Introduction
ALK-negative anaplastic large cell lymphoma (ALK(-) ALCL) is an uncommon CD30-positive T-cell lymphoma that presents a major diagnostic challenge. It affects individuals in a wide age range and a variety of nodal and extranodal sites. Both morphologically and immunohistochemically, anaplastic large cell lymphoma (ALCL) can mimic many other hematologic and non-hematologic malignancies. Lack of expression of pan T-cell markers and a spectrum of histologic appearances can be misleading. Recent research has shown that DUSP22 rearrangements occur in ALK(-) ALCL with a favorable prognosis. This article provides an overview of the many challenges associated with the diagnosis of this entity.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
No causative agents or predisposing factors have so far been demonstrated with ALCL. There is no convincing evidence for Epstein-Barr virus or human T-cell leukemia virus involvement in the origin of ALCL. In addition, the correlation between ALCL and immunologic disorders has not been well documented [1]. However, there is a clinically distinct variant of ALCL associated with breast implants [2][1][3].
Epidemiology
Anaplastic large cell lymphoma (ALCL) refers to a group of CD30-positive T-cell lymphomas that have overlapping morphologies and immunophenotypes but have different genetics and clinical behavior. The 2017 World Health Organization Classification (WHO) characterizes ALCL into three distinct subsets: ALK-positive ALCL, ALK-negative ALCL (ALK(-) ALCL), and breast implant-associated ALCL. ALK-positive refers to the presence of chromosomal rearrangements of the ALK gene at 2p23 with varied gene partners. These tumors typically have a favorable prognosis. In contrast, ALK(-) ALCL lacks ALK gene rearrangements and generally have poorer outcomes than ALK-positive ALCLs with more variable genetic and clinical features [4][5]. Primary cutaneous ALCL presents in the skin, may involve regional lymph nodes, rarely disseminates, and has typically good outcomes. In contrast to systemic ALCL, primary cutaneous ALCL is typically negative for ALK rearrangements [6]. A unique subtype of ALCL with a favorable prognosis has been described in association with breast implants [2][3]. This type of ALCL is not discussed in depth in this article. The revised WHO establishes ALK(-) ALCL as a definite entity (previously a provisional entity in the 2008 WHO), and breast implant-associated ALCL is recognized as a provisional entity [7].
In most studies, about 40% to 50% of systemic ALCLs are ALK-negative [8]. Unlike younger aged patients with ALK-positive ALCL, ALK(-) ALCL typically affects adults (40 to 65 years). Males are more commonly affected than females with a male: female ratio of 1.5:1 [8]. Patients typically present with adenopathy and B symptoms with advanced stage III to IV disease. ALK(-) ALCL usually involves lymph nodes at diagnosis (49%) and, less frequently, extranodal sites (20%) [1]. However, the disease can present in nearly any site including skin, the central nervous system (CNS), oral cavity, bladder, and liver [9][10][11][12][13]. No particular risk factors have been identified.
Histopathology
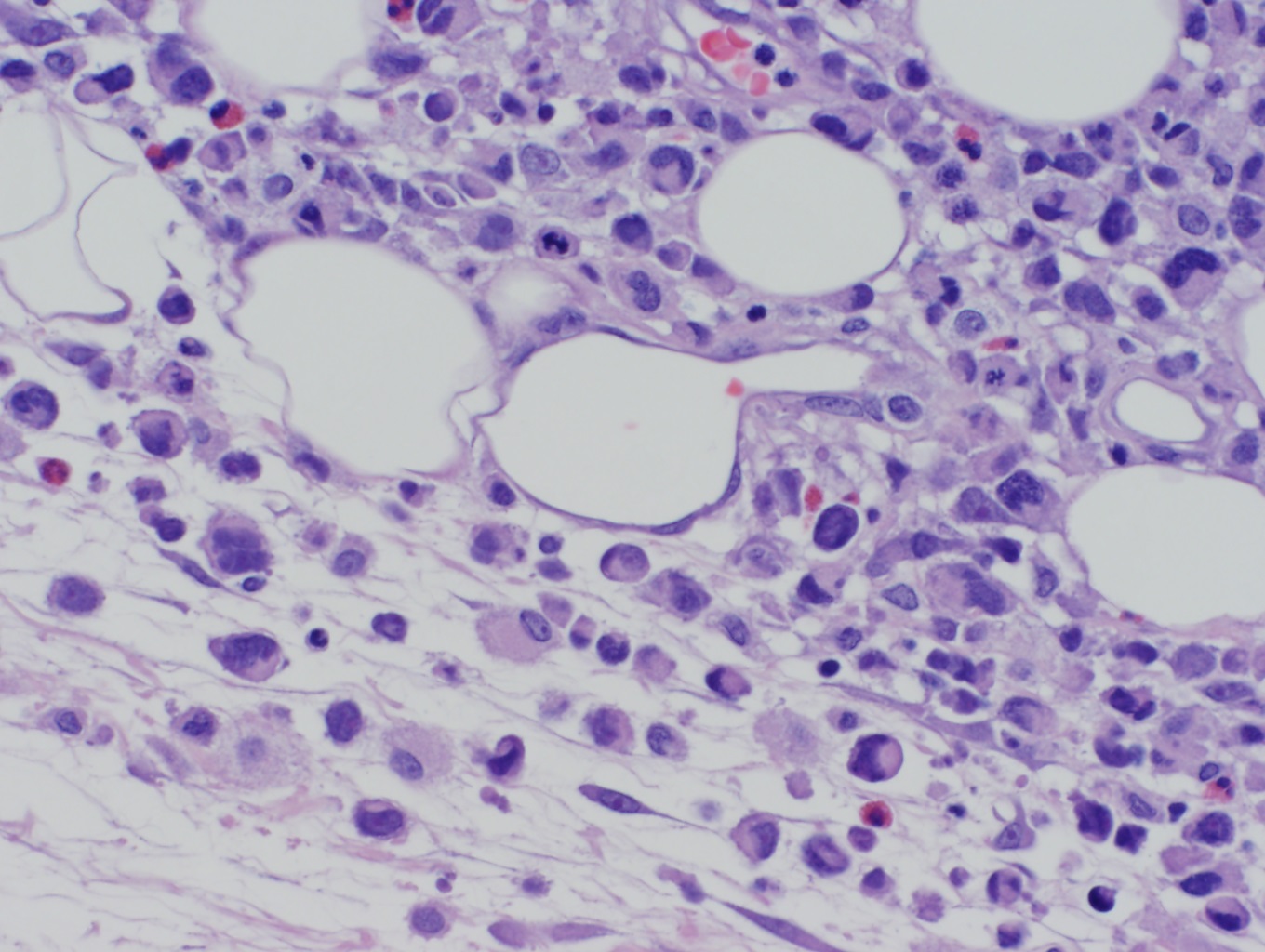
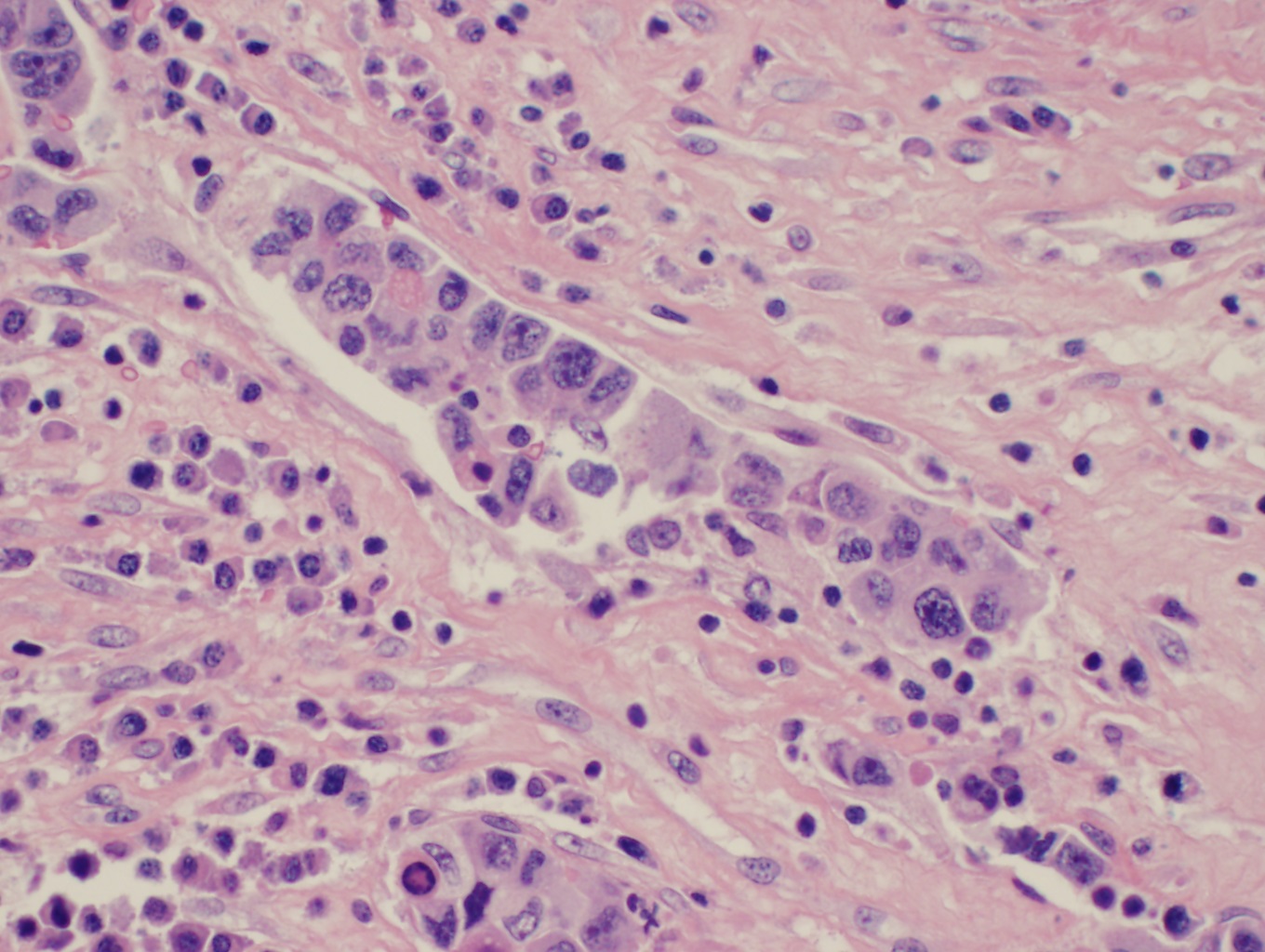
ALK(-) ALCL tends to grow cohesively and invade lymph node sinuses. The WHO classification describes several histologic patterns including common, small cell, lymphohistiocytic, and Hodgkin-like. In the common pattern, the tumor cells have abundant cytoplasm with wreath-like nuclei (see image). The small cell pattern is typically associated with ALK-positive and not ALK(-) ALCL. This pattern consists of small to medium-sized neoplastic cells with pale cytoplasm and centrally located nuclei referred to as “fried egg cells.” In the lymphohistiocytic pattern, the tumor cells are mixed with reactive histiocytes which sometimes show erythrophagocytosis. The Hodgkin-like pattern is characterized by morphological features similar to nodular sclerosis classical Hodgkin lymphoma. All patterns demonstrate some examples of a characteristic cell type known as the “hallmark” cell which is a large cell with abundant cytoplasm, containing a nucleus that is horseshoe or kidney-shaped around a prominent central Golgi zone. Sometimes, the nucleus may appear “doughnut” shaped when it envelops the Golgi [4]. The neoplastic cells in ALK(-) ALCL tend to be larger and more pleomorphic, or have a higher nuclear: cytoplasmic ratio than those seen with ALK-positive ALCL.
The architecture of involved organs or lymph nodes is usually effaced by a solid, sheet-like infiltrate of tumor cells. Frequently within lymph nodes, the neoplastic cells appear as cohesive groups within sinuses, mimicking carcinoma. When bone marrow is involved, the pattern is typically nodular but may also be interstitial [14]. Cases with exclusively interstitial involvement may be difficult to diagnose by routine stains alone due to the paucity of cells present. Extraordinary cases may present with extensive blood and bone marrow involvement, described as “leukemic phase” [15].
History and Physical
Patients usually present with B symptoms with advanced stage III to IV disease with peripheral or abdominal lymphadenopathy. [1] Lymph node involvement is most common. Extranodal spread is less common than in the ALK-positive form. The most frequent extranodal sites are skin, liver, and lung. [1] Of note, there is a primary cutaneous anaplastic large cell lymphoma that may be difficult to distinguish clinically from secondary skin involvement by systemic ALCL. [16] Clinical presentation includes solitary or localized skin lesions such as tumors, nodules, or papules. [6]
Evaluation
The most critical stain for diagnosing ALK(-) ALCL is the diffuse and strong positivity for CD30. It is usually membranous and in the Golgi staining region, although diffuse cytoplasmic positivity is also common. Usually, at least one T-cell antigen is expressed such as CD2, CD3, CD5, and CD7. If there is a loss of T-cell antigens (“null-cell phenotype”), CD43 may be useful to establish the lesion as hematolymphoid. CD45 is not reliably positive. CD4 is typically positive, and CD8 is negative. Many express the cytotoxic molecules TIA-1, Granzyme-B, perforin, and clusterin. Vimentin may be positive and should not be interpreted as evidence of mesenchymal origin. EBV-latent membrane protein type 1 and EBER in situ hybridization are negative in ALK(-) ALCL [17]. Markers of B-cell lineage are absent, including PAX5, CD19, CD20, and CD79a. However, MUM1, which is typically seen on B-cell and plasma cell neoplasms, is frequently expressed [18]. By definition, ALK immunohistochemistry is negative, which reflects the lack of an ALK rearrangement. Fluorescent in-situ hybridization (FISH) studies for ALK rearrangement are not necessary if immunohistochemistry is negative.
Until recently, the genetics of ALK(-) ALCL has been unknown. Recent studies have shown that 30% of ALK(-) ALCL have chromosomal rearrangements of DUSP22 and 8% have chromosomal rearrangements of TP63 [19]. These rearrangements are mutually exclusive of each other and are absent in ALK-positive ALCLs [5]. Furthermore, DUSP22 rearranged cases have favorable outcomes similar to ALK-positive ALCL. By contrast, TP63 rearranged ALCLs have worse outcomes. Five-year overall survival rates for DUSP22 rearranged ALCL were 90%, compared to 85% for ALK-positive ALCL. The rate for TP63 rearranged ALCL was 17%, and the rate for cases lacking all three genetic markers (ALK, DUSP22, and TP63) was 42% [19]. Testing for DUSP22 and TP63 rearrangements is not widely available at this time.
Gene expression profiling is also useful as a diagnostic tool to distinguish peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) and cutaneous ALCL from ALK(-) ALCL. IRF4 (interferon regulatory factor-4) translocations are suggestive of cutaneous ALCL over other CD30 positive lymphoproliferative disorders. Systemic ALCL tends to have other IRF4 abnormalities but no translocations [1][20]. A recent study has defined a set of three genes (TNFRSF8, BATF3, and TMOD1) whose co-expression could potentially separate ALK(-) ALCL from peripheral T-cell lymphomas. The study found that when these three genes are expressed, ALK(-), ALCL could be distinguished from PTCL-NOS with an overall accuracy near 97% in unrelated groups of patients [21].
Treatment / Management
There is no known optimal therapy for ALK(-) ALCL due to disease rarity, heterogeneity of clinical presentation, and lack of randomized trials focused on this lymphoma. ALK(-) ALCL is usually analyzed with other T-cell lymphomas, including most peripheral T-cell lymphoma categories, so there are few retrospective studies focused solely on adult patients with ALK(-) ALCL. Chemotherapy for peripheral T-cell lymphomas is derived from experiences in treating aggressive B-cell lymphomas. ALK(-) ALCL is generally responsive to doxorubicin-based chemotherapy regimens, but relapses are frequent [1]. The standard first-line treatment for systemic ALCL is cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). The standard therapy for patients with relapsed or refractory disease has not been established. Newer approaches include targeted therapy such as Brentuximab vedotin (BV), an anti-CD30 antibody-drug conjugate used for relapsed/refractory systemic ALCL [1][22]. A recent study reported a case of relapsed systemic ALK-negative ALCL that was similar to the primary disease except for the loss of CD30 expression immunohistochemically after BV therapy [22].
Differential Diagnosis
Biopsies of ALK(-) ALCL are challenging and require exclusion of other malignancies. ALK(-) ALCL can mimic other non-hematologic malignancies such as sarcomas, carcinomas, germ cell tumors, and melanomas both cytologically and histologically. This is especially true in lesions characterized predominately by anaplasia and pleomorphism when “hallmark cells” with horseshoe-shaped or wreath-like nuclei are difficult to appreciate [20]. Besides nuclear morphology, the architectural pattern of ALCL can mimic other non-hematologic malignancies as both ALK(-) ALCL, and metastatic carcinoma tend to invade lymph node sinuses. Special care should be taken in small specimens, especially the fine-needle aspiration (FNA) specimen. There have been several case reports describing cytomorphologic features of ALCL mimicking soft tissue sarcomas on FNA biopsy specimens [23][24].
In the absence of clear histologic clues to the cell of origin, a typical initial immunohistochemical workup will often attempt to broadly classify the lesion as carcinoma (with a pancytokeratin cocktail), melanoma (with S100 or more specific markers), or lymphoma (with CD45). If the initial round of stains is either negative or only positive for CD45, CD30 should be performed as part of a more directed panel. To differentiate between ALCL and metastatic carcinoma, epithelial membrane antigen (EMA) staining should be avoided as it is more common in ALK-positive ALCL (83%) than in ALK(-) ALCL (43%) [20][25]. Instead, cytokeratins should be preferred as markers of epithelial differentiation, which have only rarely been reported in ALCL [26].
Detection of diffuse and strong CD30 expression should raise a high suspicion of ALCL. Many other lymphoid neoplasms may be variably CD30 positive: classical Hodgkin lymphoma, diffuse large B-cell lymphoma, PTCL-NOS, enteropathy associated T-cell lymphoma (type 2), extranodal NK/T-cell lymphoma, nasal type, and transformed mycosis fungoides. Non-neoplastic immunoblast proliferations show variable CD30 positivity as well. Other tumors such as embryonal carcinoma are typically CD30 positive but are excluded by keratin expression. Although there are many entities which may express CD30, they typically not have the diffuse and strong pattern that is seen in ALCL.
Differentiating ALK(-) ALCL from other CD30 positive T cell lymphoproliferative disorders can be challenging because of shared morphologic and immunophenotypical features. This pattern of CD30 expression is important to distinguish ALK(-) ALCL from other peripheral T cell lymphomas that have variable CD30 expression. An exception to this rule is the variable CD30 staining seen in the small-cell variant of ALK-positive ALCL [27]. ALK-positive ALCL is easily distinguished from ALK(-) ALCL by the presence of ALK staining by immunohistochemistry. The diagnosis of primary cutaneous ALCL requires clinicopathologic correlation to rule out secondary involvement systemic ALCL.
IHC plays a relevant role in distinguishing ALK(-) ALCL from classical Hodgkin lymphoma (CHL). This is especially useful if there is a high concentration of malignant cells in a case of CHL, as is seen in the syncytial variant. CHL is recognizable for B-cell marker expression and dim PAX-5 positivity, CD15, and occasionally EBER. MUM1 does not distinguish between ALCL and CHL. Only exceptional cases of ALCL express PAX-5 [1][28]. It is important to recognize that CHL will frequently express T-cell antigens. CD4 and CD2 are seen in more than half of cases; CD3, CD5, and/or CD7 are seen in approximately one-third of cases [29]]. Cytotoxic T-cell markers, such as TIA-1 are typically positive in ALK(-) ALCL and negative in CHL.
There is no specific immunophenotypic or genetic features to define ALK(-) ALCL; the immunophenotype of primary cutaneous and systemic ALK(-) ALCL are indistinguishable [8]. Both have strong expression of CD30 with variable expression of T-cell antigens (CD2, CD3, and CD5). Both often express CD4 and cytotoxic proteins. Primary cutaneous ALCL expresses cutaneous lymphocytes antigen more frequently and EMA less frequently than ALK(-) ALCL, but these markers are not specific enough to distinguish these entities by phenotype alone [8][25]. At present, both entities share recurrent genetic abnormalities, of which the most common are DUSP22 rearrangements which occur in about 28% of cases of primary cutaneous ALCL and 30% of systemic ALK(-) ALCL which may secondarily involve the skin. Clinical staging remains necessary to distinguish DUSP22 rearranged cases of primary cutaneous ALCL from systemic ALK(-) ALCL with DUSP22 rearrangement and skin involvement. ALK-positive ALCL is molecularly characterized and can be readily diagnosed with the highly specific combination of CD30 and ALK positivity.
Prognosis
Recent studies have shown differences in overall prognosis among patients with ALK(-) ALCL based on the presence of different chromosomal rearrangements. For instance, 30% of patients with ALK(-) ALCL have been found to have the DUSP22 rearrangement with a 90% five-year overall survival rate, which is similar to that of ALK-positive ALCL. 8% of ALK(-) ALCL patients have the TP63 rearrangement with a far worse five-year overall survival rate of 17%. Finally, the 5-year overall survival rate of patients lacking ALK, DUSP22, or TP63 rearrangement is 42% [4][19]. In comparison to other CD30 positive T-cell lymphomas, patients with ALK(-) ALCL have a superior 5-year overall survival rate (49%) in comparison to PTCL-NOS (32%), but ALK-positive ALCL still has the better prognosis (70%). However, the favorable prognosis may be due to a younger age of presentation as one study found no outcome difference when the analysis was limited to ALCL patients 40 years of age and older. In comparison, primary cutaneous ALCL seems to have a more indolent course because it has a favorable 5-year overall survival rate (90%), but with a propensity to relapse with a 5-year failure-free survival of 55% [25].
Pearls and Other Issues
ALK(-) ALCL is a T-cell lymphoma characterized by large, anaplastic lymphoid cells with uniform, strong expression of CD30 but a lack of ALK protein expression. It tends to grow cohesively and invade lymph node sinuses. It also has a vast morphologic spectrum and may mimic other entities featuring pleomorphic and anaplastic cells with wreath-like or horseshoe-shaped nuclei. Morphology and immunohistochemistry are the mainstays used to distinguish this entity from other ALK-positive ALCL, CD30 positive T-cell lymphomas, and classical Hodgkin lymphoma as well as other non-hematologic malignancies such as carcinomas, sarcomas, and melanomas. ALK(-) ALCL has a poorer prognosis than ALK-positive ALCL, but it has a better prognosis than peripheral T-cell Lymphoma, NOS. Molecular studies have identified DUSP22 rearranged ALK(-) ALCL as having a prognosis similar to ALK-positive ALCL. Regarding treatment, ALK(-) ALCL is generally responsive to doxorubicin-containing chemotherapy (CHOP regimen), but relapses are frequent. CD30 is a newer therapeutic target and brentuximab vedotin (anti CD30) has been used for relapsed/refractory systemic ALCL.
Enhancing Healthcare Team Outcomes
When healthcare professionals see patients with symptoms suggestive of lymphoma, prompt referral to an oncologist/hematologist is recommended. These patients may present with solitary or localized skin lesions such as tumors, nodules. Prior to undertaking treatment, the type of lymphoma needs to be ascertained. ALK(-) ALCL is a T-cell lymphoma characterized by large, anaplastic lymphoid cells with uniform, strong expression of CD30 but a lack of ALK protein expression. It tends to grow cohesively and invade lymph node sinuses. ALK(-) ALCL has a poorer prognosis than ALK-positive ALCL, but it has a better prognosis than peripheral T-cell Lymphoma, NOS. Molecular studies have identified DUSP22 rearranged ALK(-) ALCL as having a prognosis similar to ALK-positive ALCL. Regarding treatment, ALK(-) ALCL is generally responsive to doxorubicin-containing chemotherapy (CHOP regimen), but relapses are frequent. CD30 is a newer therapeutic target and brentuximab vedotin (anti CD30) has been used for relapsed/refractory systemic ALCL.
Media
(Click Image to Enlarge)
References
Ferreri AJ, Govi S, Pileri SA, Savage KJ. Anaplastic large cell lymphoma, ALK-negative. Critical reviews in oncology/hematology. 2013 Feb:85(2):206-15. doi: 10.1016/j.critrevonc.2012.06.004. Epub 2012 Jul 11 [PubMed PMID: 22789917]
Brody GS. Anaplastic Large Cell Lymphoma Occurring in Women with Breast Implants: Analysis of 173 Cases. Plastic and reconstructive surgery. 2015 Oct:136(4):553e-554e. doi: 10.1097/PRS.0000000000001601. Epub [PubMed PMID: 26086383]
Level 3 (low-level) evidenceJewell M, Spear SL, Largent J, Oefelein MG, Adams WP Jr. Anaplastic large T-cell lymphoma and breast implants: a review of the literature. Plastic and reconstructive surgery. 2011 Sep:128(3):651-661. doi: 10.1097/PRS.0b013e318221db81. Epub [PubMed PMID: 21865998]
Level 2 (mid-level) evidenceKing RL, Dao LN, McPhail ED, Jaffe ES, Said J, Swerdlow SH, Sattler CA, Ketterling RP, Sidhu JS, Hsi ED, Karikehalli S, Jiang L, Gibson SE, Ondrejka SL, Nicolae A, Macon WR, Dasari S, Parrilla Castellar E, Feldman AL. Morphologic Features of ALK-negative Anaplastic Large Cell Lymphomas With DUSP22 Rearrangements. The American journal of surgical pathology. 2016 Jan:40(1):36-43. doi: 10.1097/PAS.0000000000000500. Epub [PubMed PMID: 26379151]
Zeng Y, Feldman AL. Genetics of anaplastic large cell lymphoma. Leukemia & lymphoma. 2016:57(1):21-7. doi: 10.3109/10428194.2015.1064530. Epub 2015 Jul 21 [PubMed PMID: 26104084]
DeCoteau JF, Butmarc JR, Kinney MC, Kadin ME. The t(2;5) chromosomal translocation is not a common feature of primary cutaneous CD30+ lymphoproliferative disorders: comparison with anaplastic large-cell lymphoma of nodal origin. Blood. 1996 Apr 15:87(8):3437-41 [PubMed PMID: 8605362]
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19:127(20):2391-405. doi: 10.1182/blood-2016-03-643544. Epub 2016 Apr 11 [PubMed PMID: 27069254]
Xing X, Feldman AL. Anaplastic large cell lymphomas: ALK positive, ALK negative, and primary cutaneous. Advances in anatomic pathology. 2015 Jan:22(1):29-49. doi: 10.1097/PAP.0000000000000047. Epub [PubMed PMID: 25461779]
Level 3 (low-level) evidenceGrandhi A, Boros AL, Berardo N, Reich RF, Freedman PD. Two cases of CD30+, anaplastic lymphoma kinase (ALK)-negative anaplastic large cell lymphoma with oral manifestations. Oral surgery, oral medicine, oral pathology and oral radiology. 2013 Feb:115(2):e41-7. doi: 10.1016/j.oooo.2012.04.010. Epub 2012 Aug 31 [PubMed PMID: 22940020]
Level 3 (low-level) evidenceKodama K, Hokama M, Kawaguchi K, Tanaka Y, Hongo K. Primary ALK-1-negative anaplastic large cell lymphoma of the brain: case report and review of the literature. Neuropathology : official journal of the Japanese Society of Neuropathology. 2009 Apr:29(2):166-71. doi: 10.1111/j.1440-1789.2008.00935.x. Epub 2008 Jun 28 [PubMed PMID: 18564100]
Level 3 (low-level) evidenceSaikia UN, Sharma N, Duseja A, Bhalla A, Joshi K. Anaplastic large cell lymphoma presenting as acute liver failure: A report of two cases with review of literature. Annals of hepatology. 2010 Oct-Dec:9(4):457-61 [PubMed PMID: 21057166]
Level 3 (low-level) evidenceLobo J, Henrique R, Monteiro P, Lobo C. ALK-negative anaplastic large cell lymphoma with urinary bladder involvement diagnosed in urine cytology: A case report and literature review. Diagnostic cytopathology. 2017 Apr:45(4):354-358. doi: 10.1002/dc.23669. Epub 2017 Jan 31 [PubMed PMID: 28139895]
Level 3 (low-level) evidenceQuerfeld C, Khan I, Mahon B, Nelson BP, Rosen ST, Evens AM. Primary cutaneous and systemic anaplastic large cell lymphoma: clinicopathologic aspects and therapeutic options. Oncology (Williston Park, N.Y.). 2010 Jun:24(7):574-87 [PubMed PMID: 20669794]
Park SH, Chi HS, Cho YU, Jang S, Park CJ. Immunohistopathological features of anaplastic large-cell lymphoma according to anaplastic lymphoma kinase expression and bone marrow involvement pattern. Histopathology. 2013 Jul:63(1):13-8. doi: 10.1111/his.12141. Epub 2013 May 8 [PubMed PMID: 23656317]
Lu Y, Zhao X, Wang E, Chen W, Huang Q. ALK-negative anaplastic large cell lymphoma with extensive peripheral blood and bone marrow involvements manifested as "leukemic phase". Leukemia research. 2010 Apr:34(4):475-82. doi: 10.1016/j.leukres.2009.07.034. Epub 2009 Aug 19 [PubMed PMID: 19695703]
Level 3 (low-level) evidenceWada DA, Law ME, Hsi ED, Dicaudo DJ, Ma L, Lim MS, Souza Ad, Comfere NI, Weenig RH, Macon WR, Erickson LA, Ozsan N, Ansell SM, Dogan A, Feldman AL. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011 Apr:24(4):596-605. doi: 10.1038/modpathol.2010.225. Epub 2010 Dec 17 [PubMed PMID: 21169992]
Level 2 (mid-level) evidenceHerling M, Rassidakis GZ, Jones D, Schmitt-Graeff A, Sarris AH, Medeiros LJ. Absence of Epstein-Barr virus in anaplastic large cell lymphoma: a study of 64 cases classified according to World Health Organization criteria. Human pathology. 2004 Apr:35(4):455-9 [PubMed PMID: 15116326]
Level 3 (low-level) evidenceKempf W, Kutzner H, Cozzio A, Sander CA, Pfaltz MC, Müller B, Pfaltz M. MUM1 expression in cutaneous CD30+ lymphoproliferative disorders: a valuable tool for the distinction between lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. The British journal of dermatology. 2008 Jun:158(6):1280-7. doi: 10.1111/j.1365-2133.2008.08566.x. Epub 2008 Apr 10 [PubMed PMID: 18410414]
Parrilla Castellar ER, Jaffe ES, Said JW, Swerdlow SH, Ketterling RP, Knudson RA, Sidhu JS, Hsi ED, Karikehalli S, Jiang L, Vasmatzis G, Gibson SE, Ondrejka S, Nicolae A, Grogg KL, Allmer C, Ristow KM, Wilson WH, Macon WR, Law ME, Cerhan JR, Habermann TM, Ansell SM, Dogan A, Maurer MJ, Feldman AL. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014 Aug 28:124(9):1473-80. doi: 10.1182/blood-2014-04-571091. Epub 2014 Jun 3 [PubMed PMID: 24894770]
Level 2 (mid-level) evidenceFornari A, Piva R, Chiarle R, Novero D, Inghirami G. Anaplastic large cell lymphoma: one or more entities among T-cell lymphoma? Hematological oncology. 2009 Dec:27(4):161-70. doi: 10.1002/hon.897. Epub [PubMed PMID: 19358142]
Agnelli L, Mereu E, Pellegrino E, Limongi T, Kwee I, Bergaggio E, Ponzoni M, Zamò A, Iqbal J, Piccaluga PP, Neri A, Chan WC, Pileri S, Bertoni F, Inghirami G, Piva R, European T-Cell Lymphoma Study Group. Identification of a 3-gene model as a powerful diagnostic tool for the recognition of ALK-negative anaplastic large-cell lymphoma. Blood. 2012 Aug 9:120(6):1274-81 [PubMed PMID: 22740451]
Level 2 (mid-level) evidenceAl-Rohil RN, Torres-Cabala CA, Patel A, Tetzlaff MT, Ivan D, Nagarajan P, Curry JL, Miranda RN, Duvic M, Prieto VG, Aung PP. Loss of CD30 expression after treatment with brentuximab vedotin in a patient with anaplastic large cell lymphoma: a novel finding. Journal of cutaneous pathology. 2016 Dec:43(12):1161-1166. doi: 10.1111/cup.12797. Epub 2016 Sep 15 [PubMed PMID: 27531242]
Hudacko R, Rapkiewicz A, Berman RS, Simsir A. ALK-negative anaplastic large cell lymphoma mimicking a soft tissue sarcoma. Journal of cytology. 2011 Oct:28(4):230-3. doi: 10.4103/0970-9371.86362. Epub [PubMed PMID: 22090705]
Level 3 (low-level) evidenceVij M, Dhir B, Verma R, Agrawal V, Agarwal V, Jaiswal S, Pandey R. Cytomorphology of ALK+ anaplastic large cell lymphoma displaying spindle cells mimicking a sarcomatous tumor: report of a case. Diagnostic cytopathology. 2011 Oct:39(10):775-9. doi: 10.1002/dc.21552. Epub 2010 Oct 1 [PubMed PMID: 20890996]
Level 3 (low-level) evidenceSavage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, Rimsza L, Pileri SA, Chhanabhai M, Gascoyne RD, Armitage JO, Weisenburger DD, International Peripheral T-Cell Lymphoma Project. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008 Jun 15:111(12):5496-504. doi: 10.1182/blood-2008-01-134270. Epub 2008 Apr 2 [PubMed PMID: 18385450]
Level 2 (mid-level) evidenceZhang Q, Ming J, Zhang S, Li B, Han X, Qiu X. Cytokeratin positivity in anaplastic large cell lymphoma: a potential diagnostic pitfall in misdiagnosis of metastatic carcinoma. International journal of clinical and experimental pathology. 2013:6(4):798-801 [PubMed PMID: 23573330]
Level 3 (low-level) evidenceKinney MC, Collins RD, Greer JP, Whitlock JA, Sioutos N, Kadin ME. A small-cell-predominant variant of primary Ki-1 (CD30)+ T-cell lymphoma. The American journal of surgical pathology. 1993 Sep:17(9):859-68 [PubMed PMID: 8394652]
Arun I, Roy P, Arora N, Bhave SJ, Nair R, Chandy M. PAX-5 Positivity in Anaplastic Lymphoma Kinase-Negative Anaplastic Large Cell Lymphoma: A Case Report and Review of Literature. International journal of surgical pathology. 2017 Jun:25(4):333-338. doi: 10.1177/1066896916683447. Epub 2016 Dec 26 [PubMed PMID: 28013563]
Level 3 (low-level) evidenceVenkataraman G, Song JY, Tzankov A, Dirnhofer S, Heinze G, Kohl M, Traverse-Glehen A, Eberle FC, Hanson JC, Raffeld MA, Pittaluga S, Jaffe ES. Aberrant T-cell antigen expression in classical Hodgkin lymphoma is associated with decreased event-free survival and overall survival. Blood. 2013 Mar 7:121(10):1795-804. doi: 10.1182/blood-2012-06-439455. Epub 2013 Jan 10 [PubMed PMID: 23305738]
Level 2 (mid-level) evidence