Introduction
The development of the female reproductive system requires an elaborate process. In females, reproductive organs divide into three main groups: gonads, reproductive ducts, and external genitalia. The female reproductive system derives from four origins: mesoderm, primordial germ cells, coelomic epithelium, and mesenchyme. The uterus forms during Mullerian organogenesis accompanied by the development of the upper third of the vagina, the cervix, and both fallopian tubes.[1][2] Knowledge of the embryology of the female reproductive tract provides insight into congenital pathologies that are related to these organs. The objective of this activity is to review uterine embryology and its clinical significance.
Development
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Development
Up to the fifth and sixth week of fetal life, the genital system remains indifferent. Two pairs of genital ducts are present at this time: the mesonephric (Wolffian duct) and paramesonephric (Mullerian duct). In females, the absence of anti-Mullerian hormone (AMH) and SRY gene conditions the regression of Wolff ducts and further differentiation of Mullerian ducts. The upper third of the vagina, the cervix, both fallopian tubes, and the uterus derive from the paramesonephric ducts.[3] During the seventh week, paired paramesonephric ducts arise from focal invaginations of the coelomic epithelium that is found on the upper pole of each mesonephros, shortly after this the Mullerian ducts grow caudally and laterally to the urogenital ridges.[4]
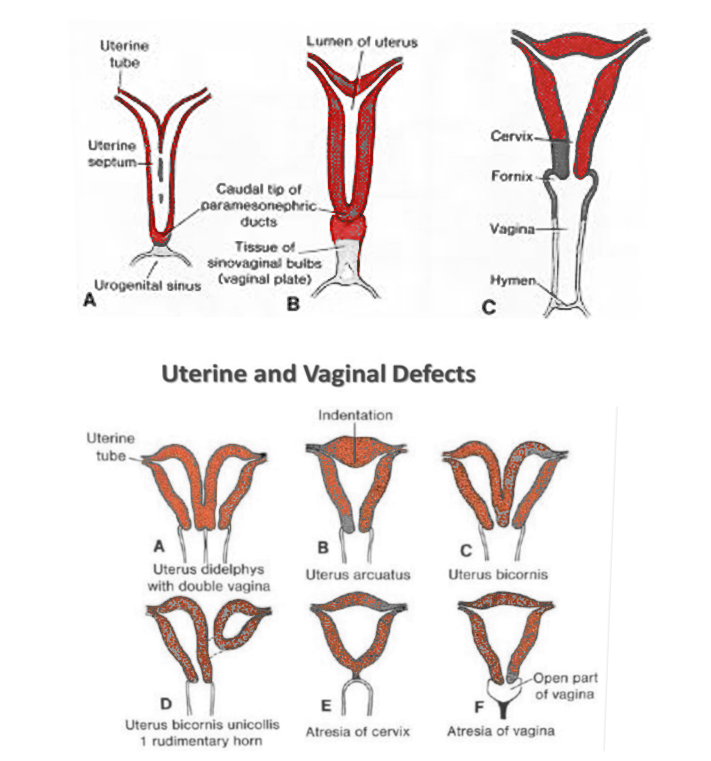
In the eighth week, a vertical fusion of paramesonephric ducts occurs. The fused cranial end gives origin to the left and right parts of what will ultimately become the uterus. This structure contains mesoderm that will form the endometrium and myometrium. The unfused cranial ends of the Mullerian ducts will develop into the fallopian tubes, the fimbrial portion of the fallopian tubes derives from the tip of this structure that remains open and acquires a funnel shape. The caudal end of the fused ducts will form the upper third of the vagina.[5] At this stage, a midline septum is present along these structures, and within the uterine cavity, this septum usually reabsorbs completely around 20 weeks, but it can persist and produce a septate uterus.[3] Regarding uterine ligaments, both the round ligament and the ovarian ligament develop from the gubernaculum, and undifferentiated mesenchymal tissue is attached to the ovary in the female fetus. The round ligament must attach to both the ovary and uterus for the ovary to be in place.[6] By the end of the first trimester development of the uterus and the other structures derived from the Mullerian ducts is complete.[3]
Molecular Level
For the first 10 weeks, the human fetus has the potential to become either female or male. The final phenotype depends on genetic information that influences differentiation in the embryonic structures. A female fetus will classically develop if there is the presence of a XX genotype. For a male fetus to develop there must be the presence of a Y chromosome that codes for SRY protein that enables testicular, epididymis, ductus deferens, ejaculatory duct, and seminal vesicles differentiation and secretion of anti-Mullerian hormone (AMH) from the Sertoli cells which will inhibit the further differentiation of paramesonephric ducts and condition their regression. In cases where this characteristic does not occur, an immature female fetus, or an intersex fetus will develop.[7]
Function
The primary function of the uterus is reproductive. The principal elements of uterine physiology are the endometrium and myometrium. The uterus accepts the ovum after fertilization, holds and provides nutrients and oxygen for the fetus and during birth, and it contracts to cause delivery. The uterus is a hormone-sensitive organ: differentiation, proliferation, exfoliation of the endometrium, and contraction during childbirth get regulated by the interaction between itself and the hypothalamus, pituitary gland, and ovaries.[8]
Testing
Assessment of uterine anatomy through imaging is a priority when Mullerian abnormalities are suspected. Magnetic resonance imaging (MRI) has proven to be highly sensitive and specific. Therefore it is considered the gold standard for these pathologies. Additionally to its diagnostic accuracy, more often than not, Mullerian remnants are present. MRI permits the identification of endometrial activity within the Mullerian structures. This imaging technique can also demonstrate urinary tract, gastrointestinal, and skeletal abnormalities that can accompany these entities. Regarding treatment decisions, MRI can aid in the differentiation between surgically correctable abnormalities and inoperable forms.[3]
Pathophysiology
Deviation from normal development of reproductive ducts can result in congenital structural anomalies referred to as Mullerian anomalies. Mullerian anomalies may result from arrested development of the paramesonephric ducts, failure of fusion of the paramesonephric ducts, or failure of resorption of the medial septum.[9] The development of the gastrointestinal and urinary system occurs closely in time and space to this phenomenon and anomalies in the development of these organ systems may also affect the female reproductive system and vice-versa.[10]
Clinical Significance
Mullerian anomalies can be either clinically asymptomatic and missed in routine gynecological examinations or manifest with infertility and amenorrhea. These pathologies represent unique challenges for establishing reproductive health. The American fertility association has classified this anomaly in seven categories [11]:
- Class I: Hypoplasia/uterine hypoplasia. (Mayer Rokitansky Kuster Hauser syndrome)
- Class II: Unicornuate uterus
- Class III: Uterus didelphys.
- Class IV: Bicornuate uterus
- Class V: Septate uterus
- Class VI: Arcuate uterus
- Class VII: T-shaped uterus resulting from the exposure to Diethylstilbestrol in fetal life
Mayer Rokitansky Kuster Hauser syndrome has a prevalence of 1 in 4000 to 5000 births and is one of the most frequent Mullerian abnormalities. It characteristically presents with uterine and vaginal agenesis or hypoplasia and can be accompanied by renal and bone abnormalities. Patients usually arrive at care due to primary amenorrhea with normal secondary sexual characteristics. Treatment of vaginal aplasia consists of the creation of a neovagina surgically or by dilation. Depression, anxiety, and female identity issues often occur in these patients, thus seeking counseling and peer support groups are recommended activities before and during treatment. Alternatives for having children include adoption and gestational surrogacy; this merits discussion during the consult.[11][12]
Also of clinical relevance after fetal life rudimentary structures derived from paramesonephric ducts can persist and be encountered in clinical practice[13]:
- Testicular appendage: From the cranial end of the fussed paramesonephric ducts in males.
- Prostatic utricle: A small sac found in the prostatic urethra in males.
- Morgagni hydatid in females from the cranial end of the paramesonephric duct that does not contribute to the fallopian tube
Media
References
Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nature reviews. Genetics. 2003 Dec:4(12):969-80 [PubMed PMID: 14631357]
Level 3 (low-level) evidenceWITSCHI E. Embryology of the uterus: normal and experimental. Annals of the New York Academy of Sciences. 1959 Jan 9:75():412-35 [PubMed PMID: 13845458]
Robbins JB, Broadwell C, Chow LC, Parry JP, Sadowski EA. Müllerian duct anomalies: embryological development, classification, and MRI assessment. Journal of magnetic resonance imaging : JMRI. 2015 Jan:41(1):1-12. doi: 10.1002/jmri.24771. Epub 2014 Oct 7 [PubMed PMID: 25288098]
Guioli S, Sekido R, Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Developmental biology. 2007 Feb 15:302(2):389-98 [PubMed PMID: 17070514]
Level 3 (low-level) evidenceWarne GL, Kanumakala S. Molecular endocrinology of sex differentiation. Seminars in reproductive medicine. 2002 Aug:20(3):169-80 [PubMed PMID: 12428197]
Level 3 (low-level) evidenceChaudhry SR, Chaudhry K. Anatomy, Abdomen and Pelvis: Uterus Round Ligament. StatPearls. 2023 Jan:(): [PubMed PMID: 29763145]
Roly ZY, Backhouse B, Cutting A, Tan TY, Sinclair AH, Ayers KL, Major AT, Smith CA. The cell biology and molecular genetics of Müllerian duct development. Wiley interdisciplinary reviews. Developmental biology. 2018 May:7(3):e310. doi: 10.1002/wdev.310. Epub 2018 Jan 19 [PubMed PMID: 29350886]
de Ziegler D, Pirtea P, Galliano D, Cicinelli E, Meldrum D. Optimal uterine anatomy and physiology necessary for normal implantation and placentation. Fertility and sterility. 2016 Apr:105(4):844-54. doi: 10.1016/j.fertnstert.2016.02.023. Epub 2016 Feb 27 [PubMed PMID: 26926252]
Chandler TM, Machan LS, Cooperberg PL, Harris AC, Chang SD. Mullerian duct anomalies: from diagnosis to intervention. The British journal of radiology. 2009 Dec:82(984):1034-42. doi: 10.1259/bjr/99354802. Epub 2009 May 11 [PubMed PMID: 19433480]
Thomas DFM. The embryology of persistent cloaca and urogenital sinus malformations. Asian journal of andrology. 2020 Mar-Apr:22(2):124-128. doi: 10.4103/aja.aja_72_19. Epub [PubMed PMID: 31322137]
Troiano RN, McCarthy SM. Mullerian duct anomalies: imaging and clinical issues. Radiology. 2004 Oct:233(1):19-34 [PubMed PMID: 15317956]
Morcel K, Camborieux L, Programme de Recherches sur les Aplasies Müllériennes, Guerrier D. Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. Orphanet journal of rare diseases. 2007 Mar 14:2():13 [PubMed PMID: 17359527]
Sajjad Y. Development of the genital ducts and external genitalia in the early human embryo. The journal of obstetrics and gynaecology research. 2010 Oct:36(5):929-37. doi: 10.1111/j.1447-0756.2010.01272.x. Epub 2010 Sep 16 [PubMed PMID: 20846260]