in the brain.[9] Studies have shown that this nucleus is involved in stress adaptation, anxiety, and pain.[10] However, the response to stress by the EWcp nucleus is thought to be separate from that of the hypothalamic-pituitary-adrenal (HPA) axis.[11]
Embryology
Nucleogenesis in the brain during embryologic development is a subject of ongoing research. The thought is that brain nuclei develop as part of neuromeres and arcs (distinct columns of cells in the brain with high levels of acetylcholinesterase).[12] As part of the midbrain, the EW nucleus develops with the midbrain arc, which controls the homeobox genes Sonic Hedgehog and FGF8.[12] Post-mortem studies in neonates who suffered prenatal hypoxia have shown there to be an expression of urocortin-1 from the EWcp as early as 34 weeks gestation—demonstrating both the development of the EW nucleus at this age and specifically the EWcp role in stress response.[8]
Blood Supply and Lymphatics
While there is not a single blood supply to the EW nucleus, it receives its blood supply via vessels that feed the midbrain and brainstem. These blood vessels are part of the vertebrobasilar circulation and include the basilar artery, superior cerebellar artery, and posterior cerebral artery [13]. The posterior cerebral artery (PCA) is intimately related to the midbrain after originating from the bifurcation of the basilar artery within the interpeduncular cistern. Thereafter, it is anatomically divided into 4 segments. The first segment (P1) extends from the origin of the PCA to the junction with the posterior communicating artery.
The second segment (P2) is characterized by an anterior and posterior part (P2A and P2P). The P2A begins at the end of the P1 and extends to the most lateral aspect of the cerebral peduncle. The P2P then continues onwards to the posterior edge of the lateral midbrain. The P3 part of the artery extends until the origin of the parieto-occipital sulcus, and the P4 parts are the branches that occupy this sulcus and the calcarine sulcus.[14] Although the P2P segment of the PCA lies closest to the dorsal part of the midbrain occupied by the Edinger-Westphal nucleus, the arterial branches that supply that part of the midbrain, in fact, branch from the P1 and P2A segments of the PCA.[15] Specifically, these are the long and short circumflex arteries (from P1) and the medial posterior choroidal artery (from P2A).[15] The lymphatic drainage of the brain consists of the perivascular pathway (basement-membrane drainage system), glymphatic pathway, cerebrospinal fluid drainage via meningeal lymphatic vessels, and cervical lymph drainage routes.[16]
Nerves
The EWpg nucleus projects parasympathetic preganglionic neurons, which synapse on the postganglionic ciliary bodies.[6][7] The axons of the postganglionic ciliary bodies are the short ciliary nerves that innervate the sphincter pupillae and the eye's ciliary muscles.[6] The EWcp nucleus sends projections to the interpeduncular nucleus (a component of the limbic midbrain), lateral hypothalamus, lateral septum, raphe, and preganglionic sympathetic neurons in the spinal cord; however, these projections are not explicitly named nerves.[5]
While the oculomotor nucleus is a separate complex, the preganglionic projections to and postganglionic projections from the ciliary ganglion do course along CN III.[6] The oculomotor nucleus houses the motor neurons that innervate the superior, inferior, and medial recti and inferior oblique and levator palpebrae ocular muscles.[3]
Muscles
As discussed above, the short ciliary nerves innervate the sphincter pupillae (miosis) and the ciliary muscles (lens accommodation secondary to zonular fiber relaxation).[6] Thus, pathology affecting the Edinger-Westphal nucleus is often clearly evident on clinical examination, as discussed later.
Physiologic Variants
Much of today's information about the EW nucleus was originally studied in monkeys, mice, and birds. For instance, cellular morphology in pigeons differs from that of mice and humans, which have similar patterns of distribution of urocortin-1-positive neurons of the EWcp.[4] However, more opportunity remains for the variations in cell populations in the EW nucleus to be studied in humans.
Surgical Considerations
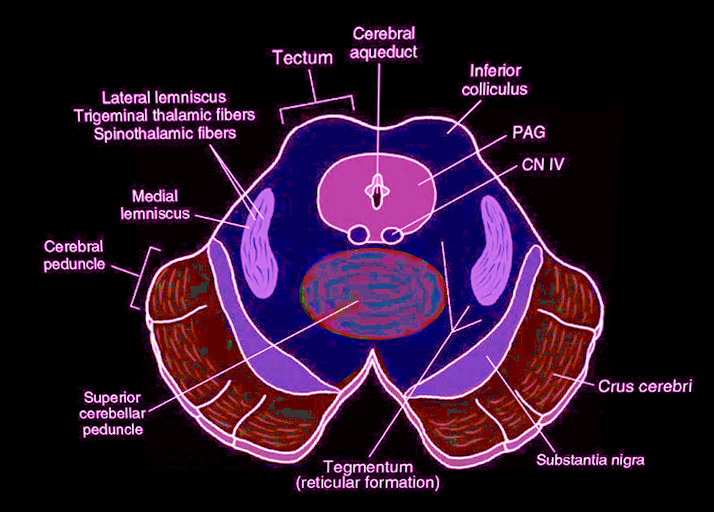
Brainstem and midbrain surgery are technically highly challenging procedures, and careful avoidance of damage to the numerous critical structures in this region is paramount to ensure that patients have as good of a quality of life following surgery.[17] The EW nucleus is situated in the dorsal part of the midbrain, or the tectum, at the level of the superior colliculus (see Image. Midbrain Cross-Sectional Anatomy).[18] It lies laterally to the periaqueductal grey matter on either side of the aqueduct of Sylvius.[19]
In adults, gliomas (malignant primary central nervous system tumors) are rare, constituting only 2% of brain malignancy in this population. The mean age of onset is in the sixth decade, and surgical treatment is often challenging and without the possibility of achieving total resection of the tumor. Adjuvant chemotherapy and radiotherapy are often employed, which has improved survival rates, but the overall median survival is still poor at 13.5 months.[20]
Clinical Significance
Alzheimer Disease
One clinical implication of the EW nucleus is its association with Alzheimer disease. Being that there are more than 5.4 million Americans estimated to be affected by Alzheimer disease and that this neurodegenerative disease is 1 of the leading causes of death in the United States, this relationship could prove to be an important finding in the understanding of the disease.[21] Degenerative changes in the early course of the disease are known to affect the hippocampus and entorhinal cortex, but another early target is the neurons of the EW nucleus.[22][21] The density of the dendritic spines of the EW neurons was significantly reduced in the post-mortem analysis of AD-affected brains compared to unaffected age-matched controls.[22] This finding clinically correlates with exaggerated pupillary responses to cholinergic antagonists seen early on in the course of the disease in individuals with Alzheimer disease, and being that the EW nucleus is an early target of degeneration, this response could be a useful tool in the early clinical diagnosis of Alzheimer disease.[22]
Weber Syndrome
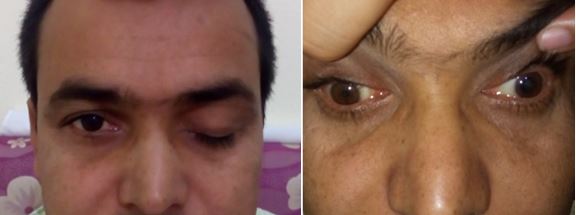
Weber syndrome is a rare brainstem infarct syndrome characterized by occlusion of 1 of the paramedian branches of the basilar artery or posterior cerebral artery. The resulting infarction affects the oculomotor nucleus and the cerebral peduncle.[18] Owing to these structures being involved, the clinical presentation typically consists of a combination of oculomotor nerve palsy (the ipsilateral eye deviated inferolateral, with diplopia and ptosis) and concurrent contralateral hemiplegia (see Image. Pupil Nonsparing Third Nerve Palsy). Despite the debilitating consequences of the presenting symptoms, with aggressive control of vascular risk factors (ie, hypertension), a good clinical recovery can be achieved.[23]
Benedikt Syndrome
Benedikt syndrome (paramedian midbrain syndrome) is the name given to the rare clinical syndrome resulting from the infarction of branches from the posterior cerebral artery that supplies oculomotor nerve fascicles and the red nucleus.[18] The red nucleus is a primitive subcortical relay between the cerebellum and the spinal cord, allowing for control of purposeful motor movements. It is involved via the rubrospinal and olivocerebellar tracts.[24][25] Similarly to Weber syndrome, the principal etiology is vascular, although traumatic and infectious cases have been reported in the literature.[26] The clinical presentation consists of ipsilateral oculomotor nerve palsy (the ipsilateral eye deviated inferolateral, with diplopia and ptosis), contralateral ataxia, and intention tremor. In patients with residual tremors following optimal medical management of the vascular risk factors, deep brain stimulation targeting both the thalamic ventral intermediate nucleus and the contralateral lenticular fasciculus has been trialed with good results reported in small case series.[27][28]
Other clinical considerations would include the presence of structural lesions that could lead to the compression of the nucleus. These lesions include tumors, aneurysms, and impaired CSF outflow, leading to cerebral aqueduct enlargement.
Other Issues
Many of the issues regarding the understanding of the EW nucleus involve the discovery of the EWcp, which changed the classic definition of the EW nucleus. For decades, it was solely associated with parasympathetic control of the eye. With time and more research, the terminology of the EWpg and EWcp gained recognition and acceptance throughout the scientific community.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Dos Santos Júnior ED, Da Silva AV, Da Silva KR, Haemmerle CA, Batagello DS, Da Silva JM, Lima LB, Da Silva RJ, Diniz GB, Sita LV, Elias CF, Bittencourt JC. The centrally projecting Edinger-Westphal nucleus--I: Efferents in the rat brain. Journal of chemical neuroanatomy. 2015 Oct:68():22-38. doi: 10.1016/j.jchemneu.2015.07.002. Epub 2015 Jul 21 [PubMed PMID: 26206178]
Kozicz T, Bittencourt JC, May PJ, Reiner A, Gamlin PD, Palkovits M, Horn AK, Toledo CA, Ryabinin AE. The Edinger-Westphal nucleus: a historical, structural, and functional perspective on a dichotomous terminology. The Journal of comparative neurology. 2011 Jun 1:519(8):1413-34. doi: 10.1002/cne.22580. Epub [PubMed PMID: 21452224]
Level 3 (low-level) evidenceChe Ngwa E, Zeeh C, Messoudi A, Büttner-Ennever JA, Horn AK. Delineation of motoneuron subgroups supplying individual eye muscles in the human oculomotor nucleus. Frontiers in neuroanatomy. 2014:8():2. doi: 10.3389/fnana.2014.00002. Epub 2014 Feb 12 [PubMed PMID: 24574976]
Ryabinin AE,Tsivkovskaia NO,Ryabinin SA, Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neuroscience. 2005; [PubMed PMID: 16039794]
Cunha RP, Reiner A, Toledo CA. Involvement of urocortinergic neurons below the midbrain central gray in the physiological response to restraint stress in pigeons. Brain research. 2007 May 25:1147():175-83 [PubMed PMID: 17320052]
Level 3 (low-level) evidenceMcDougal DH, Gamlin PD. Autonomic control of the eye. Comprehensive Physiology. 2015 Jan:5(1):439-73. doi: 10.1002/cphy.c140014. Epub [PubMed PMID: 25589275]
Level 3 (low-level) evidenceSzabadi E. Functional Organization of the Sympathetic Pathways Controlling the Pupil: Light-Inhibited and Light-Stimulated Pathways. Frontiers in neurology. 2018:9():1069. doi: 10.3389/fneur.2018.01069. Epub 2018 Dec 18 [PubMed PMID: 30619035]
Pagida MA, Konstantinidou AE, Tsekoura E, Patsouris E, Panayotacopoulou MT. Immunohistochemical demonstration of urocortin 1 in Edinger-Westphal nucleus of the human neonate: colocalization with tyrosine hydroxylase under acute perinatal hypoxia. Neuroscience letters. 2013 Oct 25:554():47-52. doi: 10.1016/j.neulet.2013.08.054. Epub 2013 Sep 4 [PubMed PMID: 24012814]
Level 3 (low-level) evidenceYamaguchi K. Development of the human oculomotor nuclear complex: Centrally-projecting Edinger-Westphal nucleus. Neuroscience letters. 2017 Apr 12:646():8-14. doi: 10.1016/j.neulet.2016.11.040. Epub 2016 Nov 21 [PubMed PMID: 27884738]
Level 3 (low-level) evidenceKorosi A, Schotanus S, Olivier B, Roubos EW, Kozicz T. Chronic ether stress-induced response of urocortin 1 neurons in the Edinger-Westphal nucleus in the mouse. Brain research. 2005 Jun 7:1046(1-2):172-9 [PubMed PMID: 15885665]
Level 3 (low-level) evidenceViau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. The Journal of comparative neurology. 2002 Apr 15:445(4):293-307 [PubMed PMID: 11920708]
Level 3 (low-level) evidenceAgarwala S, Ragsdale CW. A role for midbrain arcs in nucleogenesis. Development (Cambridge, England). 2002 Dec:129(24):5779-88 [PubMed PMID: 12421716]
Level 3 (low-level) evidencePiccinin MA, Munakomi S. Neuroanatomy, Vertebrobasilar System. StatPearls. 2024 Jan:(): [PubMed PMID: 31082039]
Párraga RG, Ribas GC, Andrade SE, de Oliveira E. Microsurgical anatomy of the posterior cerebral artery in three-dimensional images. World neurosurgery. 2011 Feb:75(2):233-57. doi: 10.1016/j.wneu.2010.10.053. Epub [PubMed PMID: 21492726]
Ciceri EF, Klucznik RP, Grossman RG, Rose JE, Mawad ME. Aneurysms of the posterior cerebral artery: classification and endovascular treatment. AJNR. American journal of neuroradiology. 2001 Jan:22(1):27-34 [PubMed PMID: 11158883]
Level 2 (mid-level) evidenceSun BL, Wang LH, Yang T, Sun JY, Mao LL, Yang MF, Yuan H, Colvin RA, Yang XY. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Progress in neurobiology. 2018 Apr-May:163-164():118-143. doi: 10.1016/j.pneurobio.2017.08.007. Epub 2017 Sep 10 [PubMed PMID: 28903061]
Mehta VS, Chandra PS, Singh PK, Garg A, Rath GK. Surgical considerations for 'intrinsic' brainstem gliomas: proposal of a modification in classification. Neurology India. 2009 May-Jun:57(3):274-81. doi: 10.4103/0028-3886.53272. Epub [PubMed PMID: 19587467]
Level 2 (mid-level) evidenceSciacca S, Lynch J, Davagnanam I, Barker R. Midbrain, Pons, and Medulla: Anatomy and Syndromes. Radiographics : a review publication of the Radiological Society of North America, Inc. 2019 Jul-Aug:39(4):1110-1125. doi: 10.1148/rg.2019180126. Epub [PubMed PMID: 31283463]
Shepherd TM, Hoch MJ. MRI-Visible Anatomy of the Brainstem. Neuroimaging clinics of North America. 2022 Aug:32(3):553-564. doi: 10.1016/j.nic.2022.04.003. Epub [PubMed PMID: 35843662]
Kitagawa H, Sasaki Y, Ishihara K, Kawakami M. Heartworm migration toward right atrium following artificial pulmonary arterial embolism or injection of heartworm body fluid. Nihon juigaku zasshi. The Japanese journal of veterinary science. 1990 Jun:52(3):591-9 [PubMed PMID: 2385039]
Level 3 (low-level) evidenceAlzheimer's Association. 2016 Alzheimer's disease facts and figures. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2016 Apr:12(4):459-509 [PubMed PMID: 27570871]
Mavroudis IA, Manani MG, Petrides F, Petsoglou C, Njau SN, Costa VG, Baloyannis SJ. Dendritic and spinal alterations of neurons from Edinger-Westphal nucleus in Alzheimer's disease. Folia neuropathologica. 2014:52(2):197-204 [PubMed PMID: 25118905]
Sheikh Hassan M, Osman Sidow N, Adam BA, Adani AA. Superior alternating hemiplegia (Weber's syndrome)- Case report. Annals of medicine and surgery (2012). 2022 May:77():103674. doi: 10.1016/j.amsu.2022.103674. Epub 2022 Apr 28 [PubMed PMID: 35638077]
Level 3 (low-level) evidenceBasile GA, Quartu M, Bertino S, Serra MP, Boi M, Bramanti A, Anastasi GP, Milardi D, Cacciola A. Red nucleus structure and function: from anatomy to clinical neurosciences. Brain structure & function. 2021 Jan:226(1):69-91. doi: 10.1007/s00429-020-02171-x. Epub 2020 Nov 12 [PubMed PMID: 33180142]
Cacciola A, Milardi D, Basile GA, Bertino S, Calamuneri A, Chillemi G, Paladina G, Impellizzeri F, Trimarchi F, Anastasi G, Bramanti A, Rizzo G. The cortico-rubral and cerebello-rubral pathways are topographically organized within the human red nucleus. Scientific reports. 2019 Aug 20:9(1):12117. doi: 10.1038/s41598-019-48164-7. Epub 2019 Aug 20 [PubMed PMID: 31431648]
Paidakakos NA, Rokas E, Theodoropoulos S, Dimogerontas G, Konstantinidis E. Posttraumatic Benedikt's syndrome: a rare entity with unclear anatomopathological correlations. World neurosurgery. 2012 Dec:78(6):715.e13-5. doi: 10.1016/j.wneu.2012.03.028. Epub 2012 Apr 3 [PubMed PMID: 22484069]
Level 3 (low-level) evidenceCheng G, Yang Y, Wang Y, Tan H, Zhang S. Deep brain stimulation of the thalamic ventral intermediate nucleus for Benedikt's syndrome mainly present as tremor: a long-term case observation. Acta neurochirurgica. 2018 Jul:160(7):1349-1353. doi: 10.1007/s00701-018-3526-8. Epub 2018 Mar 30 [PubMed PMID: 29600395]
Level 3 (low-level) evidenceBandt SK, Anderson D, Biller J. Deep brain stimulation as an effective treatment option for post-midbrain infarction-related tremor as it presents with Benedikt syndrome. Journal of neurosurgery. 2008 Oct:109(4):635-9. doi: 10.3171/JNS/2008/109/10/0635. Epub [PubMed PMID: 18826349]
Level 3 (low-level) evidence