Introduction
Humans possess three pairs of major salivary glands and approximately 600 to 1000 minor glands. The major salivary glands are the submandibular gland (SMG), sublingual gland (SLG), and the parotid gland (PG). Of these, the parotid gland is the largest and most important in terms of salivary production, providing approximately 50% of the total saliva volume. Collectively, all the major salivary glands function to secrete saliva, which contains a host of electrolytes, such as bicarbonate, and enzymes, such as salivary amylase, which break down carbohydrates in the oral cavity.
Structure
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure
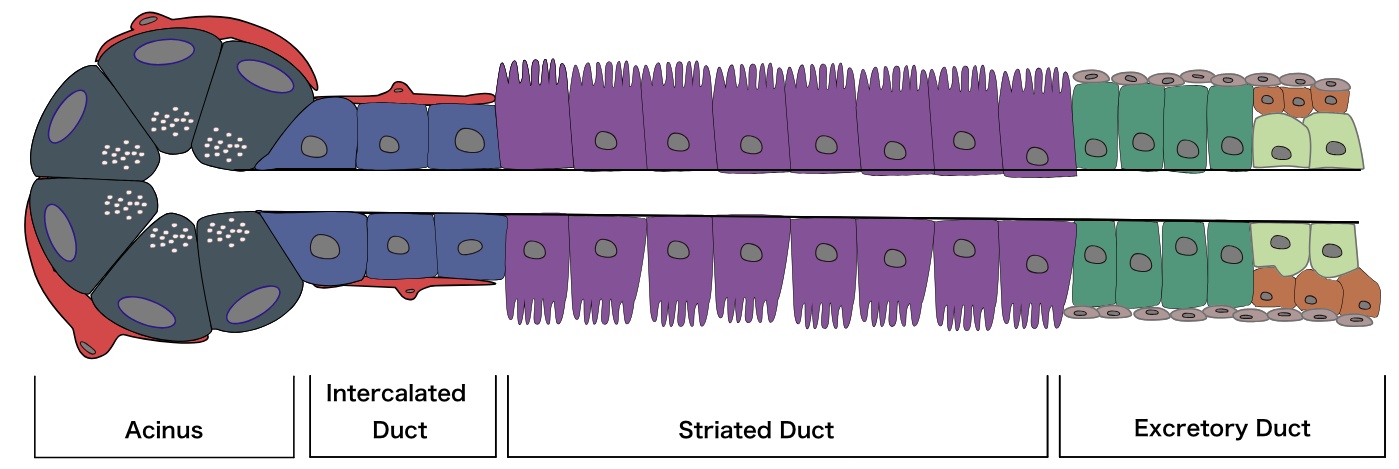
The major salivary glands have essentially the same structure: secretory end-pieces termed acini that produce saliva, which flows into arborized ducts that open into the oral cavity. All salivary glands are encapsulated and divided into divisions called lobes, which further subdivide into lobules, each of which is separated by a connective tissue septum. Each salivary gland is made up of two separate parts: a serous portion, responsible for secreting watery saliva, and a mucous portion, responsible for a more viscous fluid. The three major salivary glands are all composed of a mixture of these two types of cells. The parotid gland is composed primarily of serous acini and produces watery saliva, which lubricates the oral cavity and helps with swallowing, talking, and maintaining homeostasis. The submandibular gland has a predominance of serous cells with some mucous cells. The sublingual gland is composed of mostly mucous acini and thus produces the thickest and most viscous saliva. Both parasympathetic and sympathetic branches of the autonomic nervous system stimulate the secretion of saliva via aquaporins, specifically aquaporin 1 and 5. Electrolytes that will ultimately get released in saliva are first secreted from the acini through the intercalated ducts, into the striated ducts where they are moved out into the lumen through each gland’s respective duct via transmembrane active pumping.[1][2][3]
Function
As previously mentioned, all three major salivary glands are composed of either serous, mucous acini, or a combination of both. While the parotid gland is the largest of the three, all glands function to produce saliva to moisturize the mouth and assist in the breakdown of carbohydrates in the mouth. The submandibular gland is the primary source of basal saliva secretion, while the parotid gland is the main source of stimulated saliva secretion. The submandibular gland releases saliva through its main duct, Wharton’s duct, while the parotid gland and sublingual gland utilize Stensen’s duct and Bartholin’s duct respectively. Salivary glands also play a crucial immunologic role as their secretions contain many immunoglobins, namely IgA, that help fight bacteria and other foreign antigens in the oropharyngeal environment.[2][3]
Histochemistry and Cytochemistry
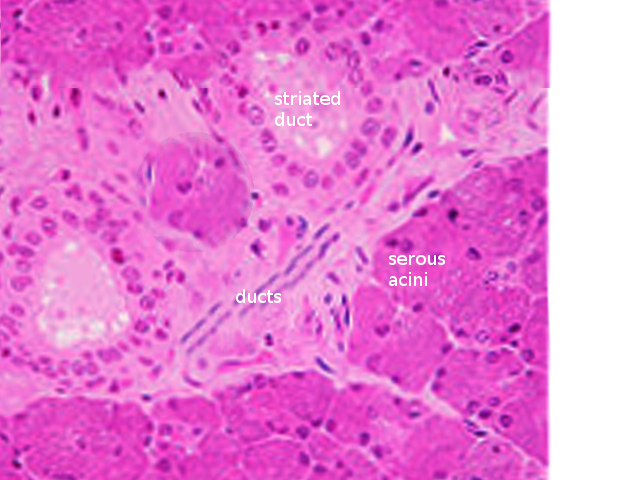
Salivary glands are made up of three cell types: acinar cells, ductal cells, and myoepithelial cells. The myoepithelial cells are a crucial component of the glands as they wrap around both the ductal cells and acinar cells and contract to squeeze the saliva out of the gland. On hematoxylin and eosin (H & E) staining, the sublingual gland stains lighter than the parotid and submandibular glands. This staining is due to the heavy presence of glycolipids found in the sublingual gland secretions that give it a clear cytoplasmic appearance histologically. The ducts are lined with simple columnar epithelium and surrounded by a basal layer of myoepithelial cells. Enzymes (such as salivary peroxidase and salivary amylase) and peptides (including defensins, agglutinins, and cystatins) are stored in granules within the acinar cells, awaiting the signal for release as a component of the saliva. These granules stain darkly with hematoxylin, gray with mucicarmine, black with anti-smooth muscle actin (SMA), and rich brown with anti-amylase. Damage to the salivary glands is often evidenced histologically as the columnar cells become almost cuboidal, and the adipose tissue and surrounding stroma occupy more space as the cells balloon outwards.[4][5]
Clinical Significance
A host of diseases impact the salivary glands, and age can often be an essential factor when determining etiology. Viral causes are more likely to be seen in young children, while autoimmune and inflammatory conditions occur more frequently in middle-aged and elderly populations. A thorough history and complete physical exam can further characterize the disease process. For example, the sialotropic Paramyxovirus that causes mumps is usually seen in unvaccinated children and would be expected to demonstrate acute swelling of both the parotid glands with or without fever. If a fever is present and there is acute swelling of only one salivary gland, usually the parotid gland or submandibular gland, this may be indicative of sialolithiasis or acute sialoadenitis, and ultrasound is necessary for confirmation. Confirmatory findings of acute bacterial sialoadenitis include hypoechoic salivary glands associated with ductal dilatation if found early or, if detected late, abscess resulting in a relatively anechoic focus. The most common cause of acute sialoadenitis is Staphlococcus aureus, but other causes can include Streptococcal species and Haemophilus influenza. If the diagnosis is confirmed, the clinician should initiate oral antibiotic therapy with amoxicillin-clavulanate over a 10-day course.[6][7]
Several chronic conditions impact salivary glands. One important chronic condition that affects the major salivary glands is Sjogren syndrome. Often seen in adults, Sjogren syndrome is an idiopathic autoimmune condition that causes inflammation and atrophy of the salivary glands and lacrimal glands, causing atrophy and resulting in xerostomia and xeropthalmia, respectively. Sjogren syndrome commonly presents with other autoimmune conditions, most frequently systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Diagnosis of Sjogren syndrome is through serological testing, positive anti-SSA (Ro) and anti-SSB (La) antibodies, physical exam findings, dry eyes and mouth for at least three consecutive months, positive Schirmer’s test, and histologically through labial biopsies. The histological hallmark of the condition is focal lymphocytic infiltration in otherwise normal-appearing glandular acini. It is worth noting that one does not biopsy the parotid glands directly to make the diagnosis of Sjogren syndrome due to the risk of damaging the facial nerve. While ultrasound can be useful, sialography or contrast-enhanced CT can be used to determine the extent of salivary involvement with Sjogren syndrome. Other causes of salivary adenopathy may include sarcoidosis, human immunodeficiency virus infection, mycobacterial infection, or radiotherapy. If chronic swelling of a single gland is present, a ductal obstruction must be ruled out via ultrasound.[8][7][6]
Neoplastic disease may also affect the salivary glands. Pleomorphic adenoma is the most common benign tumor, constituting up to two-thirds of all salivary gland tumors, while mucoepidermoid carcinoma (MEC) is the most common malignancy. Pleomorphic adenoma shows a predilection for females ages 30 to 50, most often involves the parotid gland, and is best treated by wide local excision. MEC is also most commonly seen in females ages 40 to 60 years old and predominantly affects the parotid gland as well. Similar to pleomorphic adenoma, surgical excision is also the treatment of choice for MEC.[9][10]
Conclusion
Human salivary glands represent a crucial part of oropharyngeal anatomy. Saliva contains amylase, which initiates digestion through the breakdown of carbohydrates. Salivary gland pathology may be a harbinger of systemic disease, including conditions that may shorten the life span. These glands also play a crucial role in immunologic defense as saliva contains many immunoglobins, mainly IgA, which help defend the oropharynx from microbes. Ultrasound is the gold standard for imaging of salivary glands. Histologic findings of salivary glands can be imperative in determining a diagnosis, and biopsies should be considered, especially in the setting of chronic autoimmune or inflammatory conditions.[11]
Media
(Click Image to Enlarge)
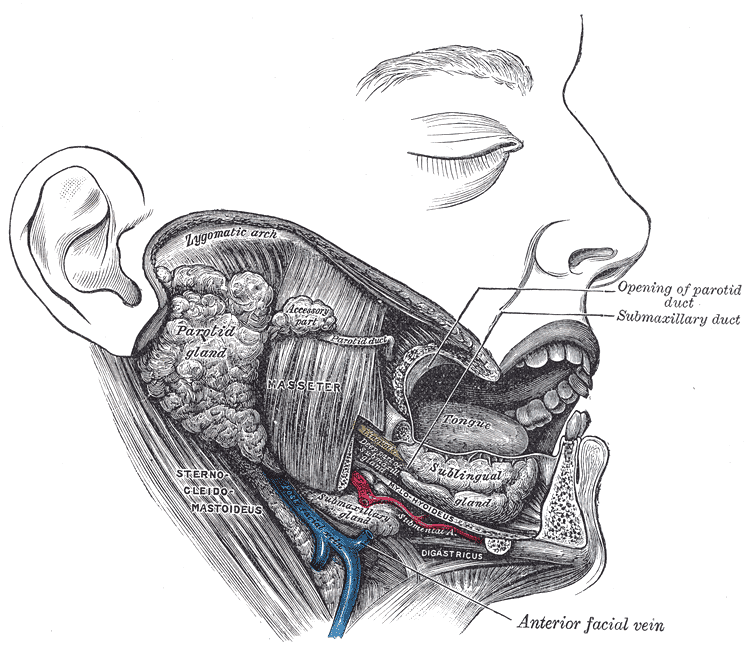
The Mouth, Dissection. Dissection of the mouth showing the salivary glands on the right side.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Delporte C, Bryla A, Perret J. Aquaporins in Salivary Glands: From Basic Research to Clinical Applications. International journal of molecular sciences. 2016 Jan 27:17(2):. doi: 10.3390/ijms17020166. Epub 2016 Jan 27 [PubMed PMID: 26828482]
Holmberg KV, Hoffman MP. Anatomy, biogenesis and regeneration of salivary glands. Monographs in oral science. 2014:24():1-13. doi: 10.1159/000358776. Epub 2014 May 23 [PubMed PMID: 24862590]
Amano O, Mizobe K, Bando Y, Sakiyama K. Anatomy and histology of rodent and human major salivary glands: -overview of the Japan salivary gland society-sponsored workshop-. Acta histochemica et cytochemica. 2012 Oct 31:45(5):241-50. doi: 10.1267/ahc.12013. Epub 2012 Sep 22 [PubMed PMID: 23209333]
Level 3 (low-level) evidenceMaruyama CL, Monroe MM, Hunt JP, Buchmann L, Baker OJ. Comparing human and mouse salivary glands: A practice guide for salivary researchers. Oral diseases. 2019 Mar:25(2):403-415. doi: 10.1111/odi.12840. Epub 2018 Apr 24 [PubMed PMID: 29383862]
Redman RS. On approaches to the functional restoration of salivary glands damaged by radiation therapy for head and neck cancer, with a review of related aspects of salivary gland morphology and development. Biotechnic & histochemistry : official publication of the Biological Stain Commission. 2008 Jun:83(3-4):103-30. doi: 10.1080/10520290802374683. Epub [PubMed PMID: 18828044]
Level 3 (low-level) evidenceAbdel Razek AAK, Mukherji S. Imaging of sialadenitis. The neuroradiology journal. 2017 Jun:30(3):205-215. doi: 10.1177/1971400916682752. Epub 2017 Jan 6 [PubMed PMID: 28059621]
Ugga L, Ravanelli M, Pallottino AA, Farina D, Maroldi R. Diagnostic work-up in obstructive and inflammatory salivary gland disorders. Acta otorhinolaryngologica Italica : organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. 2017 Apr:37(2):83-93. doi: 10.14639/0392-100X-1597. Epub [PubMed PMID: 28516970]
Stefanski AL, Tomiak C, Pleyer U, Dietrich T, Burmester GR, Dörner T. The Diagnosis and Treatment of Sjögren's Syndrome. Deutsches Arzteblatt international. 2017 May 26:114(20):354-361. doi: 10.3238/arztebl.2017.0354. Epub [PubMed PMID: 28610655]
Jain S, Hasan S, Vyas N, Shah N, Dalal S. Pleomorphic Adenoma of the Parotid Gland: Report of a Case With Review of Literature. Ethiopian journal of health sciences. 2015 Apr:25(2):189-94 [PubMed PMID: 26124628]
Level 3 (low-level) evidenceDevaraju R, Gantala R, Aitha H, Gotoor SG. Mucoepidermoid carcinoma. BMJ case reports. 2014 Aug 1:2014():bcr-2013-202776. doi: 10.1136/bcr-2013-202776. Epub 2014 Aug 1 [PubMed PMID: 25085946]
Level 3 (low-level) evidenceCascarini L, McGurk M. Epidemiology of salivary gland infections. Oral and maxillofacial surgery clinics of North America. 2009 Aug:21(3):353-7. doi: 10.1016/j.coms.2009.05.004. Epub [PubMed PMID: 19608052]