Neuroanatomy, Cranial Nerve 0 (Terminal Nerve)
Neuroanatomy, Cranial Nerve 0 (Terminal Nerve)
Introduction
Despite the plethora of literature describing the traditional 12 pairs of cranial nerves, very little has been published on the seemingly innocuous nerve known as nervus terminalis, commonly referred to as the terminal nerve, nerve nulla (n), cranial nerve zero “0,” and cranial nerve XIII. However, this nerve has been identified in numerous invertebrate and vertebrate species, including humans, for more than a century.[1] Interestingly, this structure remains largely unrecognized in the medical literature, especially because most anatomy and medical books have overlooked its existence, even though it has been identified in the human brain since 1914. Ever since, a wide repertoire of studies has described its embryology, histology, neurophysiology, and its clinical significance.
Studies in adult brains and fetuses have shown its fibers and those of the vomeronasal organ independent from the olfactory nerve embryologically, as early as stages 17 and 18. In the late 1980s, it was named “cranial nerve 0” (CN0) for its position rostral to the official 12 cranial nerves. The CN0 neurons are associated with gonadotropin-releasing hormone (GnRH), suggesting a potential role in controlling human reproductive functions and behaviors. It has been speculated to play a role in the unconscious perception of special odorants influencing autonomic and reproductive hormonal systems via the ubiquitous hypothalamic-pituitary-gonadal axis (HPG).[2] Furthermore, the literature points to a potential role in detecting pheromones for mate selection and neuromodulation of reproductive functions.[3][1]
Researchers hypothesize that CN0 may trigger hormonal responses independently or with other neuroanatomical circuits, such as the kisspeptin neural network. In females, these cells are mainly localized in the preoptic area and the infundibular regions of the hypothalamus, posing a riveting sexually dimorphic trait that may have significant clinical considerations. Thus, the presence of CN0 is relevant to medicine from a myriad of different clinical perspectives.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
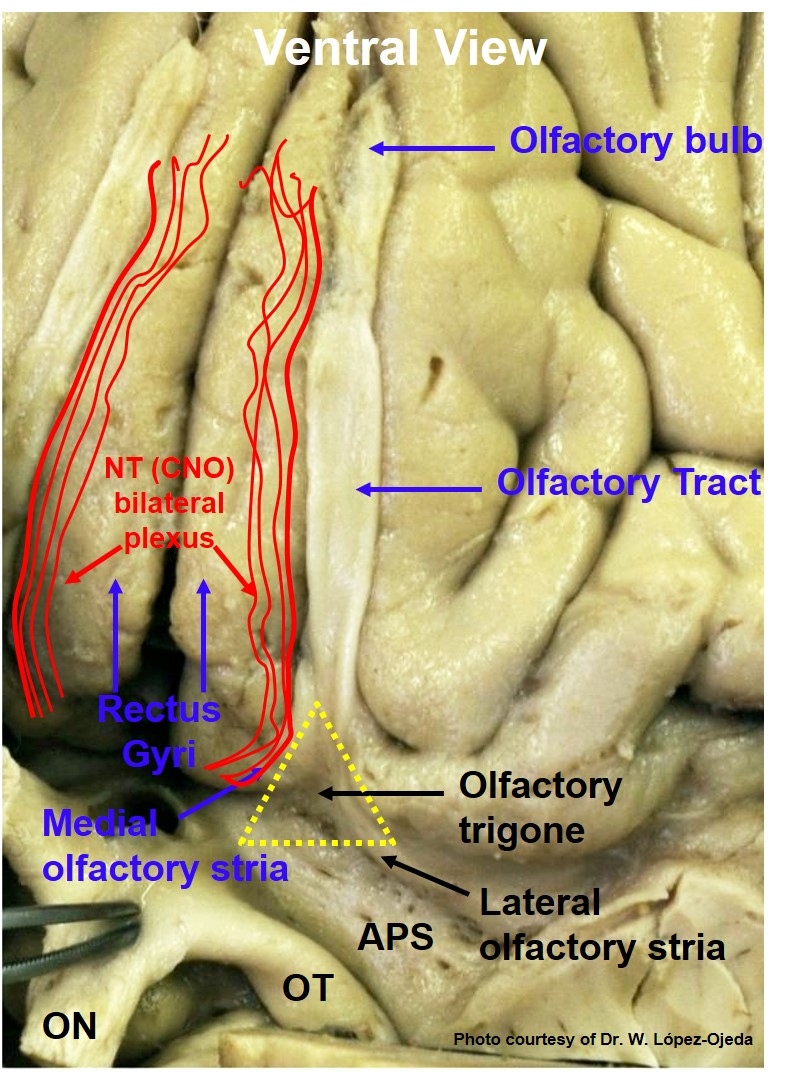
The CN0 is located on the ventral surface of the human brain. Its plexiform fibers appear to run in proximity to CNI, the olfactory nerve. CN0 rests on the anterior surface of the brain in the region of the olfactory trigone and courses anteriorly on the medial surface of the olfactory tract and bulb. Medially, it forms a plexus of fibers closely associated with the olfactory stria (medial to the anterior perforated space), from where it enters the brain independently (see Image. Basal View of a Human Brain Dissection Depicting the Location of CN0 Plexiform Fibers Over the Medial Surface of Gyri Recti). Its structural composition consists of numerous smaller strands that branch and anastomose, elaborating an elongated plexus (plexiform). Its bilateral bundle of unmyelinated nerve fibers is most evident in human fetal stages. However, it is also seen in adult brains.[4] Its fascicles can be seen within the subarachnoid space covering the gyri recti surface of the frontal lobe.
In humans and a wide range of other species, the physiology of reproductive hormones is under the regulatory control of the HPG axis, and GnRH plays a pivotal role. The latter is released from the hypothalamus upon the appropriate neuronal induction. Once GnRH is released from the hypothalamic neurons, it potentiates the synthesis and releases gonadotropins, a subset of hormones known as luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the adenohypophysis. This, in turn, regulates the production and secretion of the sex steroid hormones (e.g., estrogens, testosterone) that will ultimately command the sexual behaviors governing the biology of our reproduction.[2] The embryological migration pattern of the GnRH cells and their direct association with CN0 suggests a potential role in developing the HPG axis in humans.[5] Furthermore, it has been suggested that this nerve may have neuromodulatory implications through GnRH and the nasal mucosa blood vessels and glands, which could exert regulatory functions of human sexual behaviors.[6][7]
Embryology
Since the beginning of the 20th century, there have been many studies on the embryological origins and stages of human olfactory structures.[8][9][10][11][12] Although the embryological origin of the CN0 correlates to the rest of the human olfactory structures, it appears that the terminal nerve fibers enter the brain along with the olfactory nerves and those of the vomeronasal processes at embryological stages 17 and 18. This is based on Bossy’s works (1980) and preliminary studies presenting details on the Carnegie Collection from stages 11 to 23 in human embryos.[10] These have been pivotal in understanding the terminal nerve embryological development and other olfactory structures in human embryos. See Image. Further work from Muller et al. provided reconstructions essential for understanding and correctly interpreting the developmental happenings of the neural connections within and beyond the olfactory system structures.[8]
The specific embryological origin of CN0 remains somewhat enigmatic. While some authors have reported the origin of the nerve from the olfactory placode, from where the olfactory cells also originate, others indicate that it comes from the neural crest.[13][14] Perhaps it originates from a concatenation of migrating cells from varying embryological orders, among which the neural crest may contribute to the subset of GnRH-secreting neurons.
Physiologic Variants
Although CN0 is closely positioned next to the CNI (olfactory nerve), its functionality is distinct from the classical olfactory sensory role of perceiving odorant molecules via the olfactory receptors (ORs), which belong to a superfamily of G protein-coupled receptors (GPCRs).[15] Various reports indicate that the terminal nerve has projections to several important neuroanatomical structures, such as the medial pre-commissural septum, including the medial septal nucleus. It also sends fibers to the nasal mucosa and rostral ventral brain structures, primarily olfactory and limbic areas (i.e., amygdala, hypothalamic nuclei).[16][17] These connections provide access to the limbic system structures, such as the hypothalamus. In the hypothalamus, specifically within the preoptic (POA) and the infundibular (INF) nuclei, there is a relatively inconspicuous group of neurons collectively referred to as the "kisspeptin neuronal network" (KP).[18] This hypothalamic neuronal circuit is involved in the central endocrinologic control of puberty and human reproductive functions. The hypothalamic KP neurons act primarily by inducing GnRH secretion from the hypothalamus, which, in turn, regulates the secretion of the gonadotropin hormones LH and FSH. These adenohypophyseal hormones will ultimately influence the synthesis and release of sex steroids from the gonads.[19]
The CN0's proposed neuromodulatory role of sexual behaviors via GnRH poses an interesting connection with the hypothalamic KP neurons. While there are various elegant studies describing the human KP efferent projections, the afferents to this important hypothalamic cellular network are poorly understood.[20] Perhaps the enigmatic CN0 is the missing link in this complex scientific conundrum. The CN0 has projections to the nasal mucosa, the amygdala, and the hypothalamus, among other structures. If hypothetically, some of these projections reach the POA, INF, or both hypothalamic nuclei; these may represent a potential afferent component to the KP neurons regulating GnRH secretions, thereby controlling human sexual behaviors and functions. Although exciting, this notion is merely speculative but warrants scientific investigation.
Furthermore, during the last several decades, the idea of pheromones influencing human sexual behaviors has been a controversial topic.[21][22] This naturally includes the even more controversial vomeronasal organ (VNO), a very discrete chemosensory organ located deep into the nasal cavity of vertebrates, including humans.[23][24][25] For many years, the VNO has been held responsible for intra-specific chemical communication via pheromones, which are chemical messengers secreted externally by an individual and detected by another of the same species. Many still debate the existence of the VNO and pheromones in humans. However, the evidence coming from many reputable studies is robustly supportive.[26] These studies suggest that humans have the genes to produce at least six of the same pheromone receptors present in mice. The presence of VNO in humans has been massively described in the literature as well. The VNO is present in human fetuses by weeks 6 to 7; its ducts have openings into the nasal cavity by the 28th week. Histologically, it consists of the olfactory epithelium, lamina propria, and a rich vascularization network. A recent Bulgarian study reported the presence of the VNO in approximately 27% (males, 53%; females, 47%) of the adult population.[27] Similar findings have been reported in other populations (e.g., the United States, Canada, France, and Egypt).
Despite all these important considerations, recent studies on the VNO suggest this sexually dimorphic structure is just vestigial in adult humans, namely a remnant from our embryological differentiation stages. This suggests that although unequivocally present, this structure is not physiologically active in adult life. Accordingly, the hypothesis correlating the VNO to detecting pheromones in adult humans lacks scientific support. Alternatively, a reasonable notion may implicate the nasal mucosa nerve projections from CN0, transducing the inscrutable chemical signaling from adult human pheromones and regulating the hypothalamic GnRH secretory pulses via the KP neurons and consequentially, controlling gonadotropins and sex steroids secretions in response to the pheromones' chemical cues. However, this conceptual cascade of neuroendocrinological events is only hypothetical since the CN0 projections to the hypothalamic KP neurons are merely speculative. Considering that the VNO may lack physiological competence concerning biological pheromones detection in adults, the CN0 may be a plausible candidate for this novel physiological role independent from the VNO.
Surgical Considerations
The location of the CN0 fibers is in close relationship to the olfactory tract. Its nerve components conglomerate into a rich plexus of fibers embedded along with the dura mater in proximity to CNI and other nerve fibers. Possibly, this explains why CN0 fibers have consistently been mistaken as part of CNI during human dissections, imaging studies, and cranial surgical procedures. Furthermore, the CN0 fibers also travel through the minuscule foramina of the cribriform plate (ethmoid bone) towards the nasal cavity. The CN0 fibers then course on either side of the nasal septum and branch through the septal mucosa bilaterally. Herein, its fibers travel along the olfactory and nasopalatine nerve fibers.
The anatomical positioning of CN0 poses important clinical considerations, especially for otorhinolaryngology surgical procedures. Given its potential neuroreproductive role, damage or lacerations to CN0 during routine otolaryngological surgical procedures may compromise its structural integrity and potential functionality. In animal models, the laceration of the terminal nerve has been reported to cause GnRH deficiencies. However, there are no clinical studies to confirm these findings in humans.
Clinical Significance
In addition to the surgical considerations listed above, it is imperative to acknowledge the existence of CN0 and appreciate its potential medical relevance. For instance, Kallmann syndrome (KS) is an inherited condition associated with hypogonadotropic hypogonadism (HH) and hyposmia or anosmia affecting both sexes.[28] Interestingly, an embryological failure in the normal migratory pattern of basal forebrain GnRH cells appears to be the primary cause of the hypogonadism associated with this human genetic condition. Studies have shown point mutations and deletions in KISS1R in some HH patients.[29] This suggests that the CN0 GnRH-containing axons play a pivotal role in the development and differentiation of the hypothalamic-pituitary-adrenal (HPA) axis and hypothalamic-pituitary-gonadal (HPG) axis. It may be potentially critical for the normal sexual development of both male and female patients.
Other Issues
One persistent argument associated with this nerve is its name. There has been some debate on how to refer to it properly in scientific terminology. While some argue it should remain as the terminal nerve after its location by the lamina terminalis, others have proposed other nominations. There have been several suggestions, including CN0, CN XIII, and others.[30] Since the nerve was discovered after the other CNs had been named and classified sequentially based on their anatomical location, some agree that CN XIII is most appropriate. CN XIII seems reasonable because the nerve was discovered last. However, this term seems counterintuitive mainly because its anatomical location is rostral to all other cranial nerves. Accordingly, CN0 has been proposed as the most suitable, but some argued that it is discordant since zero "0" is not a roman numeral. Therefore, it is inconsistent with all other CNs, which were enumerated with roman numerals symbols.
Aside from the anatomical nomenclature issues, the reality is that CN0 can function as an important topic for medical teaching. Not only is CN0 anatomically relevant for its long history in the scientific literature, but it is also clinically relevant for its relationship to normal and abnormal development and function, as illustrated by its potential role in KS, commonly caused by a genetic mutation in the kisspeptin or GnRH signaling pathways. Embryologically, the development of CN0 occurs synchronized to the sensory structures of the olfactory system. Developmental disturbances in stages 17 and 18 have been linked to physiological abnormalities of the HPA axis, as described in KS. Also, traumatic avulsion of the olfactory network also plays a role in depression concomitant with anosmia in patients who experience sufficiently traumatic forces commonly associated with automobile collisions. Depression with anosmia has also been linked to symptoms such as reduced sexual drive and impaired recognition of emotion in others.[31]
Medical and health sciences students will benefit from a better understanding of the basic science and clinical aspects associated with traditional cranial nerve teaching, along with the growing body of scientific evidence on CN0. Finally, CN0 is important in principle as a topic of ongoing scientific debates that shape the nature of the evidence-based practice that underpins medicine. Scientific knowledge evolves, resulting in changes to standards of practice and treatment options requiring medicine students to be "lifelong learners" for their patients' benefit.
Media
(Click Image to Enlarge)
References
Vilensky JA. The neglected cranial nerve: nervus terminalis (cranial nerve N). Clinical anatomy (New York, N.Y.). 2014 Jan:27(1):46-53. doi: 10.1002/ca.22130. Epub 2012 Jul 26 [PubMed PMID: 22836597]
Level 3 (low-level) evidenceBiehl MJ, Raetzman LT. Developmental Origins of Hypothalamic Cells Controlling Reproduction. Seminars in reproductive medicine. 2017 Mar:35(2):121-129. doi: 10.1055/s-0037-1599083. Epub 2017 Mar 9 [PubMed PMID: 28278530]
Winkelmann A. Response to "The neglected cranial nerve: nervus terminalis (cranial nerve N)". Clinical anatomy (New York, N.Y.). 2014 Sep:27(6):806-7. doi: 10.1002/ca.22389. Epub 2014 Mar 21 [PubMed PMID: 24659085]
Level 3 (low-level) evidenceFuller GN, Burger PC. Nervus terminalis (cranial nerve zero) in the adult human. Clinical neuropathology. 1990 Nov-Dec:9(6):279-83 [PubMed PMID: 2286018]
Taroc EZM, Prasad A, Lin JM, Forni PE. The terminal nerve plays a prominent role in GnRH-1 neuronal migration independent from proper olfactory and vomeronasal connections to the olfactory bulbs. Biology open. 2017 Oct 15:6(10):1552-1568. doi: 10.1242/bio.029074. Epub 2017 Oct 15 [PubMed PMID: 28970231]
. The terminal nerve (nervus terminalis): structure, function, and evolution. Annals of the New York Academy of Sciences. 1987:519():1-469 [PubMed PMID: 3448965]
Level 3 (low-level) evidenceDemski LS, Schwanzel-Fukuda M. The terminal nerve (nervus terminalis): structure, function, and evolution. Introduction. Annals of the New York Academy of Sciences. 1987:519():ix-xi [PubMed PMID: 3448964]
Level 3 (low-level) evidenceMüller F, O'Rahilly R. Olfactory structures in staged human embryos. Cells, tissues, organs. 2004:178(2):93-116 [PubMed PMID: 15604533]
O'Rahilly R, Müller F, Hutchins GM, Moore GW. Computer ranking of the sequence of appearance of 73 features of the brain and related structures in staged human embryos during the sixth week of development. The American journal of anatomy. 1987 Sep:180(1):69-86 [PubMed PMID: 3661464]
Bossy J. Development of olfactory and related structures in staged human embryos. Anatomy and embryology. 1980:161(2):225-36 [PubMed PMID: 7469043]
Oelschläger HA, Buhl EH, Dann JF. Development of the nervus terminalis in mammals including toothed whales and humans. Annals of the New York Academy of Sciences. 1987:519():447-64 [PubMed PMID: 3448969]
Level 3 (low-level) evidenceBrown JW. The nervus terminalis in insectivorous bat embryos and notes on its presence during human ontogeny. Annals of the New York Academy of Sciences. 1987:519():184-200 [PubMed PMID: 3448966]
Level 3 (low-level) evidenceWhitlock KE, Wolf CD, Boyce ML. Gonadotropin-releasing hormone (GnRH) cells arise from cranial neural crest and adenohypophyseal regions of the neural plate in the zebrafish, Danio rerio. Developmental biology. 2003 May 1:257(1):140-52 [PubMed PMID: 12710963]
Level 3 (low-level) evidenceWhitlock KE. Development of the nervus terminalis: origin and migration. Microscopy research and technique. 2004 Sep:65(1-2):2-12 [PubMed PMID: 15570589]
Level 3 (low-level) evidenceLARSELL O. The nervus terminalis. The Annals of otology, rhinology, and laryngology. 1950 Jun:59(2):414-38 [PubMed PMID: 15426155]
Wirsig-Wiechmann CR, Lepri JJ. LHRH-immunoreactive neurons in the pterygopalatine ganglia of voles: a component of the nervus terminalis? Brain research. 1991 Dec 24:568(1-2):289-93 [PubMed PMID: 1814573]
Level 3 (low-level) evidenceDemski LS, Northcutt RG. The terminal nerve: a new chemosensory system in vertebrates? Science (New York, N.Y.). 1983 Apr 22:220(4595):435-7 [PubMed PMID: 6836287]
Level 3 (low-level) evidenceHrabovszky E. Neuroanatomy of the human hypothalamic kisspeptin system. Neuroendocrinology. 2014:99(1):33-48. doi: 10.1159/000356903. Epub 2013 Nov 8 [PubMed PMID: 24401651]
Level 3 (low-level) evidenceLehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Advances in experimental medicine and biology. 2013:784():27-62. doi: 10.1007/978-1-4614-6199-9_3. Epub [PubMed PMID: 23550001]
Level 3 (low-level) evidenceMikkelsen JD, Simonneaux V. The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides. 2009 Jan:30(1):26-33. doi: 10.1016/j.peptides.2008.09.004. Epub 2008 Sep 18 [PubMed PMID: 18840491]
Level 3 (low-level) evidenceMorozova SV, Savvateeva DM, Svistushkin VM, Toporkova LA. [The role of the vomeronasal system in the formation of the human sexual behaviour]. Vestnik otorinolaringologii. 2017:82(2):90-94. doi: 10.17116/otorino201782190-94. Epub [PubMed PMID: 28514374]
Baum MJ, Bakker J. Roles of sex and gonadal steroids in mammalian pheromonal communication. Frontiers in neuroendocrinology. 2013 Oct:34(4):268-84. doi: 10.1016/j.yfrne.2013.07.004. Epub 2013 Jul 18 [PubMed PMID: 23872334]
Level 3 (low-level) evidenceWirsig-Wiechmann CR, Wiechmann AF. The prairie vole vomeronasal organ is a target for gonadotropin-releasing hormone. Chemical senses. 2001 Nov:26(9):1193-202 [PubMed PMID: 11705805]
Level 3 (low-level) evidenceRodewald A, Mills D, Gebhart VM, Jirikowski GF. Steroidal pheromones and their potential target sites in the vomeronasal organ. Steroids. 2019 Feb:142():14-20. doi: 10.1016/j.steroids.2017.09.010. Epub 2017 Sep 28 [PubMed PMID: 28962851]
Salazar I, Barrios AW, SáNchez-Quinteiro P. Revisiting the Vomeronasal System From an Integrated Perspective. Anatomical record (Hoboken, N.J. : 2007). 2016 Nov:299(11):1488-1491. doi: 10.1002/ar.23470. Epub 2016 Sep 15 [PubMed PMID: 27594382]
Level 3 (low-level) evidenceVasuki AK, Fenn TK, Devi MN, Hebzibah TD, Jamuna M, Sundaram KK. Fate and Development of Human Vomeronasal Organ - A Microscopic Fetal Study. Journal of clinical and diagnostic research : JCDR. 2016 Mar:10(3):AC08-11. doi: 10.7860/JCDR/2016/15930.7373. Epub 2016 Mar 1 [PubMed PMID: 27134849]
Stoyanov G, Moneva K, Sapundzhiev N, Tonchev AB. The vomeronasal organ - incidence in a Bulgarian population. The Journal of laryngology and otology. 2016 Apr:130(4):344-7. doi: 10.1017/S0022215116000189. Epub 2016 Feb 2 [PubMed PMID: 26831012]
Swee DS, Quinton R, Maggi R. Recent advances in understanding and managing Kallmann syndrome. Faculty reviews. 2021:10():37. doi: 10.12703/r/10-37. Epub 2021 Apr 13 [PubMed PMID: 34046641]
Level 3 (low-level) evidencede Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America. 2003 Sep 16:100(19):10972-6 [PubMed PMID: 12944565]
Bordoni B, Zanier E. Cranial nerves XIII and XIV: nerves in the shadows. Journal of multidisciplinary healthcare. 2013:6():87-91. doi: 10.2147/JMDH.S39132. Epub 2013 Mar 13 [PubMed PMID: 23516138]
Gudziol V, Wolff-Stephan S, Aschenbrenner K, Joraschky P, Hummel T. Depression resulting from olfactory dysfunction is associated with reduced sexual appetite--a cross-sectional cohort study. The journal of sexual medicine. 2009 Jul:6(7):1924-9. doi: 10.1111/j.1743-6109.2009.01266.x. Epub 2009 Apr 23 [PubMed PMID: 19453919]
Level 2 (mid-level) evidence