Introduction
The aorta is the main artery of the human body. It arises from the left ventricle of the heart and travels superiorly to form the ascending aorta. It then loops inferiorly to form the arch of the aorta and through the thorax to form the thoracic aorta. After crossing the diaphragm into the abdomen, the aptly-named abdominal aorta eventually bifurcates into the common iliac arteries in the lower abdomen. Along the way, the aorta gives rise to the arterial branches that are responsible for transmitting oxygenated blood to all the tissues of the body. As such, pathology arising in the aorta frequently results in serious consequences. Thus, it is important for the clinician to be aware of the functional, anatomical, surgical, and clinical considerations about the aorta.[1][2]
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
The aorta is the central source of oxygenated blood from which all the arteries of the body arise before traveling distally to their destination tissues. The aorta gives rise to major arterial branches such as the left and right coronary arteries, brachiocephalic artery, left common carotid artery, left subclavian artery, celiac trunk, superior mesenteric artery, left and right renal arteries, gonadal arteries, inferior mesenteric artery, median sacral artery, and the left and right common iliac arteries.[2]
The abdominal aorta enters the abdomen posterior to the median arcuate ligament and lies anterior and slightly to the left of the anterior aspect of the lumbar vertebrae, with the inferior vena cava lying to its right.
Structures crossing the abdominal aorta:
- Between the origins of coeliac and superior mesenteric arteries - splenic vein and body of pancreas
- Between the origins of superior mesenteric and inferior mesenteric arteries - left renal vein, the uncinate process of the pancreas and 3 part of the duodenum
The branches of the abdominal aorta:
- Paired branches: the middle adrenal, renal, gonadal, inferior phrenic, and lumbar arteries
- Unpaired branches: the coeliac, superior mesenteric, inferior mesenteric, and median sacral arteries
- Terminal branches: the common iliac arteries
Embryology
The abdominal aorta arises from the fusion of the paired dorsal aortae during embryonic development. The paired dorsal aortae are continuous with the aortic arches in the embryologic head region which, in turn, arise from the aortic sac. The aortic sac gives rise to the ascending aorta. The aortic arches, also known as the pharyngeal arch arteries, are six pairs of embryologic vascular structures that give rise to the great arteries of the head and neck, including parts of the common carotid arteries, internal carotid arteries, maxillary arteries, aortic arch, pulmonary arteries, right subclavian artery, ductus arteriosus, stapedial arteries, and hyoid artery.[2][3]
Blood Supply and Lymphatics
The walls of the aorta are supplied by a network of small blood vessels known as the vasa vasorum whose function is to deliver nutrients and oxygen and remove waste products.[4] The descending aorta lacks vasa vasorum below the renal arteries and relies on diffusion for its metabolic needs. As a result, the infra-renal aorta is markedly thinner. This leads to an increased incidence of infrarenal aortic aneurysms when compared to other species such as dogs, whose infrarenal aortae have associated vasa vasorum.[5]
Nerves
The vascular smooth muscle of blood vessels, including the aorta, is innervated primarily by the autonomic nervous system (ANS), particularly the sympathetic nervous system. Activation of alpha-1 receptors causes vasoconstriction in response to low blood pressure. Activation of beta-2 receptors causes vasodilation.[6]
Muscles
The wall of the aorta consists of 3 layers known as the tunica adventitia, tunica media, and tunica intima. The innermost layer, the tunica intima, is lined by a single layer of endothelial cells and bound by the internal elastic lamina. The middle layer, the tunica media, is composed of smooth muscle cells, collagen, elastin, and fibroblasts. These components control vasodilation and vasoconstriction by way of signals from the autonomic nervous system. Elastin fibers in the media of the aorta provide tensile strength and the ability to withstand the pulsatile nature of the circulation. The outermost layer, the adventitia, is a thin layer containing connective tissue, fibroblasts, and the vasa vasorum.[7]
Physiologic Variants
Knowledge of anatomic variability of the aorta and its branches is important in medicine, particularly in the surgical and radiologic fields. The clinician should be able to recognize the various physiological variants of the aorta on imaging and correlate with clinical findings to guide management. Anomalies are often visible on computed tomography (CT), including CT angiography, but magnetic resonance imaging (MRI) is widely considered the preferred standard for imaging of the aortic arch and its arterial branching pattern.[8]
Dextrocardia
Dextrocardia refers to the occurrence of a right-sided cardiac apex. Dextrocardia of embryonic arrest is an isolated form in which the heart is simply placed further to the right than is normal. This form is often associated with severe heart defects.[9] Dextrocardia with situs inversus is a form in which the position of the heart is a mirror image of the normal anatomy. Additionally, visceral organs tend to be mirrored as well. Dextrocardia with situs inversus totalis refers to mirroring of the heart and all visceral organs.
Bovine Aortic Arch
A bovine arch is the most common physiologic variant of the aortic arch. It occurs when the brachiocephalic artery shares a common origin with the left common carotid artery. It is a misnomer as this branching pattern is found in many animals such as canines, felines, and Macaque monkeys, but differs from the true branching pattern of bovine.[10]
Aberrant Right Subclavian Artery
Also known as arteria lusoria, this branching pattern occurs when the right subclavian artery arises distal to the left subclavian artery (rather than arising from the brachiocephalic artery) and hooks back to reach the right side. It can be associated with trisomy 21 as well as other chromosomal abnormalities.[11][12][13]
Innominate Artery Compression Syndrome
Innominate artery compression syndrome occurs when the brachiocephalic trunk (innominate artery) is located further to the left than normal in a location that is directly anterior to the trachea, thus causing compression. Compression can be seen on tracheography, CT, or MRI. There is a tendency for the origin of the brachiocephalic trunk to become more rightward with age, thus leading to the resolution of symptoms in many cases. Symptomatic patients are treated with aortopexy or reimplantation of the innominate artery.[14]
Right Arch Mirror Image
This variant occurs when the aortic arch travels posterior and rightward rather than posterior and leftward. The arch passes over the right main-stem bronchus rather than the left. Although asymptomatic, the majority of patients have associated congenital heart disease.[15] When this variant is accompanied by a left subclavian artery as the first branch of the aorta, it is termed a right arch with an aberrant left subclavian artery. This variant is an obstructing vascular ring as the left subclavian artery must cross the midline to the left side of the body. The left subclavian artery may travel anteriorly and compress the trachea, or it may travel posteriorly and travel behind the esophagus and trachea. The ligamentum ductus arteriosus between the arch of the aorta and the left pulmonary artery completes the obstructing ring.
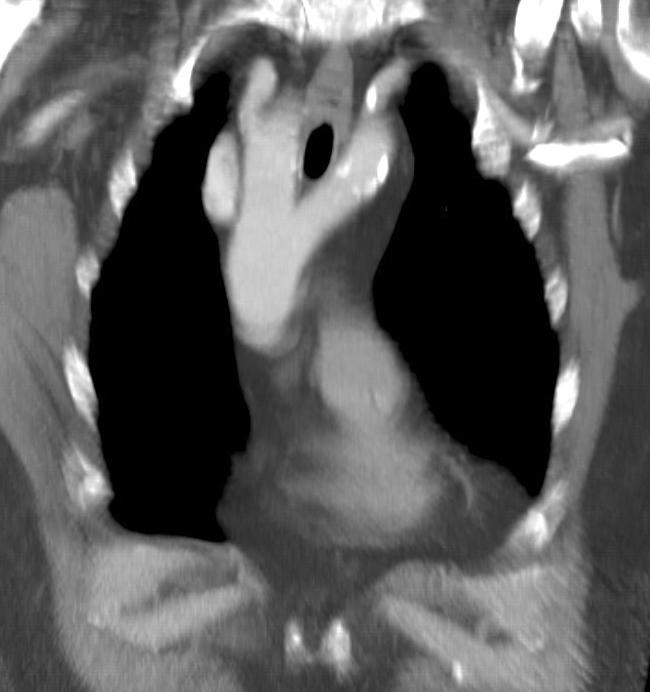
Double Aortic Arch
A double aortic arch (DAA) accounts for up to 45% to 65% of patients undergoing repair of a vascular ring. Many subtypes exist, but all types commonly result in compression due to the splitting of the ascending aorta into 2 vessels that pass on either side of the trachea and esophagus. The 2 vessels then join to form a single descending aorta. See Image 1.
Surgical Considerations
For the repair of an abdominal aortic aneurysm, the vessel is approached either transabdominal or retroperitoneal. Nowadays, many aneurysms are being repaired with the help of minimally invasive endovascular approaches.
Clinical Significance
Vascular Ring
Any of the subtypes of vascular rings may cause compression of the tracheobronchial tree and/or esophagus leading to respiratory and gastrointestinal symptoms. Complete vascular rings completely encircle both structures while incomplete vascular rings do not. In retrospective reviews and case series, DAA along with the right aortic arch with an aberrant left subclavian artery accounts for up to 90% of reported cases of vascular rings causing significant symptoms.[16][17][18][19][20] Tracheal symptoms include stridor, recurrent respiratory infections, respiratory distress, cough, and wheezing. Esophageal symptoms include vomiting, feeding difficulty, and dysphagia.[21] However, the spectrum of clinical presentation ranges from critical neonatal airway obstruction to coincidental findings in asymptomatic adults.
Aortic Aneurysm
Aortic aneurysm (AA) describes an increased diameter in the ascending aorta to greater than 5.0 cm or descending aorta to greater than 4.0 cm (increase in diameter >50% from baseline).[22] They are relatively common with a prevalence of approximately 7.5% in patients over 65. An abdominal aortic aneurysm (AAA) is much more common than a thoracic aortic aneurysm.[23] Risk factors include increasing age, smoking, male gender, and family history. AA is a distinct degenerative process involving all layers of the vessel wall. The pathophysiology involves infiltration of the vessel wall by lymphocytes and macrophages, destruction of elastic components in the media and adventitia by proteases, and loss of smooth muscle cells within the tunica media.[24] The clinical significance of aortic aneurysms is their tendency to expand and rupture. Rupture is the most feared complication and a surgical emergency. The US Preventative Services Task Force (USPSTF) recommends one-time screening with ultrasonography in men ages 65 to 75 years who have ever smoked. Surveillance is accomplished with interval ultrasonography with frequency dependent on the size of an aortic aneurysm. Elective abdominal aortic aneurysm repair is the most effective treatment to prevent rupture. The Society for Vascular Surgery recommends elective repair in asymptomatic patients with aortic aneurysm diameter greater than 5.5 cm or rapidly expanding abdominal aortic aneurysm.
Aortic Coarctation
Aortic coarctation (AC) occurs when there is a narrowing of the lumen at the distal arch or descending aorta. AC accounts for 4 to 6% of all congenital heart defects with a reported prevalence of approximately 4 per 10,000 live births.[25] Associations include a bicuspid aortic valve, ventricular septal defect, mitral valve defects, patent ductus arteriosus, and Turner syndrome. The vast majority of cases are congenital with an unknown pathophysiologic mechanism. The two main theories include reduced antegrade intrauterine blood flow and migration of extension of ductal tissue into the wall of the thoracic aorta.[25][26][27][28] Clinical manifestations of AC range from asymptomatic to heart failure and shock shortly after birth with the closure of the patent ductus arteriosus. The patients classically present with hypertension with diminished or delayed pulses in the lower extremities. Management includes corrective surgery, preferably in early childhood. The estimated average survival age of patients with unoperated coarctation is approximately 35 years of age, mostly due to complications of hypertension, accelerated coronary artery disease, heart failure, and stroke.[29]
Aortic Dissection
Aortic dissection (AD) occurs when blood enters the medial layer of the aortic wall through a tear in the intimal layer forming a second blood-filled pathway called a false lumen. The majority of intimal tears originate in the ascending aorta. The dissection can propagate proximally or distally to affect structures such as the aortic valve, cerebral arteries, coronary arteries, renal arteries, or pericardial space resulting in ischemic manifestations such as aortic regurgitation, acute coronary syndrome, stroke, and cardiac tamponade. The incidence of AD is estimated at 2.6 to 3.5 per 100,000.[30] The most important risk factor for AD is hypertension. Other risk factors include collagen disorders (i.e., Marfan syndrome, Ehlers-Danlos syndrome), preexisting aortic aneurysm, bicuspid aortic valve, AC, Turner syndrome, vasculitis, and aortic instrumentation or surgery. AD can be diagnosed with magnetic resonance angiography, computed tomography, or transesophageal echocardiography. Management is dependent on the level of aortic involvement. Involvement of the ascending aorta, termed Stanford A dissection, is a surgical emergency. Dissections without the involvement of the ascending aorta are classified as Stanford B and are generally managed medically.
Aortitis
Aortitis describes inflammation of the aorta and encompasses a wide range of conditions. The most common causes of aortitis are large vessel vasculitides, including giant cell arteritis (GCA) and Takayasu arteritis (TA). Other causes include anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, rheumatoid arthritis, systemic lupus erythematosus, seronegative spondyloarthropathies, sarcoidosis, and infectious aortitis.[31] Clinical manifestations vary widely depending on the etiology. The spectrum of clinical presentation of aortitis includes back pain, fever, aneurysm, aortic regurgitation, acute coronary syndrome, aortic thrombosis with embolization, aortic dissection, and hypertension.[31] The approach to management involves treatment of the underlying cause. Management is aimed at the immediate treatment of aortic inflammation or infection and the surveillance and treatment of complications.
Atherosclerosis
Atherosclerosis is a pathological process involving the deposition of fatty and fibrous plaques on the inner walls of the systemic circulatory vessels. The process can begin as early as childhood with the development of fatty streaks. In the United States, 1 in 6 teenagers was found to have abnormal intimal thickening on ultrasound.[32] Risk factors for atherosclerosis include hypertension, high levels of low-density lipoprotein cholesterol (LDL), smoking, obesity, and diabetes mellitus. The pathophysiology involves endothelial cell dysfunction, dyslipidemia, inflammatory factors, and plaque rupture. Oxidized LDL is known to cause endothelial cell dysfunction leading to loss of nitric oxide-induced vasodilation. Macrophages that have taken up oxidized LDL release inflammatory cytokines and growth factors.[33] Atherosclerosis generally remains asymptomatic until luminal diameter reaches 70% to 80% of normal. Symptoms vary depending on the organ systems distal to the diseased vessels. Atherosclerosis of the aorta itself is generally asymptomatic due to its large caliber. However, marked stenosis of the coronary arteries is responsible for unstable angina and myocardial infarction. Atherosclerosis of the carotid arteries can result in cerebral ischemia, leading to symptoms of a stroke. Peripheral arterial atherosclerosis, also known as peripheral artery disease, can result in limb claudication, tingling, numbness, and non-healing ulcers. Atherosclerosis of the renal arteries often results in chronic kidney disease and renovascular hypertension. Preventative measures include diet, exercise, weight loss, and smoking cessation. Statins, blood pressure medications, and aspirin are the mainstays of treatment. Vascular bypass surgeries can re-establish flow in the limbs, brain, and heart.
Trauma (Traumatic Aortic Rupture)
Traumatic aortic rupture (TAR) most commonly occurs at a site immediately distal to the left subclavian artery known as the aortic isthmus. This part of the aorta is tethered by the ligamentum arteriosum making it prone to injury during sudden deceleration, such as during an automobile collision.[34] TAR is frequently fatal due to resulting hemodynamic shock and is the second leading cause of trauma-related death behind head injury.[35] Management has shifted from open surgical repair to endovascular approaches.[36]
Media
(Click Image to Enlarge)
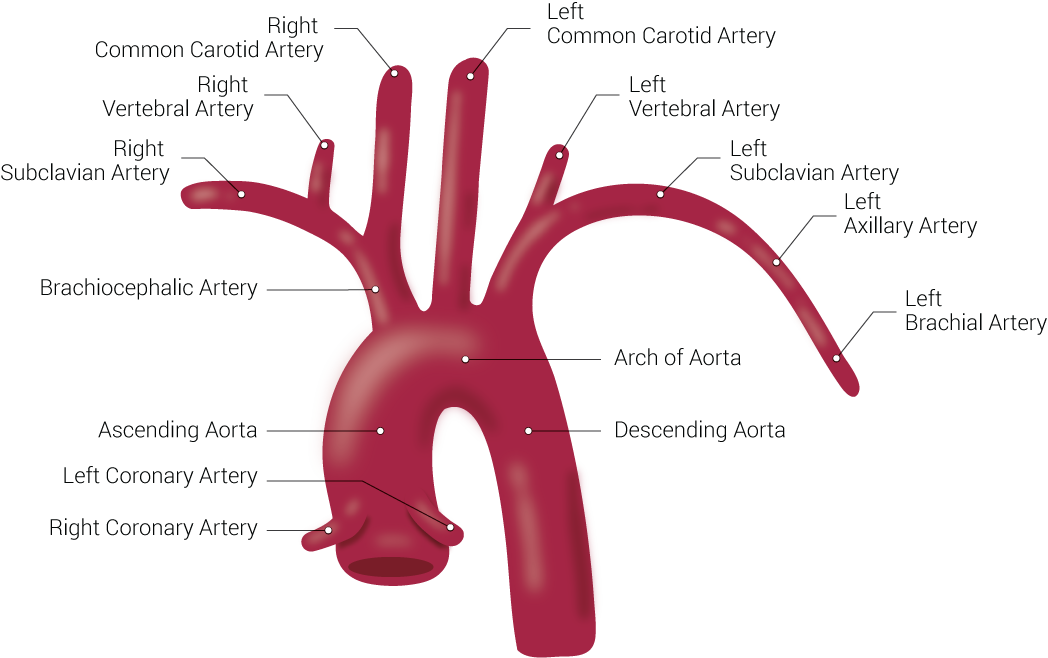
Branches of the Aorta. This illustration includes the right common carotid artery, right vertebral artery, right subclavian artery, brachiocephalic artery, ascending aorta, left coronary artery, right coronary artery, left common carotid artery, left vertebral artery, left subclavian artery, left axillary artery, left brachial artery, arch of aorta, and descending aorta.
Contributed by Beckie Palmer
(Click Image to Enlarge)
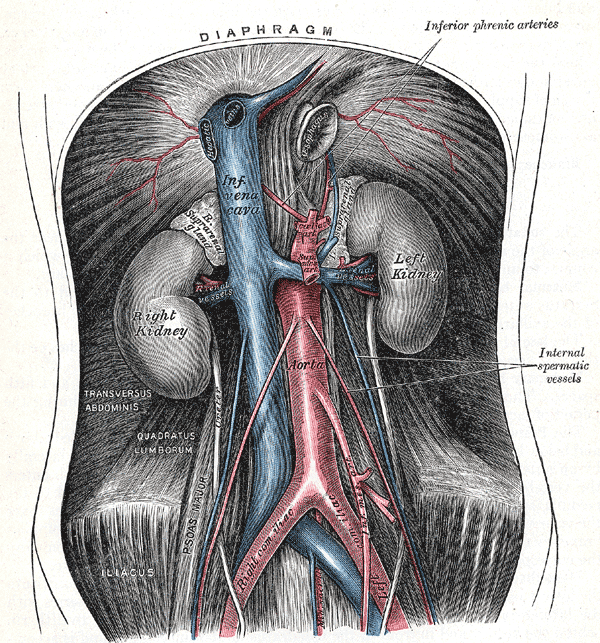
Retroperitoneal Organs. This image shows the anatomical relationships between the diaphragm, right and left suprarenal glands, right and left gonadal vessels, celiac trunk, superior and inferior mesenteric arteries, inferior phrenic arteries, internal spermatic vessels, median sacral arteries, left and right kidneys, inferior vena cava, abdominal aorta, right and left renal vessels, transversus abdominis, quadratus lumborum, right and left common Iliac vessels, psoas major, and iliacus.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
References
Loukas M, Bilinsky E, Bilinsky S, Blaak C, Tubbs RS, Anderson RH. The anatomy of the aortic root. Clinical anatomy (New York, N.Y.). 2014 Jul:27(5):748-56. doi: 10.1002/ca.22295. Epub 2013 Sep 2 [PubMed PMID: 24000000]
Murillo H, Lane MJ, Punn R, Fleischmann D, Restrepo CS. Imaging of the aorta: embryology and anatomy. Seminars in ultrasound, CT, and MR. 2012 Jun:33(3):169-90. doi: 10.1053/j.sult.2012.01.013. Epub [PubMed PMID: 22624964]
Schleich JM. Images in cardiology. Development of the human heart: days 15-21. Heart (British Cardiac Society). 2002 May:87(5):487 [PubMed PMID: 11997429]
Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovascular research. 2007 Sep 1:75(4):649-58 [PubMed PMID: 17631284]
Level 3 (low-level) evidenceWolinsky H, Glagov S. Comparison of abdominal and thoracic aortic medial structure in mammals. Deviation of man from the usual pattern. Circulation research. 1969 Dec:25(6):677-86 [PubMed PMID: 5364644]
Level 3 (low-level) evidenceBennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circulation research. 2016 Feb 19:118(4):692-702. doi: 10.1161/CIRCRESAHA.115.306361. Epub [PubMed PMID: 26892967]
Wang D, Wang Z, Zhang L, Wang Y. Roles of Cells from the Arterial Vessel Wall in Atherosclerosis. Mediators of inflammation. 2017:2017():8135934. doi: 10.1155/2017/8135934. Epub 2017 Jun 7 [PubMed PMID: 28680196]
Kau T, Sinzig M, Gasser J, Lesnik G, Rabitsch E, Celedin S, Eicher W, Illiasch H, Hausegger KA. Aortic development and anomalies. Seminars in interventional radiology. 2007 Jun:24(2):141-52. doi: 10.1055/s-2007-980040. Epub [PubMed PMID: 21326792]
Maldjian PD, Saric M. Approach to dextrocardia in adults: review. AJR. American journal of roentgenology. 2007 Jun:188(6 Suppl):S39-49; quiz S35-8 [PubMed PMID: 17515336]
Arnáiz-García ME, González-Santos JM, López-Rodriguez J, Dalmau-Sorli MJ, Bueno-Codoñer M, Arévalo-Abascal A, Fdez García-Hierro JM, Arnáiz-García AM, Arnáiz J. A bovine aortic arch in humans. Indian heart journal. 2014 May-Jun:66(3):390-1. doi: 10.1016/j.ihj.2014.03.021. Epub 2014 Apr 24 [PubMed PMID: 24973853]
Level 3 (low-level) evidenceScala C, Leone Roberti Maggiore U, Candiani M, Venturini PL, Ferrero S, Greco T, Cavoretto P. Aberrant right subclavian artery in fetuses with Down syndrome: a systematic review and meta-analysis. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2015 Sep:46(3):266-76. doi: 10.1002/uog.14774. Epub 2015 Aug 6 [PubMed PMID: 25586729]
Level 1 (high-level) evidenceGul A, Corbacioglu A, Bakirci IT, Ceylan Y. Associated anomalies and outcome of fetal aberrant right subclavian artery. Archives of gynecology and obstetrics. 2012 Jan:285(1):27-30. doi: 10.1007/s00404-011-1907-9. Epub 2011 Apr 13 [PubMed PMID: 21487731]
Carles D, Pelluard F, André G, Nocart N, Sauvestre F. [Aberrant right subclavian artery (arteria lusoria) and the risk for trisomy 21. Retrospective study of 11,479 fetopathological examinations]. Journal de gynecologie, obstetrique et biologie de la reproduction. 2014 Nov:43(9):698-703. doi: 10.1016/j.jgyn.2013.10.001. Epub 2013 Dec 12 [PubMed PMID: 24332742]
Level 2 (mid-level) evidenceGrimmer JF, Herway S, Hawkins JA, Park AH, Kouretas PC. Long-term results of innominate artery reimplantation for tracheal compression. Archives of otolaryngology--head & neck surgery. 2009 Jan:135(1):80-4. doi: 10.1001/archoto.2008.517. Epub [PubMed PMID: 19153311]
Level 3 (low-level) evidenceMcElhinney DB, Hoydu AK, Gaynor JW, Spray TL, Goldmuntz E, Weinberg PM. Patterns of right aortic arch and mirror-image branching of the brachiocephalic vessels without associated anomalies. Pediatric cardiology. 2001 Jul-Aug:22(4):285-91 [PubMed PMID: 11455394]
Shah RK, Mora BN, Bacha E, Sena LM, Buonomo C, Del Nido P, Rahbar R. The presentation and management of vascular rings: an otolaryngology perspective. International journal of pediatric otorhinolaryngology. 2007 Jan:71(1):57-62 [PubMed PMID: 17034866]
Level 2 (mid-level) evidenceHumphrey C, Duncan K, Fletcher S. Decade of experience with vascular rings at a single institution. Pediatrics. 2006 May:117(5):e903-8 [PubMed PMID: 16585275]
Woods RK, Sharp RJ, Holcomb GW 3rd, Snyder CL, Lofland GK, Ashcraft KW, Holder TM. Vascular anomalies and tracheoesophageal compression: a single institution's 25-year experience. The Annals of thoracic surgery. 2001 Aug:72(2):434-8; discussion 438-9 [PubMed PMID: 11515879]
Level 2 (mid-level) evidenceBacker CL, Mavroudis C, Rigsby CK, Holinger LD. Trends in vascular ring surgery. The Journal of thoracic and cardiovascular surgery. 2005 Jun:129(6):1339-47 [PubMed PMID: 15942575]
Turner A, Gavel G, Coutts J. Vascular rings--presentation, investigation and outcome. European journal of pediatrics. 2005 May:164(5):266-70 [PubMed PMID: 15666159]
Level 2 (mid-level) evidenceLicari A, Manca E, Rispoli GA, Mannarino S, Pelizzo G, Marseglia GL. Congenital vascular rings: a clinical challenge for the pediatrician. Pediatric pulmonology. 2015 May:50(5):511-24. doi: 10.1002/ppul.23152. Epub 2015 Jan 20 [PubMed PMID: 25604054]
Munden RF, Carter BW, Chiles C, MacMahon H, Black WC, Ko JP, McAdams HP, Rossi SE, Leung AN, Boiselle PM, Kent MS, Brown K, Dyer DS, Hartman TE, Goodman EM, Naidich DP, Kazerooni EA, Berland LL, Pandharipande PV. Managing Incidental Findings on Thoracic CT: Mediastinal and Cardiovascular Findings. A White Paper of the ACR Incidental Findings Committee. Journal of the American College of Radiology : JACR. 2018 Aug:15(8):1087-1096. doi: 10.1016/j.jacr.2018.04.029. Epub 2018 Jun 22 [PubMed PMID: 29941240]
Assar AN, Zarins CK. Ruptured abdominal aortic aneurysm: a surgical emergency with many clinical presentations. Postgraduate medical journal. 2009 May:85(1003):268-73. doi: 10.1136/pgmj.2008.074666. Epub [PubMed PMID: 19520879]
Mathur A, Mohan V, Ameta D, Gaurav B, Haranahalli P. Aortic aneurysm. Journal of translational internal medicine. 2016 Apr 1:4(1):35-41. doi: 10.1515/jtim-2016-0008. Epub 2016 Apr 14 [PubMed PMID: 28191516]
Rudolph AM, Heymann MA, Spitznas U. Hemodynamic considerations in the development of narrowing of the aorta. The American journal of cardiology. 1972 Oct:30(5):514-25 [PubMed PMID: 4672503]
Level 3 (low-level) evidenceWielenga G, Dankmeijer J. Coarctation of the aorta. The Journal of pathology and bacteriology. 1968 Jan:95(1):265-74 [PubMed PMID: 5643456]
Russell GA, Berry PJ, Watterson K, Dhasmana JP, Wisheart JD. Patterns of ductal tissue in coarctation of the aorta in the first three months of life. The Journal of thoracic and cardiovascular surgery. 1991 Oct:102(4):596-601 [PubMed PMID: 1921436]
Ho SY, Anderson RH. Coarctation, tubular hypoplasia, and the ductus arteriosus. Histological study of 35 specimens. British heart journal. 1979 Mar:41(3):268-74 [PubMed PMID: 426975]
Jenkins NP, Ward C. Coarctation of the aorta: natural history and outcome after surgical treatment. QJM : monthly journal of the Association of Physicians. 1999 Jul:92(7):365-71 [PubMed PMID: 10627885]
Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, Szép L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000 May:117(5):1271-8 [PubMed PMID: 10807810]
Level 2 (mid-level) evidenceGornik HL, Creager MA. Aortitis. Circulation. 2008 Jun 10:117(23):3039-51. doi: 10.1161/CIRCULATIONAHA.107.760686. Epub [PubMed PMID: 18541754]
Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, Young JB, Nissen SE. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001 Jun 5:103(22):2705-10 [PubMed PMID: 11390341]
Level 2 (mid-level) evidenceBerliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995 May 1:91(9):2488-96 [PubMed PMID: 7729036]
Moar JJ. Traumatic rupture of the thoracic aorta. An autopsy and histopathological study. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1985 Mar 9:67(10):383-5 [PubMed PMID: 3983713]
Plummer D, Petro K, Akbari C, O'Donnell S. Endovascular repair of traumatic thoracic aortic disruption. Perspectives in vascular surgery and endovascular therapy. 2006 Jun:18(2):132-9 [PubMed PMID: 17060230]
Level 3 (low-level) evidenceWeidenhagen R, Bombien R, Meimarakis G, Geisler G, Koeppel TA. Management of thoracic aortic lesions--the future is endovascular. VASA. Zeitschrift fur Gefasskrankheiten. 2012 May:41(3):163-76. doi: 10.1024/0301-1526/a000183. Epub [PubMed PMID: 22565618]