Introduction
Galen initially described the ventricular system of the brain. As more information arose about the anatomy of the brain, anatomists described the cerebral aqueduct as a narrow communication duct between the third and fourth ventricles. The word aqueduct comes from the Latin word “aqueductus" which translates to a canal used for taking water through a structure to another location. It is unclear when the eponym ‘Aqueduct of Sylvius’ first appeared; it is believed to be traced back to the well-known anatomist Franciscus Sylvius.[1] The cerebral aqueduct (of Sylvius) plays an essential role in the ventricular system of the brain and when disrupted can have some significant clinical manifestations.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
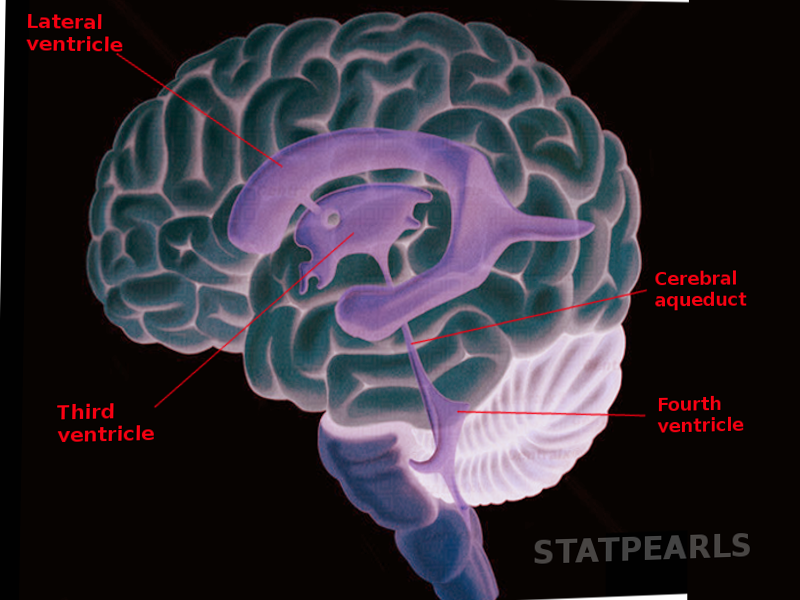
The cerebral aqueduct is a narrow 15 mm conduit that allows for cerebrospinal fluid (CSF) to flow between the third ventricle and the fourth ventricle.[1][2] It has undergone subdivision into many different sections by different anatomists but most commonly is described as having a pars anterior, antrum, and pars posterior.
The pars anterior is a dorsally based triangle under the posterior commissure that connects to the third ventricle and runs ventrally to the tectum of the midbrain. It is followed by the antrum that is flanked on either side by constrictions formed by the superior and inferior colliculus. Finally, the pars posterior has the narrowest lumen and connects to the rhomboid fossa of the fourth ventricle.[1][3][4] The aqueduct is highly variable in its size, but the impression caused by the inferior colliculi creates the smallest diameter in the aqueduct.[2] Phase-contrast magnetic resonance imaging (MRI) can be used to analyze the flow of CSF throughout the ventricular system. The CSF flow velocity increases as it flows from the pars anterior to the smaller diameter pars posterior.[5]
HISTOLOGY
The brain's ventricular system is lined by a ciliated cuboidal to columnar epithelial cells, known as ependymal cells, which lack tight junctions. Subependymal glial cells lie below the ependymal cells and together with astrocyte processes and blood vessels form the blood-brain barrier. At both the rostral and caudal boundaries of the cerebral aqueduct are the third and fourth ventricles which are both associated with a choroid plexus. The choroid plexus is a collection of specialized ependymal cells and a collection of blood vessels that produce the CSF that flows through the ventricular system.[2]
Embryology
During neurulation, the neural tube closes, and three dilations develop on the cephalic end of the neural tube. These dilations include the prosencephalon, mesencephalon, and rhombencephalon which collectively make up the embryonic brain vesicles. Within the evolving vesicles there remains a lumen that later will become the ventricular system of the brain. The cavity of the mesencephalon narrows forming the cerebral aqueduct. As the fetus develops the aqueduct lumen decreases in size and continues to contract up until ten weeks after birth. Both neuroepithelial and non-neuroepithelial cells secrete embryonic CSF which helps maintain constant intraluminal pressure to help keep the ventricles open. The embryonic CSF is rich in proteins and growth factors which further support the proliferation and differentiation of the ventricles.[1]
Blood Supply and Lymphatics
Specialized structure of ependymal cells and blood vessels, collectively known as choroid plexus, produce the CSF that flows through the cerebral aqueduct. Throughout the ventricles of the brain, tufts of fenestrated capillaries make up the choroid plexus. At the rostral and caudal ends of the cerebral aqueduct lies a choroid plexus. In the third ventricle, branches of the posterior choroidal arteries contribute to the formation of the choroid plexus. In the fourth ventricle, branches of the anterior and posterior cerebellar arteries contribute to the formation of the choroid plexus.[2] The cerebral aqueduct runs through the mesencephalon and requires an adequate blood supply to the surrounding mesencephalon.
Nerves
The oculomotor or the third nerve nuclei are in the dorsal midbrain, ventral to the cerebral aqueduct. Multiple subnuclei of the third nerve complex are found ventral to the periaqueductal gray matter in the midbrain. Branches from the multiple nuclei converge to form a fascicle that travels ventrally through the midbrain, exiting from the medial portion of the cerebral peduncle to travel through the subarachnoid space in the interpeduncular cistern, traveling medially to the posterior communicating artery.
The trochlear nerve nuclei lie ventral to the cerebral aqueduct. Fibers of trochlear nerve exit the trochlear nucleus, course dorsally, wrap around the cerebral aqueduct and then decussate in the superior medullary velum. Distal to their decussation, trochlear nerves then exit the dorsal aspect of midbrain inferior to the contralateral inferior colliculus, then travel around brainstem between the superior cerebellar and posterior cerebral arteries (smaller than oculomotor nerve but similarly passing between these arteries).[6]
Physiologic Variants
Using phase-contrast MR imaging, CSF flow through the cerebral aqueduct has some physiologic age and sex-related variants. The maximum aqueduct CSF flow in the aqueduct is higher in younger individuals compared to CSF flow in the elderly. Peak CSF velocity also decreases with age in both the caudal and cranial direction. As the brain ages, the parenchyma begins to soften causing decreased elasticity. CSF flow dynamics show a smooth sine wave-form that develops with age due to this physiologic change. Males have higher aqueduct CSF stroke volumes as well as higher average aqueduct CSF flow rates compared to their female counterparts. These physiological variants are essential to consider when looking at aqueduct pathological changes.[7]
Surgical Considerations
Ventriculostomy or endoscopic third ventriculostomy (ETV) is a procedure that has decreased morbidity and mortality in the treatment of hydrocephalus secondary to aqueduct stenosis with success rates of greater than 80%. A small opening is incised on the floor of the third ventricle to facilitate the drainage of CSF fluid as well as monitor intracranial pressure.[6][8]
Ventricular shunting is a way to remove excess CSF in patients with hydrocephalus. The role of the shunt is to drain excess CSF from the ventricles to another body cavity where it can be reabsorbed and returned to circulation. The most common shunt is the ventriculoperitoneal (VP) shunt which drains into the peritoneal cavity. A ventriculoatrial (VA) shunt is when the peritoneal cavity is not suitable for catheter placement. Instead, a VA shunt drains the fluid into the right atrium of the heart.[1]
The cerebral aqueduct can be an access point for flexible endoscopy to reach the fourth ventricle which can be helpful for the removal of ventricle cysts and clots from the fourth ventricle.[4]
Clinical Significance
The cerebral aqueduct is the narrowest portion of the ventricular system, and aqueductal stenosis or other changes to its structure are a common cause of hydrocephalus. There is a bimodal distribution with the first peak happening before one year of age and the other peaking after 12 years of age. Early-onset aqueductal stenosis usually presents with increased head circumference, tense anterior fontanelle, and sunset eyes. Compare this presentation to late-onset aqueduct stenosis, which presents with headache, visual deterioration, urinary incontinence, abnormal gait, and unusual behavior. Treatments for hydrocephalus associated with aqueductal stenosis treatments include ventriculoperitoneal shunt or endoscopic third ventriculostomy.[9][10]
Early-onset aqueductal stenosis is hypothesized to be due to intrauterine infection, atresia, or inherited as a hereditary sex-linked recessive condition.[11][12] During early development, the cerebral aqueduct is large and subsequently narrows with time. Atresia is when the narrowing causes complete obliteration of the aqueductal conduit. Full closure of the aqueduct appears in conjunction with CNS defects including Arnold-Chiari malformation, spina bifida, Dandy-Walker malformation, retrocerebellar or supracollicular cysts, and vascular malformation. X-linked hydrocephalus has also been a cause of congenital aqueductal stenosis. Along with the narrowing of the aqueduct, other midline brain abnormalities are often seen in these patients, although there are no homogenous collection of structural changes.[3]
Onset in patients over 1 year of age may have many different etiologies, including, gliosis, forking, and mechanical compression, which all lead to obstructive non-communicating hydrocephalus.[13] Gliosis is a protective reaction by the body to prevent injury due to noxious stimuli like infection or toxins by inducing glial proliferation. A few cases of gliosis appear as a post-viral complication of individuals infected with human myxoviruses. Acute and subacute phase inflammatory reaction to the virus may cause further constriction of the lumen of the aqueduct.[3] Studies of hamsters and mice infected with mumps, influenza A and parainfluenza II confirmed this phenomenon.[14] Abnormal development of the median fissure may lead to a septum to form within the aqueduct. This septum formation causes the development of two or more separate canals which is known as forking. Forking may be seen independently or in combination with other congenital CNS abnormalities including spina bifida and holoprosencephaly.[3][13] The separate lumens may both be open to the fourth ventricle or end in blind pouches which can lead to obstruction.[3] Structural changes to the aqueduct seen in patients diagnosed with hydrocephalus associated with myelomeningocele may also cause a similar phenomenon of forking as the aqueduct shortens and forms a beak-like configuration at the dorsal end of the aqueduct. Tumors of the mesencephalon can cause compression of the aqueduct leading to aqueductal stenosis.[15]
Tumors that may cause aqueductal obstruction include tectal plate gliomas and growths within the pineal region. Tectal plate gliomas are a form of midbrain gliomas that are slow-growing and low-grade tumors of the oligodendrocytic histological subtype. Tectal plate gliomas are often asymptomatic but can slowly invade into the cerebral aqueduct and cause delayed-onset aqueduct stenosis. MR imaging can provide strong evidence to support the diagnosis, but a definitive diagnosis requires histopathological analysis.[16] Pineal tumors most often arise as solid growths in children and are classified into pineal parenchymal tumors, germinal tumors, glial tumors, or pineal cysts. The pineal gland sits adjacent to the cerebral aqueduct so expansion within this region can obstruct the aqueduct.[17]
Compression of the aqueduct by the vein of Galen or Vein of Galen aneurysmal malformation (VGAM) is a congenital vascular malformation that accounts for 30% of all pediatric vascular abnormalities. The vascular defect causes the shunting of arterial blood from choroidal circulation to the prosencephalic vein of Markowski (MProsV of Markowski). In healthy development, the MProsV of Markowski completely closes off as their role is taken over by the internal cerebral veins. Formation of arteriovenous shunts connecting the choroidal circulation with the MProsV of Markowski cause increase flow through the MProsV of Markowski and prevent its closure. The continued patency of the MProsV of Markowski is known as a VGAM. Hydrocephalus and congestive heart failure are both presenting features of VGAM. Both a decrease absorption of CSF as well as a compression of the cerebral aqueduct can cause the clinical picture of hydrocephalus. In infants, a trans-fontanelle ultrasound can be used to evaluate the brain anatomy and identify a VGAM. MR imaging can help make a definitive diagnosis and assess comorbid conditions such as brain infractions, atrophy, and hydrocephalus.[18]
Media
References
Mortazavi MM, Adeeb N, Griessenauer CJ, Sheikh H, Shahidi S, Tubbs RI, Tubbs RS. The ventricular system of the brain: a comprehensive review of its history, anatomy, histology, embryology, and surgical considerations. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2014 Jan:30(1):19-35. doi: 10.1007/s00381-013-2321-3. Epub 2013 Nov 16 [PubMed PMID: 24240520]
Level 3 (low-level) evidenceJellinger G. Anatomopathology of non-tumoral aqueductal stenosis. Journal of neurosurgical sciences. 1986 Jan-Jun:30(1-2):1-16 [PubMed PMID: 3772492]
Level 3 (low-level) evidenceLongatti P, Fiorindi A, Perin A, Martinuzzi A. Endoscopic anatomy of the cerebral aqueduct. Neurosurgery. 2007 Sep:61(3 Suppl):1-5; discussion 5-6 [PubMed PMID: 17876227]
Longatti P, Fiorindi A, Feletti A, D'Avella D, Martinuzzi A. Endoscopic anatomy of the fourth ventricle. Journal of neurosurgery. 2008 Sep:109(3):530-5. doi: 10.3171/JNS/2008/109/9/0530. Epub [PubMed PMID: 18759587]
Level 2 (mid-level) evidenceLee JH, Lee HK, Kim JK, Kim HJ, Park JK, Choi CG. CSF flow quantification of the cerebral aqueduct in normal volunteers using phase contrast cine MR imaging. Korean journal of radiology. 2004 Apr-Jun:5(2):81-6 [PubMed PMID: 15235231]
Tisell M, Almström O, Stephensen H, Tullberg M, Wikkelsö C. How effective is endoscopic third ventriculostomy in treating adult hydrocephalus caused by primary aqueductal stenosis? Neurosurgery. 2000 Jan:46(1):104-10; discussion 110-1 [PubMed PMID: 10626941]
Schmid Daners M, Knobloch V, Soellinger M, Boesiger P, Seifert B, Guzzella L, Kurtcuoglu V. Age-specific characteristics and coupling of cerebral arterial inflow and cerebrospinal fluid dynamics. PloS one. 2012:7(5):e37502. doi: 10.1371/journal.pone.0037502. Epub 2012 May 30 [PubMed PMID: 22666360]
Deopujari CE, Karmarkar VS, Shaikh ST. Endoscopic Third Ventriculostomy: Success and Failure. Journal of Korean Neurosurgical Society. 2017 May:60(3):306-314. doi: 10.3340/jkns.2017.0202.013. Epub 2017 May 1 [PubMed PMID: 28490157]
Kaur LP, Munyiri NJ, Dismus WV. Clinical analysis of aqueductal stenosis in patients with hydrocephalus in a Kenyan setting. The Pan African medical journal. 2017:26():106. doi: 10.11604/pamj.2017.26.106.11050. Epub 2017 Feb 28 [PubMed PMID: 28533829]
Fukuhara T, Luciano MG. Clinical features of late-onset idiopathic aqueductal stenosis. Surgical neurology. 2001 Mar:55(3):132-6; discussion 136-7 [PubMed PMID: 11311904]
BICKERS DS, ADAMS RD. Hereditary stenosis of the aqueduct of Sylvius as a cause of congenital hydrocephalus. Brain : a journal of neurology. 1949 Jun:72(Pt. 2):246-62 [PubMed PMID: 18136715]
WARREN MC, LU AT, ZIERING WH. SEX-LINKED HYDROCEPHALUS WITH AQUEDUCTAL STENOSIS. The Journal of pediatrics. 1963 Dec:63():1104-10 [PubMed PMID: 14089815]
Feletti A, Fiorindi A, Longatti P. Split cerebral aqueduct: a neuroendoscopic illustration. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2016 Jan:32(1):199-203. doi: 10.1007/s00381-015-2827-y. Epub 2015 Aug 1 [PubMed PMID: 26231565]
Johnson RT, Johnson KP. Hydrocephalus as a sequela of experimental myxovirus infections. Experimental and molecular pathology. 1969 Feb:10(1):68-80 [PubMed PMID: 4303572]
Level 3 (low-level) evidenceSolá J, Arcas I, Martínez-Lage JF, Martínez Pérez M, Esteban JA, Poza M. Astrocytoma of the cerebral aqueduct. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1987:3(5):294-6 [PubMed PMID: 3427574]
Level 3 (low-level) evidenceIgboechi C, Vaddiparti A, Sorenson EP, Rozzelle CJ, Tubbs RS, Loukas M. Tectal plate gliomas: a review. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2013 Oct:29(10):1827-33. doi: 10.1007/s00381-013-2110-z. Epub 2013 Apr 24 [PubMed PMID: 23612874]
Rousselle C, des Portes V, Berlier P, Mottolese C. Pineal region tumors: Clinical symptoms and syndromes. Neuro-Chirurgie. 2015 Apr-Jun:61(2-3):106-12. doi: 10.1016/j.neuchi.2013.08.009. Epub 2014 Jan 17 [PubMed PMID: 24439798]
Recinos PF, Rahmathulla G, Pearl M, Recinos VR, Jallo GI, Gailloud P, Ahn ES. Vein of Galen malformations: epidemiology, clinical presentations, management. Neurosurgery clinics of North America. 2012 Jan:23(1):165-77. doi: 10.1016/j.nec.2011.09.006. Epub [PubMed PMID: 22107867]