 38-Year-Old Male Presents With Lower Extremity Edema
38-Year-Old Male Presents With Lower Extremity Edema
Case Presentation
A 38-year-old African American male, who emigrated from Somalia 10 years ago, presented with a 6-month history of lower extremity swelling, 20-pound (9 kg) weight gain over 4 weeks, and new-onset microscopic hematuria. He had no prior history of edema, proteinuria, or hematuria. There was no prior history of any systemic illness or any heart, lung, or kidney disease. He reported no over-the-counter drug use, herbal medication use, or NSAID intake. His review of systems was positive for lethargy, fatigue, and edema at his lower extremities. His family history was negative for any kidney disease. He reported a monogamous relationship with his wife and no current or past alcohol or drug use. His medical history included recently diagnosed hypertension, latent tuberculosis, and anemia. His vital signs included a blood pressure of 160/90 and a heart rate of 78.
His physical exam revealed clear lungs and a normal heart exam on auscultation. His abdomen was soft and non-tender, and his skin was warm and dry with no rash. He had 2 plus pitting edema at the ankles. Initial laboratory data was consistent with acute kidney injury (AKI) with worsening creatinine from 1.2 to 1.6 over 1 month and nephrotic range proteinuria. Serologies for hepatitis B, hepatitis C, anti-nuclear antibody (ANA), c-ANCA, serine protease 3 (PR3) IgG, myeloperoxidase antibody (MPO), and complement levels were sent. He was started on losartan for proteinuria with plans for renal biopsy; however, he was lost to follow up. The patient then presented two weeks later with worsening edema to his waist, continued weight gain, mild hemoptysis, and streaks of blood in the stool. Physical examination now showed worsening pitting edema up to his waist with new non-pruritic, erythematous excoriations on the medial aspect of his thighs bilaterally. He was admitted to the hospital with concern for worsening vasculitis. He was started on oral prednisone and underwent a renal biopsy. For his edema, he was started on diuretics to which he responded well. Due to persistent leukopenia, thrombocytopenia, and anemia, human immunodeficiency virus (HIV) testing was performed to complete the workup.
Initial Evaluation
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Initial Evaluation
His initial serological testing sent at the time of his first visit was negative except for low C3 levels and a positive c-ANCA and PR3 IgG, a marker for granulomatosis with polyangiitis (GPA), formerly known as Wegener granulomatosis.[1] Laboratory studies at the time of admission revealed a white-cell count of 4100 /mm, platelets 119,000/dL, hemoglobin 9.9 g/dL, worsening serum creatinine at 2.3, and total urine protein 6.95 every 24 hours.
Differential Diagnosis
The differential diagnosis for acute kidney injury associated with nephrotic range proteinuria and a low C3 level and a positive cANCA was confusing as ANCA related vasculitis is not associated with low complements and his infectious and autoimmune workup typically causing low complements was negative.
His differential diagnosis included:
Vasculitis
Vasculitis including granulomatosis with polyangiitis, polyarteritis nodosa, Churg-Strauss Syndrome, IgA vasculitis, cryoglobulinemia, immune complex-associated vasculitis (infectious, SLE, or drug hypersensitivity), giant cell arteritis, leukocytoclastic vasculitis.
Glomerular Disease
Primary Glomerular Disease due to:
- Focal segmental glomerulosclerosis
- Membranous nephropathy
- Other proliferative glomerulonephritides (focal, “pure mesangial,” IgA nephropathy)
- Membranoproliferative glomerulonephritis and dense deposit disease
- Minimal change disease
Secondary Glomerular Disease due to:
- Systemic lupus erythematosus (SLE)
- Drugs (NSAIDs, penicillamine, heroin)
- Infection (schistosomiasis, malaria, syphilis, hepatitis B & C, HIV)
- Malignant disease (carcinoma, lymphoma)
- Diabetes mellitus
- Hereditary nephritis.
Confirmatory Evaluation
HIV ELISA and confirmatory Western blot were positive for HIV-1.[2] His CD4 count was 16 cells/mm and HIV RNA level was over 378,000 copies/mL. Syphilis and Schistosoma serology were negative. Renal biopsy showed an immune complex-associated proliferative glomerulonephritis with membranoproliferative features (Figures 1a-1d). In this patient with HIV, these pathologic findings were highly suggestive of HIV-associated immune complex kidney disease (HIVICK), a lupus-like nephritis variant. Evidence of collapsing focal segmental glomerulosclerosis (HIV-associated nephropathy) was not identified.
Diagnosis
Based on pathologic findings a diagnosis of HIV-associated immune complex kidney disease (HIVICK) was established.
Management
He was discharged on trimethoprim/sulfamethoxazole and azithromycin prophylaxis and started on antiretroviral therapy (ART) once his HIV genotype was determined. Throughout the hospitalization, diuresis was continued with furosemide, and his renal function improved with his creatinine down to 1.7 before starting abacavir-dolutegravir-lamivudine.
Discussion
Once infected with HIV-1, the development of AIDS can be delayed up to 10 to 20 years.[3] Many individuals who seroconvert to HIV have generalized symptoms of fatigue, fever, rash, lymphadenopathy, weight loss, and myalgias.[4][5] This is an unusual presentation of HIV immune complex kidney disease (HIVICK) with newly diagnosed AIDS. This case is also atypical due to c-ANCA positivity in the presence of HIV disease.
It is important to know the limitations of laboratory data when approaching a patient with newly diagnosed renal failure. Positivity of ANCA has been found secondary to HIV infection in up to 20% to 83% of cases, with a proposed mechanism being polyclonal activation of B cells. The sensitivity of c-ANCA for GPA is 64% with indirect immunofluorescence.[6]
Renal disease in the setting of HIV manifests in several ways. HIV-associated nephropathy (HIVAN) is a form of focal segmental glomerulosclerosis with interstitial inflammation and microcysts involving direct infiltration of renal tubular and glomerular cells by HIV.[7] HIVAN is more common in advanced HIV and the black race and is associated with significant proteinuria and a rapid decline in renal function. Among the different risk factors for developing end-stage renal disease (ESRD) in HIV-infected individuals, black race is the most important factor due to the ApoL1 gene mutation. In the United States from 2002 to 2006, 88% of HIV patients with ESRD were black.[8][9] These patients almost always have HIVAN. HIVAN is rarely associated with patients already on ART with normal CD4 counts. These individuals benefit from ART and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, but glucocorticoids have not been found to be helpful. The prognosis is poor and patients often reach ESRD with hemodialysis dependence. Despite ART, RNA viral expression can persist with the kidneys as an important reservoir for HIV-1.[10]
Another form of renal disease in the setting of HIV is called HIV immune complex kidney disease (HIVICK). It is also usually seen in advanced HIV and black race. The prognosis is also poor with 32% of patients reaching ESRD status within two years.[11] Unlike HIVAN, the beneficial role of ART has not been shown. Other forms of renal disease in the setting of HIV include IgA nephropathy, membranoproliferative glomerulonephritis, renal tubular acidosis, amyloid, acute postinfectious glomerulonephritis, membranous nephropathy, focal and segmental necrotizing glomerulonephritis, immunotactoid nephropathy, and thrombotic microangiopathy.[12]
Before starting ART in an antiretroviral naïve patient with impaired renal function, there are several considerations to keep in mind. First, certain ART medications require renal adjustment or are contraindicated with impaired renal function.[13] Second, after ART is initiated, follow up to monitor renal function for new onset or worsening renal disease is crucial as this can modify therapy. Third, screening for the HLA-B*5701 allele must be addressed before starting ART as HLA-B*5701 positivity predisposes the patient to a hypersensitivity reaction (abacavir) that can be life-threatening.[14] This can pose a challenging dilemma for cost, compliance, and efficacy as abacavir is included in many of the current one-pill ART regimens.
Having HIV is no longer a contraindication for renal transplantation. The Infectious Diseases Society of America (IDSA) strongly recommends the evaluation of HIV patients with ESRD or imminent ESRD for kidney transplantation after considering comorbidities, history of opportunistic infections, and virologic response with ART.[13]
Pearls of Wisdom
The initial approach to a patient with new-onset lower extremity edema can be challenging, with the initial workup, often focusing on the hepatic, cardiovascular or renal system. Screening for a vasculitic process is often performed, particularly when it involves the renal or pulmonary systems. It is important to understand the significance and limitations of serological markers when evaluating the patient. This case aims to highlight the potential pitfalls of positive serologic markers and the importance of keeping a broad differential in new-onset lower extremity swelling.
This review separating vasculitic renal disease from infectious etiology highlights the importance of not empirically treating a patient with positive serological markers for vasculitis with cytotoxic agents as this could be disastrous for a patient with HIV. This case also presents an atypical presentation of HIV infection and the need to have a high index of suspicion for the disease, even when risk factors are not apparent. Treatment regimens will also differ for an HIV patient with concomitant renal impairment and special considerations must be given before starting ART.
Enhancing Healthcare Team Outcomes
This case presents an excellent opportunity to discuss the importance of an interprofessional team management in managing patients with renal failure and HIV. Having either HIV or renal failure can overwhelm patients due to the complexity of care required for proper management and the need for long-term treatment. Patients with renal failure who depend on hemodialysis experience extraordinarily high burdens of stress which adversely affect treatment compliance and subsequent outcomes. Thankfully, interprofessional management of dialysis patients delivers a multitude of benefits including better clinical outcomes, cost savings, and improved patient quality of life.[15] An HIV diagnosis carries a significant stigma that negatively affects treatment outcomes and quality of life metrics. HIV care teams composed of physicians, pharmacists, nurses, counselors, psychologists, and social workers provide necessary holistic support to patients with demonstrable clinical improvements.[16] Complex HIV medication regimens are best managed by the interprofessional involvement of pharmacists to help anticipate and avoid drug-drug interactions which can cause significant side effects or reduce the efficacy of treatment.[17]
Media
(Click Image to Enlarge)
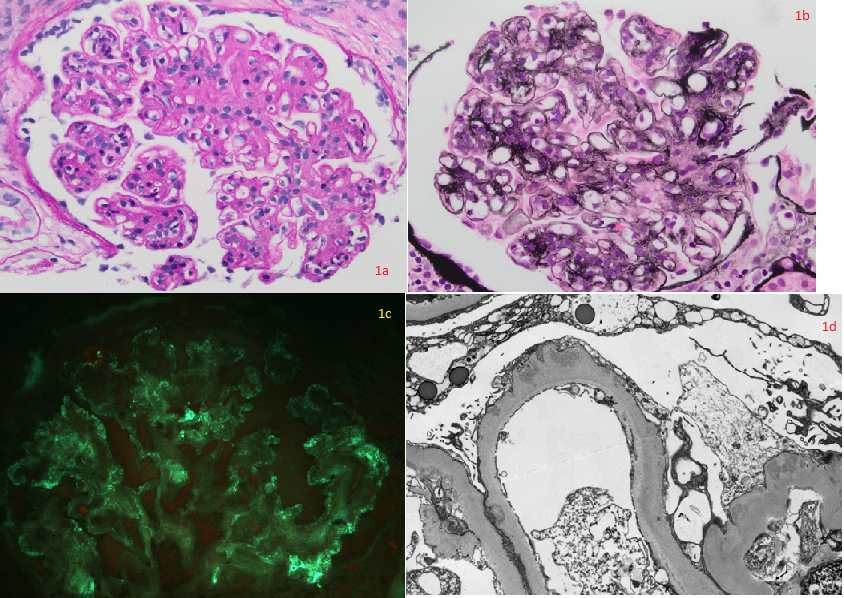
Fig. 1a (PAS x400) and 1b (Jones methenamine silver x400): Hyperlobulated glomeruli showing mesangial and segmental endocapillary hypercellularity, mesangial matrix expansion, and segmental duplication of basement membranes with mesangial cell interposition. Some peripheral capillary loops appear "wire loop“-like. Fig. 1c (FITC x400) Immunofluorescence microscopy showing segmental granular IgG staining. C3 and C1q showed similar staining. 1d: Ultrastructural examination showing thickened glomerular basement membranes with subendothelial and subepithelial electron-dense deposits consistent with immune complexes within peripheral loops, and diffuse foot process effacement. Contributed by Agnes Colanta, MD
References
Rasmussen N, Wiik A, Jayne DR. A historical essay on detection of anti-neutrophil cytoplasmic antibodies. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015 Apr:30 Suppl 1():i8-13. doi: 10.1093/ndt/gfv070. Epub [PubMed PMID: 25805749]
Wesolowski LG, Wroblewski K, Bennett SB, Parker MM, Hagan C, Ethridge SF, Rhodes J, Sullivan TJ, Ignacio-Hernando I, Werner BG, Owen SM. Nucleic acid testing by public health referral laboratories for public health laboratories using the U.S. HIV diagnostic testing algorithm. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2015 Apr:65():6-10. doi: 10.1016/j.jcv.2015.01.017. Epub 2015 Jan 24 [PubMed PMID: 25766979]
Muñoz A, Kirby AJ, He YD, Margolick JB, Visscher BR, Rinaldo CR, Kaslow RA, Phair JP. Long-term survivors with HIV-1 infection: incubation period and longitudinal patterns of CD4+ lymphocytes. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1995 Apr 15:8(5):496-505 [PubMed PMID: 7697447]
Level 2 (mid-level) evidenceKahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. The New England journal of medicine. 1998 Jul 2:339(1):33-9 [PubMed PMID: 9647878]
Tindall B, Barker S, Donovan B, Barnes T, Roberts J, Kronenberg C, Gold J, Penny R, Cooper D. Characterization of the acute clinical illness associated with human immunodeficiency virus infection. Archives of internal medicine. 1988 Apr:148(4):945-9 [PubMed PMID: 3258508]
Jansen TL, van Houte D, de Vries T, Wolthuis A. ANCA seropositivity in HIV: a serological pitfall. The Netherlands journal of medicine. 2005 Jul-Aug:63(7):270-4 [PubMed PMID: 16093579]
Level 3 (low-level) evidenceRoss MJ, Bruggeman LA, Wilson PD, Klotman PE. Microcyst formation and HIV-1 gene expression occur in multiple nephron segments in HIV-associated nephropathy. Journal of the American Society of Nephrology : JASN. 2001 Dec:12(12):2645-2651. doi: 10.1681/ASN.V12122645. Epub [PubMed PMID: 11729233]
Level 3 (low-level) evidenceWinston JA. HIV and CKD epidemiology. Advances in chronic kidney disease. 2010 Jan:17(1):19-25. doi: 10.1053/j.ackd.2009.08.006. Epub [PubMed PMID: 20005485]
Level 3 (low-level) evidenceGenovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science (New York, N.Y.). 2010 Aug 13:329(5993):841-5. doi: 10.1126/science.1193032. Epub 2010 Jul 15 [PubMed PMID: 20647424]
Level 2 (mid-level) evidenceWinston JA, Bruggeman LA, Ross MD, Jacobson J, Ross L, D'Agati VD, Klotman PE, Klotman ME. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. The New England journal of medicine. 2001 Jun 28:344(26):1979-84 [PubMed PMID: 11430327]
Level 3 (low-level) evidenceFoy MC, Estrella MM, Lucas GM, Tahir F, Fine DM, Moore RD, Atta MG. Comparison of risk factors and outcomes in HIV immune complex kidney disease and HIV-associated nephropathy. Clinical journal of the American Society of Nephrology : CJASN. 2013 Sep:8(9):1524-32. doi: 10.2215/CJN.10991012. Epub 2013 May 16 [PubMed PMID: 23685946]
Level 2 (mid-level) evidenceD'Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Seminars in nephrology. 1998 Jul:18(4):406-21 [PubMed PMID: 9692353]
Lucas GM,Ross MJ,Stock PG,Shlipak MG,Wyatt CM,Gupta SK,Atta MG,Wools-Kaloustian KK,Pham PA,Bruggeman LA,Lennox JL,Ray PE,Kalayjian RC, Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 Nov 1 [PubMed PMID: 25234519]
Level 1 (high-level) evidenceMallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jägel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A, PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. The New England journal of medicine. 2008 Feb 7:358(6):568-79. doi: 10.1056/NEJMoa0706135. Epub [PubMed PMID: 18256392]
Level 1 (high-level) evidenceHashemi MS, Irajpour A, Abazari P. Improving Quality of Care in Hemodialysis: a Content Analysis. Journal of caring sciences. 2018 Sep:7(3):149-155. doi: 10.15171/jcs.2018.024. Epub 2018 Sep 1 [PubMed PMID: 30283760]
Level 2 (mid-level) evidenceElgalib A, Al-Sawafi H, Kamble B, Al-Harthy S, Al-Sariri Q. Multidisciplinary care model for HIV improves treatment outcome: a single-centre experience from the Middle East. AIDS care. 2018 Sep:30(9):1114-1119. doi: 10.1080/09540121.2018.1479028. Epub 2018 May 24 [PubMed PMID: 29792340]
Molas E, Luque S, Retamero A, Echeverría-Esnal D, Guelar A, Montero M, Guerri R, Sorli L, Lerma E, Villar J, Knobel H. Frequency and severity of potential drug interactions in a cohort of HIV-infected patients Identified through a Multidisciplinary team. HIV clinical trials. 2018 Feb:19(1):1-7. doi: 10.1080/15284336.2017.1404690. Epub 2017 Nov 28 [PubMed PMID: 29179644]