Mycoplasma pneumoniae–Induced Rash and Mucositis (MIRM)
Mycoplasma pneumoniae–Induced Rash and Mucositis (MIRM)
Introduction
Mycoplasma pneumoniae is a common respiratory pathogen responsible for approximately 10% of all cases of community-acquired pneumonia, with rates rising as high as 37% among children in certain studies and geographic areas.[1][2] This bacterium is more prevalent in children and young adults. Aside from respiratory manifestations, M pneumoniae causes extrapulmonary syndromes, affecting approximately 25% of patients.[2][3] These syndromes include cold-agglutinin hemolytic anemia, arthritis, pericarditis, thrombosis, and mucocutaneous manifestations. Mucocutaneous eruptions, characterized by rashes affecting both mucous membrane and skin, have varied inciting etiologies, including infectious, frequently viral, drug-induced reactions, and autoimmune factors.[4][5]
Previously, M pneumoniae infections have been associated with rashes and mucocutaneous manifestations such as urticaria, erythema multiforme, Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS).[6][7] However, accurately classifying these rashes associated with M pneumoniae infections has proven challenging, leading to considerable controversy in the literature. Recently, a distinct pattern of mucocutaneous rash linked to M pneumoniae respiratory infections has spurred the proposal for a novel mucocutaneous entity, coined Mycoplasma pneumoniae–Induced rash and mucositis (or MIRM) by Canavan et al in 2015 in a systematic review.[8][9][10]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
M pneumoniae infections can lead to both pulmonary and extra-pulmonary diseases.[11] These extra-pulmonary conditions encompass vasculitides, neurological complications, immunological disorders, thrombotic events, and mucocutaneous manifestations. Notably, mucocutaneous manifestations occur in approximately 25% of patients with M pneumonia infections.[12]
MIRM, a recently identified mucocutaneous entity, was introduced and coined in a 2015 systematic review by Canavan et al.[13] This review, comprising 202 cases of M pneumoniae-associated erythema multiforme, SJS, and mucositis without rash, delineated the distinct features of MIRM.[13] MIRM is characterized by prominent mucositis with comparatively less cutaneous involvement than other mucocutaneous syndromes associated with M pneumoniae, such as urticaria, erythema nodosum, erythema multiforme, SJS, TEN, and DRESS.[6][7][14]
Epidemiology
M pneumoniae is a prevalent respiratory pathogen, accounting for about 10% of all cases of community-acquired pneumonia. This bacterium can lead to extrapulmonary syndromes in roughly 25% of patients, which include cold-agglutinin hemolytic anemia, arthritis, pericarditis, thrombosis, and mucocutaneous manifestations.[2][3] The hallmark of MIRM is the involvement of the mucosa, which typically manifests in the urogenital and oral regions with ulcerations, vesicles, bullae, and ocular involvement. These symptoms often include conjunctivitis and, in severe instances, may lead to conjunctival ulceration and pseudomembranous formation.[15]
M pneumoniae most frequently causes community-acquired pneumonia, particularly in children aged 5 or older, with some regions reporting it as the cause in up to 37% of pediatric cases.[1][2] Both M pneumoniae and MIRM occur mostly during the winter months.[16] A systematic review by Canavan et al, using their novel proposed definition, found that MIRM was mostly reported in children and young adolescents with a mean age of 12.[13] However, a more recent systematic review by Lofgren and Leinkeit found that MIRM cases ranged between the ages of 4 and 46, with a mean age of 16.[16] However, MIRM has also been reported in young adults.[17][18] In the systematic review by Canavan et al, 60% of the identified cases of MIRM occurred in males, with 47% of patients experiencing mucositis without significant skin involvement and 34% presenting with mucositis alone without any skin involvement.[13]
The exact incidence of MIRM is not known for various reasons. Importantly, until now, a distinct definition distinguishing it from other causes of mucocutaneous syndromes associated with M pneumoniae infection does not exist, leading to misclassification of the syndrome. Other contributing factors include potential underreporting, often due to M pneumoniae not being considered in the initial diagnosis, limited availability of resources for testing, failure to identify a definitive cause, and possible lack of reporting. Until 2015, the lack of a discreet definition for MIRM led to inconsistent naming conventions in publications describing cases of M pneumoniae infection. Examples include M pneumoniae–associated SJS, Fuchs syndrome, and SJS without skin lesions.[17][19][20]
Pathophysiology
The exact pathogenesis of MIRM is not completely elucidated. Proposed mechanisms include immune activation, resulting in polyclonal B-cell and antibody production, leading to immune complex deposition and complement activation, subsequently causing skin lesions. Other causes could be molecular mimicry between Mycoplasma P1-adhesion molecules and the host's keratinocytes, potentially inducing injury via antibodies or cytotoxic T cells.[12][21] These processes differ from the mechanisms underlying erythema multiforme and SJS/TEN, which are mediated by delayed hypersensitivity reaction and Fas ligand–mediated toxicity.[22] These differences can aid in the differentiation of MIRM from other cutaneous reactions.[13]
Histopathology
The presence of histopathological features unique to MIRM that differentiate it from erythema multiforme, SJS, and TEN remains uncertain. Erythema multiforme, SJS, and MIRM exhibit similar and overlapping histopathological characteristics, including apoptotic keratinocytes and sparse perivascular dermal infiltrates. The existence of distinct biopsy features enabling histopathological differentiation between these diseases remains controversial.
Rzany et al investigated specimens from erythema multiforme, SJS, and TEN and did not observe significant, consistent histologic distinctions. However, Wetter and Camilleri identified histopathologic features in drug-induced SJS that were absent in MIRM or immunization-induced SJS in some examined specimens.[23][24] Specifically, SJS may exhibit more necrotic keratinocytes, denser dermal infiltrates, microscopic red blood cell extravasation, pigment incontinence, and parakeratosis compared to MIRM.[23]
History and Physical
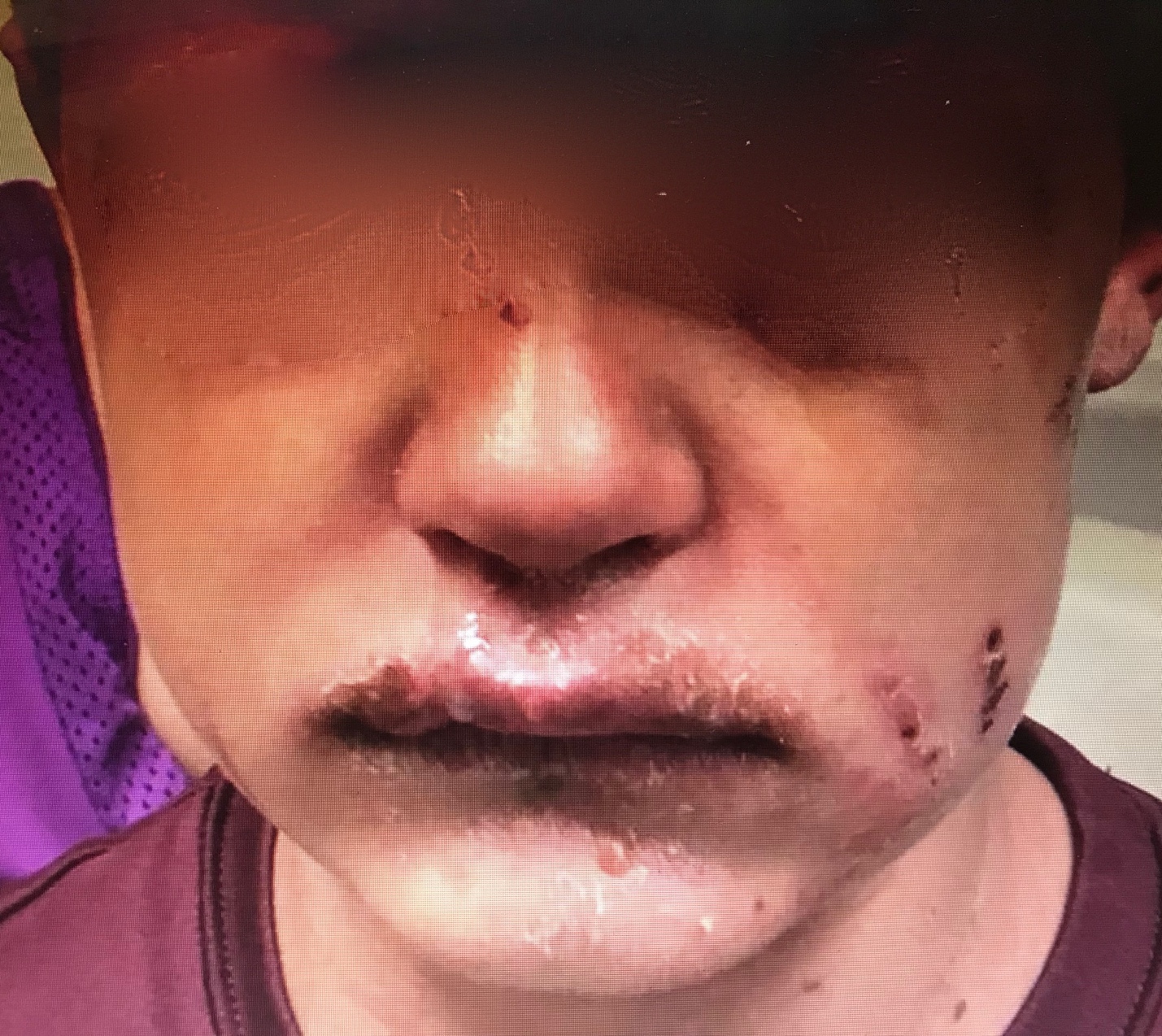
Obtaining a thorough and detailed history of the present illness is particularly crucial. Such information can provide valuable clues aiding in the differentiation of MIRM from other mucocutaneous eruptions, such as erythema multiforme and SJS/TEN. In the case of MIRM, nearly all patients typically experience prodromal symptoms, such as fever, cough, and malaise, approximately 1 week before the onset of the rash.[13] SJS/TEN, viewed as different spectra of the same underlying process, are often preceded by a prodrome of fever and upper respiratory infection symptoms. Additionally, patients with SJS/TEN usually have a history of recent exposure to new medications, such as antibiotics, nonsteroidal anti-inflammatory drugs, allopurinol, antiepileptics, and nevirapine.[25] This medication history may sometimes lead to a lower index of suspicion for infection during differential diagnosis evaluation (see Image. Mycoplasma pneumoniae–Induced Rash and Mucositis).
Physical examination findings in MIRM commonly reveal a predominance of mucosal rashes, with involvement of the oral mucosa (94%), ocular region (82%), and urogenital area (63%). Other mucosal sites, such as the nares and anus, may also be affected.[26] Mucosal lesions are typically characterized as ulcerative or hemorrhagic and may cause discomfort. Nasal involvement may present as dense hemorrhagic crusts, sometimes manifesting as blood on the tissue. Lesions in the anus, part of the gastrointestinal mucosa, can lead to pain during defecation (see Image. Mycoplasma pneumoniae–Induced Rash and Mucositis [MIRM]).[26]
Cutaneous non-mucosal rashes have been reported in 47% of MIRM cases, and if absent, the condition is classified as MIRM sine rash. When present, the cutaneous rash in MIRM displays distinct features compared to other mucocutaneous eruptions. MIRM rashes are typically sparse in distribution and are more commonly located in the acral regions (46%) than in the trunk (23%). The predominant morphology of the cutaneous rash in MIRM is described as vesiculobullous in 77% of cases. Additionally, both typical target lesions, characterized by 3 circumferential demarcation zones, and atypical target lesions, featuring 2 color zones, are observed in 48% of cases. Less commonly, rashes are described as papules (14%), macules (12%), or morbilliform (9%). The extent of detached skin typically involves less than 10% of the body surface area.[13]
In contrast, erythema multiforme presents initially as a cutaneous acral rash with macules that evolve into papules, plaques, and typical target lesions. These target lesions spread centripetally to the trunk and face. Erythema multiforme minor has little or no mucous membrane involvement, and erythema multiforme major has a rash on one or more mucous membranes. SJS/TEN manifests as a rash characterized by macules, purpura, diffuse erythema, atypical target lesions, and numerous flaccid blisters. These lesions are extensive in number and initially more concentrated centrally and gradually coalesce, spreading to involve the face and limbs. Extensive mucous membrane involvement affecting 2 or more mucosal sites is common. The amount of skin detachment determines the extent of SJS/TEN—SJS involves less than 10% skin detachment. Skin detachment ranging from 10% to 30% overlaps between SJS and TEN, whereas skin detachment exceeding 30% meets the criteria for TEN.[27]
Evaluation
Diagnosing MIRM involves the presence or recent occurrence of pulmonary symptoms, often leading to clinical findings suggestive of pneumonia, which may be confirmed through clinical examination and/or chest radiography. Laboratory assessment for the cause of pneumonia should include testing for elevated M pneumoniae IgM antibodies, detecting M pneumoniae from oropharyngeal or polymerase chain reaction (PCR) or bullae cultures, or obtaining serum cold agglutinins.[16]
The proposed definition, as outlined by Canavan et al,[13] and the classic diagnostic criteria for MIRM are mentioned below.[11]
- Evidence of atypical M pneumoniae pneumonia includes:
- Signs: Fever, cough, and auscultatory or radiographic findings.
- Laboratory findings: Increased M pneumoniae IgM antibodies, detection of M pneumoniae in oropharyngeal or bullae cultures or PCR, and/or serial cold agglutinins.
-
Skin detachment involving less than 10% of the body surface area.
- Involvement of at least 2 mucosal sites.
- Few vesiculobullous lesions or scattered atypical targets may be observed.
Within MIRM, 3 types differ in their cutaneous, non-mucosal rash patterns [13][22][16]
Classic MIRM: This subtype meets the classic criteria outlined above and additionally presents with a non-mucosal rash, characterized by vesiculobullous lesions (77%), scattered target lesions (48%), papules (14%), macules (12%), and morbilliform eruptions (9%).
MIRM sine rash: This subtype fulfills the classic criteria mentioned above but lacks significant cutaneous, non-mucosal rash presence, although a few fleeting morbilliform lesions or a few vesicles may be present.
Severe MIRM: This subtype meets the above classic criteria, with the involvement of more than 2 mucosal sites reported. Additionally, the cutaneous rash is extensive, featuring widespread non-mucosal blisters or flat atypical target lesions.
Treatment / Management
In the acute care setting, distinguishing MIRM from other mucocutaneous eruptions can be challenging for clinicians. Therefore, consultation with an infectious disease physician or dermatologist, or transfer to a facility with appropriate resources, may be warranted. Patients with MIRM require supportive care, including pain management for skin lesions and oral ulcerations (such as "magic mouthwash" solution or sucralfate), mucosal care, and correction of any fluid and nutritional deficiencies resulting from reduced oral intake. Severe cases of MIRM with extensive skin detachment may necessitate early transfer to a burn center.[16] In addition, lesions in particular mucosal areas may warrant specialty consultation with ophthalmology, otolaryngology, gastroenterology, and urology to mitigate long-term complications. (A1)
Although specific treatment guidelines are not tailored for MIRM, patients diagnosed with the condition often exhibit evidence of atypical pneumonia and may, therefore, benefit from antibiotic treatment.[28] Common oral antibiotic options for atypical pneumonia, such as macrolides, tetracyclines, and fluoroquinolones, are typically recommended, with macrolides being the preferred choice.[28] Reports of macrolide resistance have been increasing globally, with varying prevalence rates across different regions. The highest resistance to the lowest prevalence of resistance is observed in the following regions—the Western Pacific, South East Asia, the United States of America, Europe, and the East Mediterranean.[29] Therefore, clinicians must remain vigilant, particularly in cases of refractory Mycoplasma pneumoniae pneumonia, as alternative antibiotic regimens may need to be considered. (A1)
Empirical administration of corticosteroids and other immunosuppressive agents has been documented, particularly in patients with severe MIRM. Intravenous immunoglobulin (IVIG) has also been utilized in cases of MIRM with severe mucositis. In a study by Canavan et al, 35% of patients received systemic corticosteroids, and 8% received IVIG.[13] In a more recent systematic review by Lofgren and Lenkeit, 77% of patients received antibiotics, 37% were treated with corticosteroids, and 11% received IVIG.[16](A1)
Differential Diagnosis
Common differential considerations for MIRM include other conditions that can cause similar skin findings and/or mucosal manifestations[30][31]:
- Erythema multiforme major
- SJS/TEN
- DRESS
- Staphylococcal scalded skin syndrome
- Hand-foot-and-mouth disease
- Kawasaki disease
- Herpetic gingivostomatitis
- Severe cutaneous adverse reactions (eg, drug hypersensitivity syndrome)
- Bullous systemic lupus erythematosus
- Plasma cell stomatitis
- Coxsackie virus and other enteroviruses
- SARS-CoV-2 infection
- Autoimmune diseases, such as Behçet disease and systemic lupus erythematosus
Prognosis
Overall, the prognosis of MIRM is generally favorable. Only 4% of patients required admission to intensive care, with 81% experiencing complete recovery.[13] The recurrence rate of MIRM is 8%.[32] In contrast, patients afflicted with SJS/TEN often necessitate intensive care more frequently and face higher mortality rates.[33][24]
Complications
Although 81% of patients with MIRM experience a full recovery,[13] there are potential long-term sequelae, mostly involving the mucosa. Ocular mucosal damage occurs in 8.9% of patients with MIRM, resulting in conjunctival shrinkage, corneal ulcerations, blindness, ocular synechiae, and loss of eyelashes.[13] Postinflammatory pigmentary changes occurred in 5.6% of patients, while oral and genital mucosal synechiae were reported in 0.8% and 0.8% of patients, respectively. [13] Rare complications include persistent cutaneous lesions, B-cell lymphopenia, restrictive lung disease, and chronic obliterative bronchitis. However, while the mortality of MIRM is low, experts suspect that the vast majority of mild cases are underreported in the literature, suggesting that the true rate of morbidity and mortality is likely far lower.
Consultations
When considering the diagnosis of MIRM, consultation with infectious diseases specialists or dermatologists may be beneficial. Additionally, obtaining consultations with otolaryngologists, ophthalmologists, gastroenterologists, and urologists may be helpful for site-dependent mucosal lesions to mitigate long-term sequelae. In cases with extensive cutaneous lesions and detachment, consulting burn specialists is advisable.
Deterrence and Patient Education
Patients diagnosed with MIRM should be informed about the potential for painful mucosal lesions, which can lead to dehydration and reduced nutrient intake. In addition, it is important to keep cutaneous lesions clean to minimize the risk of secondary bacterial infection. Although the exact transmission patterns of MIRM are not well established, it is advisable for patients and close contacts of suspected M pneumoniae pulmonary infection to implement basic infection control measures, including droplet transmission precautions.
Pearls and Other Issues
- MIRM is considered a clinically distinct entity from other mucocutaneous eruptions.
- Historical features suggesting MIRM include a pulmonary infection approximately 1 week before the onset of the rash.
- Other mucocutaneous reactions, such as erythema multiforme, often develop after a herpes simplex infection.
- Although the rash of SJS/TEN may occur in the context of new medication use, it is crucial to include MIRM in the differential diagnosis.
- The rash pattern of MIRM may differ from that of other conditions, often characterized by mucous membrane involvement. In MIRM, the cutaneous rash often tends to be sparse rather than consolidated, frequently appearing in acral areas. Typically, the rash in MIRM is usually vesiculobullous or targets lesions.
- Although MIRM may result in some long-term sequelae, such as mucous membrane damage and cutaneous skin changes, it generally follows a more benign course than other conditions, including SJS/TEN.
- Currently, no specific guidelines exist for treating MIRM, but patients may benefit from antibiotics targeting atypical pneumonia and immunosuppressive therapy with corticosteroids. Additionally, IVIG administration should be considered as part of the treatment approach.
- Consulting with an infectious disease specialist or dermatologist can provide valuable guidance in directing patient care.
Enhancing Healthcare Team Outcomes
MIRM management is optimally conducted by an interprofessional team comprising infectious disease specialists, internists, primary care providers, and dermatologists. Collaborative efforts involving diagnosis, education, and patient support are crucial components of the interprofessional approach. The interprofessional team can achieve optimal outcomes by coordinating care and offering comprehensive patient education and support.
Patients diagnosed with MIRM should be informed about the potential for painful mucosal lesions, which can lead to dehydration and limit oral nutrient intake. In addition, it is important to keep cutaneous lesions as clean as possible to prevent secondary bacterial infection. Although the transmission patterns of MIRM are not well-established, it is advisable for patients and close contacts of suspected M pneumoniae pulmonary infection to implement basic infection control measures, including droplet transmission precautions.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Marchello C, Dale AP, Thai TN, Han DS, Ebell MH. Prevalence of Atypical Pathogens in Patients With Cough and Community-Acquired Pneumonia: A Meta-Analysis. Annals of family medicine. 2016 Nov:14(6):552-566. doi: 10.1370/afm.1993. Epub [PubMed PMID: 28376442]
Level 1 (high-level) evidenceGao LW, Yin J, Hu YH, Liu XY, Feng XL, He JX, Liu J, Guo Y, Xu BP, Shen KL. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China: 2006 to 2016. Epidemiology and infection. 2019 Jan:147():e192. doi: 10.1017/S0950268819000839. Epub [PubMed PMID: 31364532]
Poddighe D. Extra-pulmonary diseases related to Mycoplasma pneumoniae in children: recent insights into the pathogenesis. Current opinion in rheumatology. 2018 Jul:30(4):380-387. doi: 10.1097/BOR.0000000000000494. Epub [PubMed PMID: 29432224]
Level 3 (low-level) evidenceDrago F, Ciccarese G, Merlo G, Trave I, Javor S, Rebora A, Parodi A. Oral and cutaneous manifestations of viral and bacterial infections: Not only COVID-19 disease. Clinics in dermatology. 2021 May-Jun:39(3):384-404. doi: 10.1016/j.clindermatol.2021.01.021. Epub 2021 Feb 1 [PubMed PMID: 34517997]
Chiewchengchol D, Murphy R, Edwards SW, Beresford MW. Mucocutaneous manifestations in juvenile-onset systemic lupus erythematosus: a review of literature. Pediatric rheumatology online journal. 2015:13():1. doi: 10.1186/1546-0096-13-1. Epub 2015 Jan 5 [PubMed PMID: 25587243]
Marquart E, Kinaciyan T. Overlapping clinical presentation of Mycoplasma-induced rash and mucositis and drug-induced Stevens Johnson Syndrome: A case report. IDCases. 2023:33():e01888. doi: 10.1016/j.idcr.2023.e01888. Epub 2023 Aug 30 [PubMed PMID: 37693950]
Level 3 (low-level) evidenceFrantz R, Huang S, Are A, Motaparthi K. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Review of Diagnosis and Management. Medicina (Kaunas, Lithuania). 2021 Aug 28:57(9):. doi: 10.3390/medicina57090895. Epub 2021 Aug 28 [PubMed PMID: 34577817]
Vujic I, Shroff A, Grzelka M, Posch C, Monshi B, Sanlorenzo M, Ortiz-Urda S, Rappersberger K. Mycoplasma pneumoniae-associated mucositis--case report and systematic review of literature. Journal of the European Academy of Dermatology and Venereology : JEADV. 2015 Mar:29(3):595-8. doi: 10.1111/jdv.12392. Epub 2014 Feb 17 [PubMed PMID: 24665876]
Level 3 (low-level) evidenceSantos RP, Silva M, Vieira AP, Brito C. Mycoplasma pneumoniae-induced rash and mucositis: a recently described entity. BMJ case reports. 2017 Aug 22:2017():. pii: bcr-2017-220768. doi: 10.1136/bcr-2017-220768. Epub 2017 Aug 22 [PubMed PMID: 28830900]
Level 3 (low-level) evidenceVarghese C, Sharain K, Skalski J, Ramar K. Mycoplasma pneumonia-associated mucositis. BMJ case reports. 2014 Mar 13:2014():. doi: 10.1136/bcr-2014-203795. Epub 2014 Mar 13 [PubMed PMID: 24626386]
Level 3 (low-level) evidenceNarita M. Classification of Extrapulmonary Manifestations Due to Mycoplasma pneumoniae Infection on the Basis of Possible Pathogenesis. Frontiers in microbiology. 2016:7():23. doi: 10.3389/fmicb.2016.00023. Epub 2016 Jan 28 [PubMed PMID: 26858701]
Meyer Sauteur PM, Theiler M, Buettcher M, Seiler M, Weibel L, Berger C. Frequency and Clinical Presentation of Mucocutaneous Disease Due to Mycoplasma pneumoniae Infection in Children With Community-Acquired Pneumonia. JAMA dermatology. 2020 Feb 1:156(2):144-150. doi: 10.1001/jamadermatol.2019.3602. Epub [PubMed PMID: 31851288]
Canavan TN, Mathes EF, Frieden I, Shinkai K. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. Journal of the American Academy of Dermatology. 2015 Feb:72(2):239-45. doi: 10.1016/j.jaad.2014.06.026. Epub [PubMed PMID: 25592340]
Level 1 (high-level) evidenceBastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Archives of dermatology. 1993 Jan:129(1):92-6 [PubMed PMID: 8420497]
Level 3 (low-level) evidenceGandelman JS, Kim EY, Grzegorczyk AM, Zejnullahu K, Edson RS. Mycoplasma pneumoniae-Induced Rash and Mucositis in a Previously Healthy Man: A Case Report and Brief Review of the Literature. Open forum infectious diseases. 2020 Oct:7(10):ofaa437. doi: 10.1093/ofid/ofaa437. Epub 2020 Sep 14 [PubMed PMID: 33094121]
Level 3 (low-level) evidenceLofgren D, Lenkeit C. Mycoplasma Pneumoniae-Induced Rash and Mucositis: A Systematic Review of the Literature. Spartan medical research journal. 2021:6(2):25284. doi: 10.51894/001c.25284. Epub 2021 Aug 30 [PubMed PMID: 34532621]
Level 1 (high-level) evidenceAlcántara-Reifs CM, García-Nieto AV. Mycoplasma pneumoniae-associated mucositis. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2016 Jul 12:188(10):753. doi: 10.1503/cmaj.151017. Epub 2016 Feb 29 [PubMed PMID: 26927973]
Figueira-Coelho J, Lourenço S, Pires AC, Mendonça P, Malhado JA. Mycoplasma pneumoniae-associated mucositis with minimal skin manifestations. American journal of clinical dermatology. 2008:9(6):399-403. doi: 10.2165/0128071-200809060-00008. Epub [PubMed PMID: 18973408]
Level 3 (low-level) evidenceTay YK, Huff JC, Weston WL. Mycoplasma pneumoniae infection is associated with Stevens-Johnson syndrome, not erythema multiforme (von Hebra). Journal of the American Academy of Dermatology. 1996 Nov:35(5 Pt 1):757-60 [PubMed PMID: 8912572]
Šternberský J, Tichý M. Fuchs' syndrome (Stevens-Johnson syndrome without skin involvement) in an adult male--a case report and general characteristics of the sporadically diagnosed disease. Acta dermatovenerologica Croatica : ADC. 2014:22(4):284-7 [PubMed PMID: 25580788]
Level 3 (low-level) evidenceMazori DR, Nagarajan S, Glick SA. Recurrent reactive infectious mucocutaneous eruption (RIME): Insights from a child with three episodes. Pediatric dermatology. 2020 May:37(3):545-547. doi: 10.1111/pde.14142. Epub 2020 Mar 15 [PubMed PMID: 32172537]
Martínez-Pérez M, Imbernón-Moya A, Lobato-Berezo A, Churruca-Grijelmo M. Mycoplasma pneumoniae-Induced Mucocutaneous Rash: A New Syndrome Distinct from Erythema Multiforme? Report of a New Case and Review of the Literature. Actas dermo-sifiliograficas. 2016 Sep:107(7):e47-51. doi: 10.1016/j.ad.2015.09.023. Epub 2016 Mar 31 [PubMed PMID: 27040303]
Level 3 (low-level) evidenceRzany B, Hering O, Mockenhaupt M, Schröder W, Goerttler E, Ring J, Schöpf E. Histopathological and epidemiological characteristics of patients with erythema exudativum multiforme major, Stevens-Johnson syndrome and toxic epidermal necrolysis. The British journal of dermatology. 1996 Jul:135(1):6-11 [PubMed PMID: 8776350]
Level 2 (mid-level) evidenceWetter DA, Camilleri MJ. Clinical, etiologic, and histopathologic features of Stevens-Johnson syndrome during an 8-year period at Mayo Clinic. Mayo Clinic proceedings. 2010 Feb:85(2):131-8. doi: 10.4065/mcp.2009.0379. Epub [PubMed PMID: 20118388]
Level 2 (mid-level) evidenceChatproedprai S, Wutticharoenwong V, Tempark T, Wananukul S. Clinical Features and Treatment Outcomes among Children with Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A 20-Year Study in a Tertiary Referral Hospital. Dermatology research and practice. 2018:2018():3061084. doi: 10.1155/2018/3061084. Epub 2018 May 7 [PubMed PMID: 29853855]
Norton SA. Diagnosing Mycoplasma pneumoniae-induced rash and mucositis (MIRM) in the emergency room. Journal of the American Academy of Dermatology. 2015 Aug:73(2):e67. doi: 10.1016/j.jaad.2015.03.060. Epub [PubMed PMID: 26184002]
Noe MH, Micheletti RG. Diagnosis and management of Stevens-Johnson syndrome/toxic epidermal necrolysis. Clinics in dermatology. 2020 Nov-Dec:38(6):607-612. doi: 10.1016/j.clindermatol.2020.06.016. Epub 2020 Jun 30 [PubMed PMID: 33341195]
Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, Kaplan SL, Mace SE, McCracken GH Jr, Moore MR, St Peter SD, Stockwell JA, Swanson JT, Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Executive summary: the management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Oct:53(7):617-30. doi: 10.1093/cid/cir625. Epub [PubMed PMID: 21890766]
Level 1 (high-level) evidenceKim K, Jung S, Kim M, Park S, Yang HJ, Lee E. Global Trends in the Proportion of Macrolide-Resistant Mycoplasma pneumoniae Infections: A Systematic Review and Meta-analysis. JAMA network open. 2022 Jul 1:5(7):e2220949. doi: 10.1001/jamanetworkopen.2022.20949. Epub 2022 Jul 1 [PubMed PMID: 35816304]
Level 1 (high-level) evidenceMortazavi H, Safi Y, Baharvand M, Rahmani S. Diagnostic Features of Common Oral Ulcerative Lesions: An Updated Decision Tree. International journal of dentistry. 2016:2016():7278925 [PubMed PMID: 27781066]
Jain H, Singh G, Endy T. Mycoplasma pneumoniae-Induced Rash and Mucositis (MIRM) Mimicking Behçet's Disease and Paraneoplastic Pemphigus (PNP). Case reports in infectious diseases. 2022:2022():1013922. doi: 10.1155/2022/1013922. Epub 2022 Aug 22 [PubMed PMID: 36046665]
Level 3 (low-level) evidenceJelić D, Antolović R. From Erythromycin to Azithromycin and New Potential Ribosome-Binding Antimicrobials. Antibiotics (Basel, Switzerland). 2016 Sep 1:5(3):. doi: 10.3390/antibiotics5030029. Epub 2016 Sep 1 [PubMed PMID: 27598215]
Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens-Johnson syndrome: a review. Critical care medicine. 2011 Jun:39(6):1521-32. doi: 10.1097/CCM.0b013e31821201ed. Epub [PubMed PMID: 21358399]