Introduction
A tubo-ovarian abscess (TOA) is a complex infectious mass of the adnexa that forms as a sequela of pelvic inflammatory disease. Classically, a TOA manifests with an adnexal mass, fever, elevated white blood cell count, lower abdominal-pelvic pain, and/or vaginal discharge; however, presentations of this disease can be highly variable. Should the abscess rupture, life-threatening sepsis can result, thus any clinical concern for this diagnosis requires prompt evaluation and treatment. [1][2][3]
The majority of patients are women in their reproductive age, who are sexually active.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Most commonly, these abscesses arise as a late complication of pelvic inflammatory disease (PID). Pathogens from the cervical infection or vaginal infection ascend first to the endometrium and then travel through the fallopian tubes into the peritoneal cavity where they form a walled-off mass. The majority of cases have associated peritonitis. Lastly, TOAs can arise from the extension of an infected adjacent organ, most commonly the appendix, less commonly, hematogenous spread from a distant nidus of infection, or as an association with pelvic organ cancer.[4]
Risk factors for a TOA are similar to those of PID and include reproductive age, IUD insertion, multiple sexual partners, and a history of a prior episode of PID. The differential diagnosis for TOA often includes appendicitis, diverticulitis, inflammatory bowel disease, PID, ovarian torsion, ectopic pregnancy, ruptured ovarian cyst, pyelonephritis, and cystitis.
Epidemiology
These abscesses most commonly are found in reproductive-age women after an upper genital tract infection. However, a TOA also can occur without a preceding episode of PID or sexual activity and occasionally can develop as a complication of a hysterectomy.[5]
Previously, nearly 20% of hospitalized PID cases were found to have a TOA. However, in 2002, the Centers for Disease Control and Prevention (CDC) released new guidelines for the evaluation and treatment of sexually transmitted diseases, which increased the number of patients being diagnosed with and treated for PID and reduced the prevalence of TOA to a mere 2.3%.
Of note, women who are HIV positive with PID generally have a slower clinical resolution of the disease and therefore an increased risk for the development of a TOA.
Pathophysiology
Bacteria from the lower genital tract ascend to create an inflammatory mass involving the fallopian tube, ovary, and potentially other adjacent pelvic organs. Tubo-ovarian abscesses are often polymicrobial and typically contain a predominance of anaerobic bacteria. Although associated with sexually transmitted infections, the most commonly recovered bacteria from a TOA include Escherichia coli, Bacteroides fragilis, other Bacteroides species, Peptostreptococcus, Peptococcus, and aerobic streptococci. Interestingly, neither Neisseria gonorrhea nor Chlamydia trachomatis is typically isolated from a TOA.
History and Physical
The classic presentation of a TOA includes abdominal pain, pelvic mass on examination, fever, and leukocytosis. However, Landers and Sweet (1983) found that 35% of women with a TOA were afebrile and 23% had a normal white blood cell count.[6] Furthermore, only 50% of women with a TOA presented with a complaint of fever and chills, 28% with vaginal discharge, 26% with nausea, and 21% with abnormal vaginal bleeding.[6] Thus, if clinical concern exists for this disease, a prompt diagnostic evaluation must be undertaken.
A complete physical examination, including a thorough pelvic exam, must be performed. The speculum and bimanual exam should assess the consistency, size, and mobility of the uterus and both adnexa.[5] Mucopurulent discharge and cervical motion tenderness are indicative of PID, and concomitant uterine or adnexal tenderness should raise concern for a TOA.[5] A detailed abdominal examination and careful attention to vital signs will help determine associated acute abdomen or systemic inflammatory response syndrome (SIRS).[5] Routine blood work may demonstrate leukocytosis with a left shift and urine, cervical and blood cultures may show bacterial growth.[5] A wet mount of vaginal discharge may show clue cells.[5] A urine pregnancy test should be performed to rule out an intrauterine or ectopic pregnancy.[5]
Evaluation
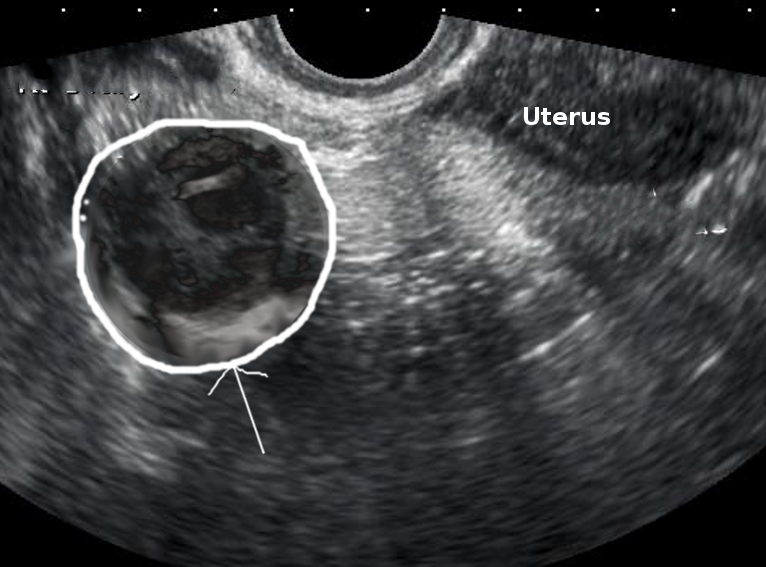
A tubo-ovarian abscess can be found on imaging with sonography, computed tomography (CT), or magnetic resonance imaging (MRI). Ha, et al. (1995) demonstrated that MRI has a superior ability to evaluate the extent of disease, the characteristics of the lesion, and to make the diagnosis of a TOA.[7] Although not commonly used, laparoscopy is still considered the gold standard for diagnosing PID and TOA. Additionally, laparoscopy can facilitate the drainage and culture of a TOA.[8][9][10]
Typically, either a transvaginal ultrasound or CT scan of the abdomen and pelvis is used to evaluate the concern for a TOA. CT with oral and IV contrast has been shown to have improved sensitivity (78% to 100%) over ultrasound (75% to 82%), but due to its low cost and lack of exposure to ionizing radiation, ultrasonography is an ideal imaging method for women of reproductive age. Landers and Sweet found that ultrasound correctly diagnosed TOA in 90% of surgically confirmed cases.[6]
In 2005, Hiller stated that CT imaging of a TOA would demonstrate a thick, uniform, enhancing abscess wall (95%) that is often multilocular (89%) with an increased fluid density (95%).[11] In contrast, ultrasound typically demonstrates complete obliteration of the normal architecture of one or both of the adnexa.[11] Fallopian tube wall thickness will be greater than 5 mm, fluid will be present in the cul-de-sac, and incomplete septae will be present within the fallopian tube.[11] Often, TOAs appears as a complex multilocular mass that contains internal echoes consistent with inflammatory debris.[11]
Treatment / Management
Historically, TOAs were treated with a total abdominal hysterectomy and bilateral salpingo-oophorectomy.[5] Management has changed drastically with the advent of broad-spectrum antibiotics, improved imaging, and drainage techniques. The majority of studies have shown a success rate of 70% or better, even with conservative management of TOAs. Daily complete blood counts should be obtained to trend the leukocytosis in hopes of improvement.[12][13][14](B2)
Any woman found to have a TOA should have a gynecological consultation and be hospitalized for further care. If the TOA is discovered before it has ruptured, treatment can begin with a course of intravenous antibiotics. In an analysis by Landers and Sweet, only 31% of patients treated with antibiotics alone later required surgical intervention.
Typically, management consists of antimicrobial therapy, with surgery reserved for cases of suspected TOA rupture or cases with a poor response to antibiotics.[5] Reed and colleagues (1991) demonstrated an inversely proportional correlation between the success of medical management and TOA size, with TOAs greater than 10 cm having greater than a 60% chance of requiring surgery (compared to only 20% in a mass sized 4 cm to 6 cm).[15]
The CDC recommendations for parenteral treatment of PID provide excellent TOA coverage as well. Often, metronidazole or clindamycin is added due to their excellent anaerobic coverage and success in abscess wall penetration.[5] Landers and Sweet also support adding clindamycin to the regimen.[6] They found that 68% of patients treated with clindamycin (versus 36.5% without) had a decrease in the size of their TOA.[5][6][5]
CDC Guidelines for the Treatment of PID
- Cefotetan 2 g (intravenously) IV every 12 hours and doxycycline 100 mg orally or IV every 12 hours
- Cefoxitin 2 g IV every 6 hours and Doxycycline 100 mg orally or IV every 12 hours
- Clindamycin 900 mg orally or IV every 12 hours and Gentamicin loading dose IV or intramuscularly (IM) (2 mg/kg) followed by 1.5 mg/kg every 8 hours
Women with a TOA should be given parenteral antibiotics until significant resolution of pain and tenderness, defervescence of fever, normalization of leukocytosis, and stability or decrease in the size of the mass is noted on imaging studies. At this point, antibiotics can be transitioned to an oral regimen until there is complete resolution of the TOA upon repeat imaging studies.[6]
Effective Antibiotic Treatment Regimens for TOA
- Cefotetan 2 g IV every 12 hours or Cefoxitin 2 grams IV every 6 hours and Doxycycline 100 mg orally or IV every 12 hours
- Ampicillin 2 grams IV every 6 hours and Gentamicin 2 mg/kg loading dose IV, then 1.5 mg/kg IV every 8 hours and Clindamycin 900 mg IV every 8 hours
- Ampicillin/sulbactam 3 gm IV every 6 hours and doxycycline 100 mg IV or oral every 12 hours
Repeat imaging should be obtained if the patient has worsening symptoms, a deteriorating clinical exam, or at two weeks of therapy.[5] If repeat imaging shows a worsening of the TOA or if a rupture has occurred, treatment consists of 24 hours of inpatient parenteral antibiotics followed by surgical removal of the abscess as well as the affected ovary and fallopian tube. After discharge from the hospital, oral antibiotics are continued, and the patient is followed to confirm that the infection has cleared.
Gjelland et al. (2005) evaluated the role of transvaginal ultrasound-guided aspiration combined with antibiotics in the treatment of a TOA.[16] Gjelland and colleagues found that 93.4% of women who were administered antibiotics and subsequent transvaginal ultrasound-guided drainage had a successful recovery with 62.3% of these women reporting a complete resolution of their pain within 48 hours of the first drainage.[16] Their research produced such a high success rate that they suggest that this regimen should be considered as a first-line procedure.[16](B3)
Surgical options to control the infection include laparoscopy/laparotomy, drainage of the abscess and salpingectomy. However, today percutaneous drainage under radiological guidance can be used to manage most patients.
To date, no large-scale randomized controlled trials have been performed to help clarify the precise role of imaging-guided drainage procedures in the management of TOAs; therefore, at this time, the choice of surgery versus imaging-guided drainage should remain individualized and based on local expertise.
Differential Diagnosis
- Renal stone
- Appendicitis
- Cholecystitis
- Inguinal hernia
- Obturator hernia
- Bowel obstruction
Prognosis
While most patients can be managed with antibiotics and percutaneous drainage, the recovery is slow. Patients often require hospital admission and parenteral antibiotics. Even those who recover may develop chronic pelvic pain and have a higher risk for ectopic pregnancies.
Complications
- Chronic pelvic pain
- Distortion of the pelvic anatomy
- Ectopic pregnancy in future
- Infertility
- Recurrent PID
Enhancing Healthcare Team Outcomes
The presentation of a TOA can be confused with several other causes like appendicitis, a ureteral stone, cystitis, or an obturator hernia. Any delay in treatment can lead to high morbidity. Thus, an interprofessional team approach is essential for the diagnosis and treatment of TOA.
The majority of patients first present to the emergency department and hence the triage nurse should be fully aware of immediate admission and notifying the physician. The radiologist plays a vital role in the diagnosis. Any time a tubo-ovarian abscess is suspected, the gynecologist must be consulted.
The key to TOA is prevention and this requires patient education. The nurse should educate the patient on the risk factors for TOA and how to avoid them. Women should be told about safe sex and avoidance of multiple partners. Use of a condom should be encouraged. The pharmacist should encourage compliance with antibiotics to ensure that the abscess resolves without sequelae.
A retrospective review of tubo-ovarian abscesses by Ginsburg et al. found that prognosis was predictable based on the extent of disease at diagnosis and the initial response to medical therapy.[17] Long-term complications include infertility, ectopic pregnancy, ovarian vein thrombosis, pelvic thrombophlebitis, and chronic pelvic pain. Of these complications, infertility is the most commonly observed. Sadly, none of the patients who were found to have bilateral TOAs maintained fertility after treatment.[17][3]
Rupture of a TOA is a surgical emergency. These patients present with diffuse peritonitis, which, unfortunately, can quickly lead to overwhelming sepsis and death.[18]
The members of the team should communicate with each other so that the outcomes can be improved.[19]
Media
References
Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: a population-based nested controlled study. Clinics (Sao Paulo, Brazil). 2018 Aug 9:73():e364. doi: 10.6061/clinics/2018/e364. Epub 2018 Aug 9 [PubMed PMID: 30110069]
Fouks Y, Cohen A, Shapira U, Solomon N, Almog B, Levin I. Surgical Intervention in Patients with Tubo-Ovarian Abscess: Clinical Predictors and a Simple Risk Score. Journal of minimally invasive gynecology. 2019 Mar-Apr:26(3):535-543. doi: 10.1016/j.jmig.2018.06.013. Epub 2018 Aug 11 [PubMed PMID: 29966713]
Fouks Y, Cohen Y, Tulandi T, Meiri A, Levin I, Almog B, Cohen A. Complicated Clinical Course and Poor Reproductive Outcomes of Women with Tubo-Ovarian Abscess after Fertility Treatments. Journal of minimally invasive gynecology. 2019 Jan:26(1):162-168. doi: 10.1016/j.jmig.2018.06.004. Epub 2018 Jun 8 [PubMed PMID: 29890350]
Inal ZO, Inal HA, Gorkem U. Experience of Tubo-Ovarian Abscess: A Retrospective Clinical Analysis of 318 Patients in a Single Tertiary Center in Middle Turkey. Surgical infections. 2018 Jan:19(1):54-60. doi: 10.1089/sur.2017.215. Epub 2017 Nov 17 [PubMed PMID: 29148955]
Level 2 (mid-level) evidenceLareau SM, Beigi RH. Pelvic inflammatory disease and tubo-ovarian abscess. Infectious disease clinics of North America. 2008 Dec:22(4):693-708. doi: 10.1016/j.idc.2008.05.008. Epub [PubMed PMID: 18954759]
Landers DV, Sweet RL. Tubo-ovarian abscess: contemporary approach to management. Reviews of infectious diseases. 1983 Sep-Oct:5(5):876-84 [PubMed PMID: 6635426]
Ha HK, Lim GY, Cha ES, Lee HG, Ro HJ, Kim HS, Kim HH, Joo SW, Jee MK. MR imaging of tubo-ovarian abscess. Acta radiologica (Stockholm, Sweden : 1987). 1995 Sep:36(5):510-4 [PubMed PMID: 7640096]
Hiller N, Fux T, Finkelstein A, Mezeh H, Simanovsky N. CT differentiation between tubo-ovarian and appendiceal origin of right lower quadrant abscess: CT, clinical, and laboratory correlation. Emergency radiology. 2016 Apr:23(2):133-9. doi: 10.1007/s10140-015-1372-z. Epub 2015 Dec 30 [PubMed PMID: 26719159]
Cho HW, Koo YJ, Min KJ, Hong JH, Lee JK. Pelvic Inflammatory Disease in Virgin Women With Tubo-ovarian Abscess: A Single-Center Experience and Literature Review. Journal of pediatric and adolescent gynecology. 2017 Apr:30(2):203-208. doi: 10.1016/j.jpag.2015.08.001. Epub 2015 Aug 7 [PubMed PMID: 26260586]
Sayasneh A, Kaijser J, Preisler J, Smith AA, Raslan F, Johnson S, Husicka R, Ferrara L, Stalder C, Ghaem-Maghami S, Timmerman D, Bourne T. Accuracy of ultrasonography performed by examiners with varied training and experience in predicting specific pathology of adnexal masses. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2015 May:45(5):605-12. doi: 10.1002/uog.14675. Epub 2015 Apr 6 [PubMed PMID: 25270506]
Level 2 (mid-level) evidenceHiller N, Sella T, Lev-Sagi A, Fields S, Lieberman S. Computed tomographic features of tuboovarian abscess. The Journal of reproductive medicine. 2005 Mar:50(3):203-8 [PubMed PMID: 15841934]
Level 2 (mid-level) evidenceBrun JL, Graesslin O, Fauconnier A, Verdon R, Agostini A, Bourret A, Derniaux E, Garbin O, Huchon C, Lamy C, Quentin R, Judlin P, Collège National des Gynécologues Obstétriciens Français. Updated French guidelines for diagnosis and management of pelvic inflammatory disease. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2016 Aug:134(2):121-5. doi: 10.1016/j.ijgo.2015.11.028. Epub 2016 Apr 19 [PubMed PMID: 27170602]
Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert review of anti-infective therapy. 2011 Jan:9(1):61-70. doi: 10.1586/eri.10.156. Epub [PubMed PMID: 21171878]
Mollen CJ, Pletcher JR, Bellah RD, Lavelle JM. Prevalence of tubo-ovarian abscess in adolescents diagnosed with pelvic inflammatory disease in a pediatric emergency department. Pediatric emergency care. 2006 Sep:22(9):621-5 [PubMed PMID: 16983244]
Level 2 (mid-level) evidenceReed SD, Landers DV, Sweet RL. Antibiotic treatment of tuboovarian abscess: comparison of broad-spectrum beta-lactam agents versus clindamycin-containing regimens. American journal of obstetrics and gynecology. 1991 Jun:164(6 Pt 1):1556-61; discussion 1561-2 [PubMed PMID: 2048603]
Gjelland K, Ekerhovd E, Granberg S. Transvaginal ultrasound-guided aspiration for treatment of tubo-ovarian abscess: a study of 302 cases. American journal of obstetrics and gynecology. 2005 Oct:193(4):1323-30 [PubMed PMID: 16202721]
Level 3 (low-level) evidenceGinsburg DS, Stern JL, Hamod KA, Genadry R, Spence MR. Tubo-ovarian abscess: a retrospective review. American journal of obstetrics and gynecology. 1980 Dec 1:138(7 Pt 2):1055-8 [PubMed PMID: 7468668]
Level 2 (mid-level) evidenceChu L, Ma H, Liang J, Li L, Shen A, Wang J, Li H, Tong X. Effectiveness and Adverse Events of Early Laparoscopic Therapy versus Conservative Treatment for Tubo-Ovarian or Pelvic Abscess: A Single-Center Retrospective Cohort Study. Gynecologic and obstetric investigation. 2019:84(4):334-342. doi: 10.1159/000493855. Epub 2019 Jan 4 [PubMed PMID: 30612130]
Level 2 (mid-level) evidenceDulin JD, Akers MC. Pelvic inflammatory disease and sepsis. Critical care nursing clinics of North America. 2003 Mar:15(1):63-70 [PubMed PMID: 12597041]
Level 3 (low-level) evidence